Abstract
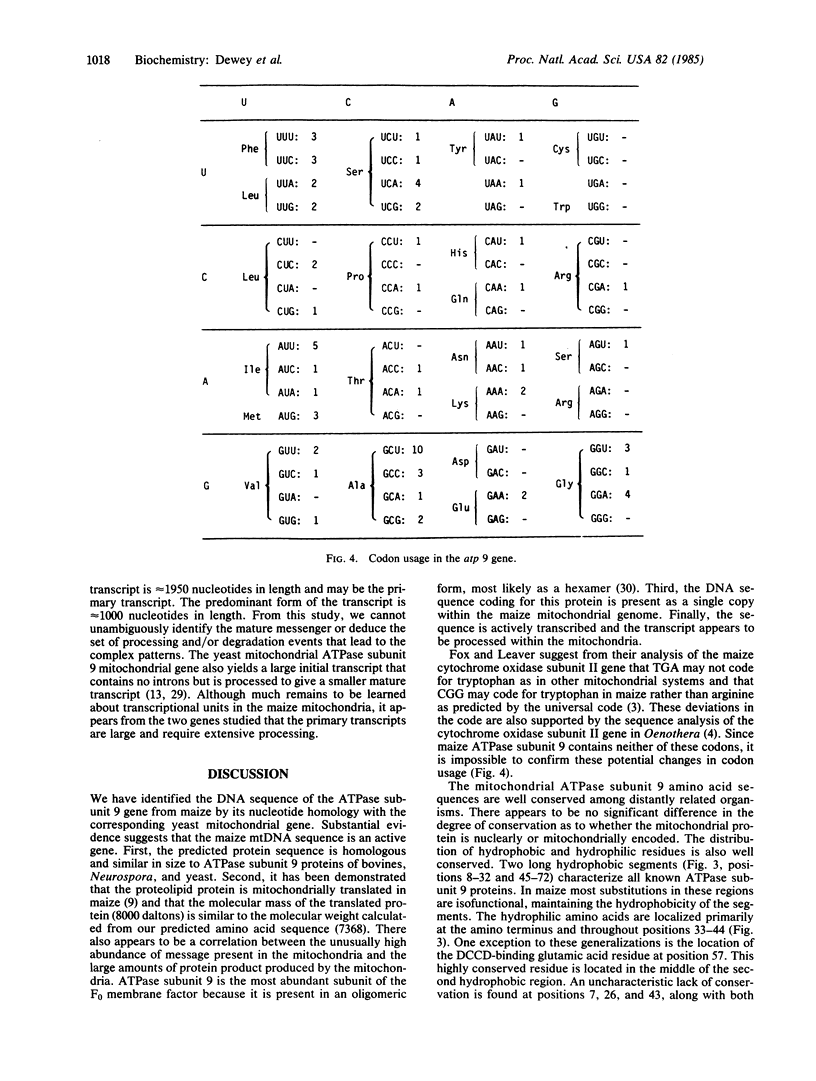
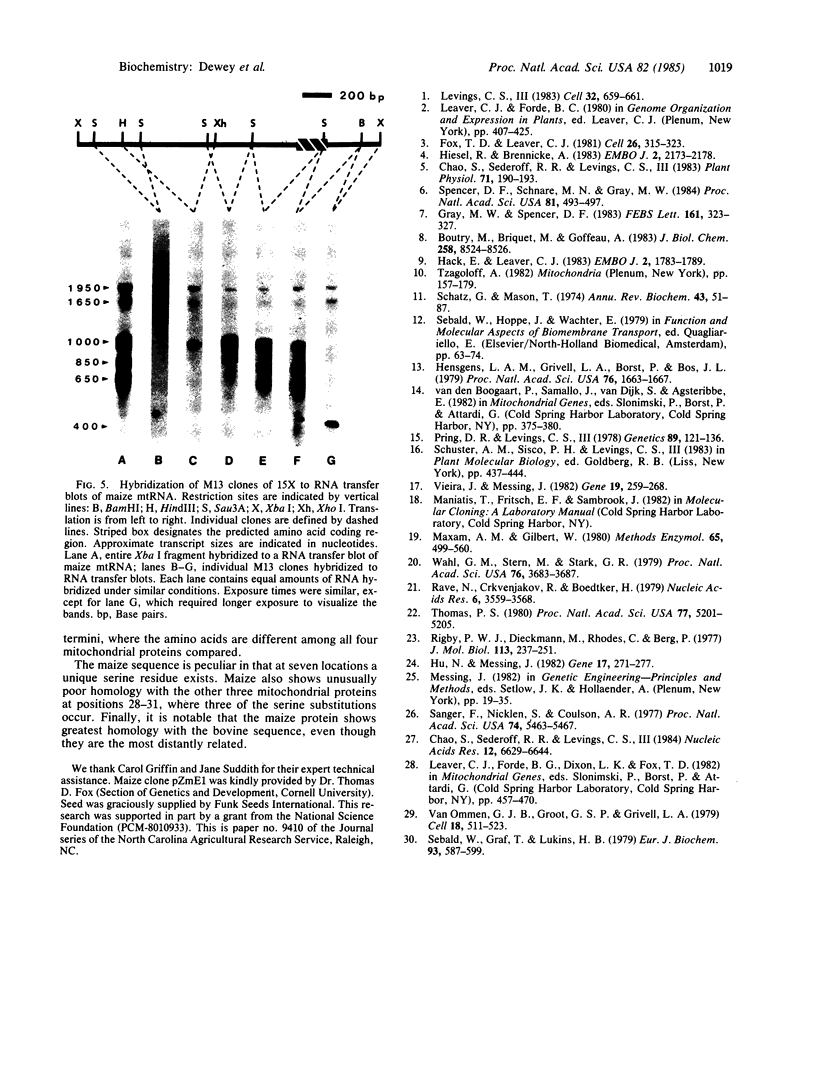
The F0-ATPase proteolipid, also referred to as subunit 9 or the dicyclohexylcarbodiimide-binding protein, is encoded by a mitochondrial gene in maize that we have designated atp 9. The clone containing atp 9 was selected for investigation from a mitochondrial DNA library because of its abundant transcript in total maize mitochondrial RNA preparations. Sequence analysis of the clone revealed an open reading frame that was readily identified by its nucleotide homology with the ATPase subunit 9 gene of yeast. As deduced from the nucleotide sequence, the maize ATPase subunit 9 protein contains 74 amino acids with a molecular weight of 7368. Substantial amino acid sequence homology is conserved among maize, yeast, bovine, and Neurospora mitochondrial ATPase subunit 9 proteins, regardless of whether the gene is nuclearly encoded (bovine and Neurospora) or mitochondrially encoded (yeast and maize). RNA transfer blot analysis indicated that the gene sequence is actively transcribed, producing an initial transcript that is large and extensively processed.
Keywords: dicyclohexylcarbodiimide-binding protein, amino acid sequence
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boutry M., Briquet M., Goffeau A. The alpha subunit of a plant mitochondrial F1-ATPase is translated in mitochondria. J Biol Chem. 1983 Jul 25;258(14):8524–8526. [PubMed] [Google Scholar]
- Chao S., Sederoff R. R., Levings C. S. Partial Sequence Analysis of the 5S to 18S rRNA Gene Region of the Maize Mitochondrial Genome. Plant Physiol. 1983 Jan;71(1):190–193. doi: 10.1104/pp.71.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao S., Sederoff R., Levings C. S., 3rd Nucleotide sequence and evolution of the 18S ribosomal RNA gene in maize mitochondria. Nucleic Acids Res. 1984 Aug 24;12(16):6629–6644. doi: 10.1093/nar/12.16.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Leaver C. J. The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell. 1981 Nov;26(3 Pt 1):315–323. doi: 10.1016/0092-8674(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Hack E., Leaver C. J. The alpha-subunit of the maize F(1)-ATPase is synthesised in the mitochondrion. EMBO J. 1983;2(10):1783–1789. doi: 10.1002/j.1460-2075.1983.tb01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensgens L. A., Grivell L. A., Borst P., Bos J. L. Nucleotide sequence of the mitochondrial structural gene for subunit 9 of yeast ATPase complex. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1663–1667. doi: 10.1073/pnas.76.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesel R., Brennicke A. Cytochrome oxidase subunit II gene in mitochondria of Oenothera has no intron. EMBO J. 1983;2(12):2173–2178. doi: 10.1002/j.1460-2075.1983.tb01719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Levings C. S., 3rd The plant mitochondrial genome and its mutants. Cell. 1983 Mar;32(3):659–661. doi: 10.1016/0092-8674(83)90051-x. [DOI] [PubMed] [Google Scholar]
- Pring D. R., Levings C. S. Heterogeneity of Maize Cytoplasmic Genomes among Male-Sterile Cytoplasms. Genetics. 1978 May;89(1):121–136. doi: 10.1093/genetics/89.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebald W., Graf T., Lukins H. B. The dicyclohexylcarbodiimide-binding protein of the mitochondrial ATPase complex from Neurospora crassa and Saccharomyces cerevisiae. Identification and isolation. Eur J Biochem. 1979 Feb 1;93(3):587–599. doi: 10.1111/j.1432-1033.1979.tb12859.x. [DOI] [PubMed] [Google Scholar]
- Spencer D. F., Schnare M. N., Gray M. W. Pronounced structural similarities between the small subunit ribosomal RNA genes of wheat mitochondria and Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(2):493–497. doi: 10.1073/pnas.81.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ommen G. J., Groot G. S., Grivell L. A. Transcription maps of mtDNAs of two strains of saccharomyces: transcription of strain-specific insertions; Complex RNA maturation and splicing. Cell. 1979 Oct;18(2):511–523. doi: 10.1016/0092-8674(79)90068-0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]










