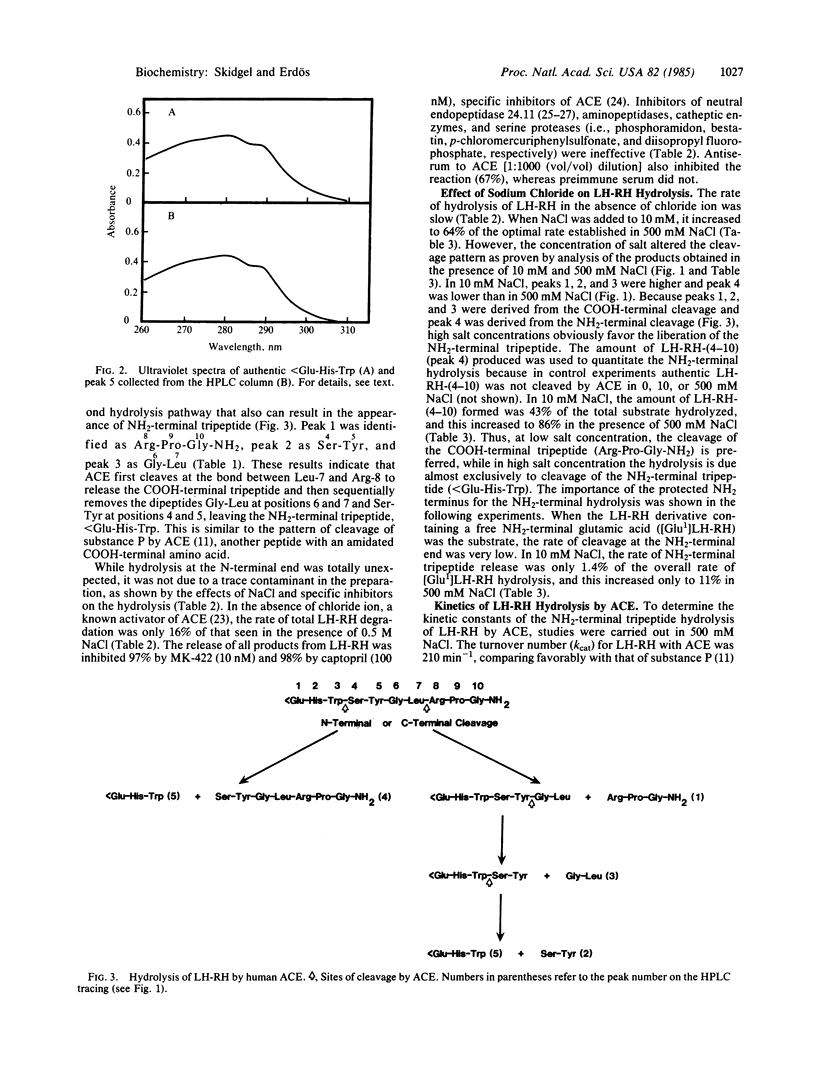
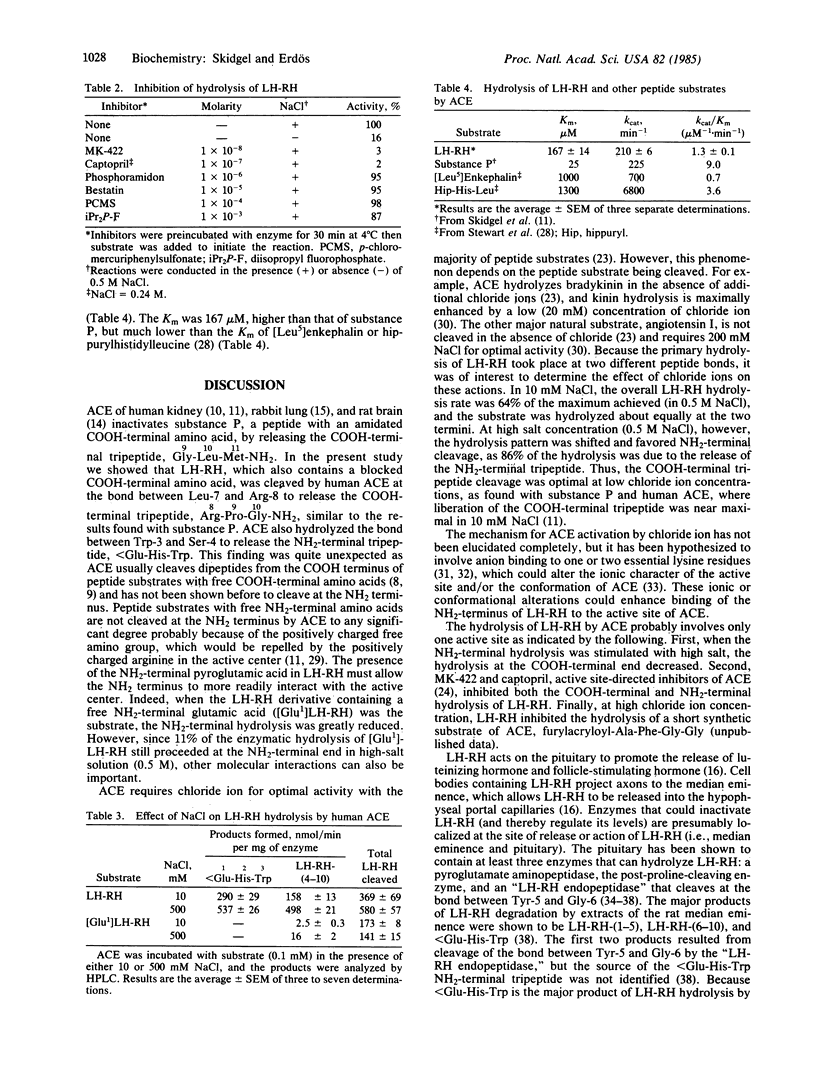
Abstract
Angiotensin I converting enzyme (ACE; kininase II; peptidyldipeptide hydrolase, EC 3.4.15.1) cleaves COOH-terminal dipeptides from active peptides containing a free COOH terminus. We investigated the hydrolysis of luteinizing hormone-releasing hormone (LH-RH) by homogeneous human ACE. Although this decapeptide is blocked at both the NH2 and COOH termini, it was metabolized to several peptides, which were separated by HPLC and identified by amino acid analysis. A major product was the NH2-terminal tripeptide, less than Glu-His-Trp, and another was LH-RH-(4-10) heptapeptide, indicating that the Trp-Ser bond is cleaved to release the NH2-terminal tripeptide. ACE also released the COOH-terminal tripeptide, Arg-Pro-Gly-NH2, and then sequentially the dipeptides Gly-Leu and Ser-Try, leaving less than Glu-His-Trp intact. Thus, less than Glu-His-Trp was formed by both NH2- and COOH-terminal hydrolysis. The cleavage of LH-RH was inhibited by specific ACE inhibitors and by antibody to ACE but not by inhibitors of other enzymes, showing that the hydrolysis was indeed due to ACE. In the absence of chloride, the hydrolysis proceeded at only 16% of the maximal rate (in 500 mM NaCl), but in 10 mM NaCl it increased to 64%. In 500 mM NaCl solution, 86% of the hydrolysis was accounted for by the release of the NH2-terminal tripeptide, whereas in 10 mM NaCl, the COOH-terminal and NH2-terminal cleavage occurred about equally. The Km of LH-RH in 500 mM NaCl was 167 microM and the catalytic constant kcat was 210 min-1. When the NH2-terminal pyroglutamic acid was replaced with glutamic acid ([Glu1]LH-RH), ACE liberated almost exclusively the COOH-terminal tripeptide in 10 mM NaCl. Thus, human ACE, although it is named peptidyl dipeptidase or dipeptidyl carboxypeptidase, can cleave a protected peptide at the NH2 or COOH terminus. The enzyme could be involved in the in vivo metabolism of LH-RH and possibly other blocked peptides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Advis J. P., Krause J. E., McKelvy J. F. Luteinizing hormone-releasing hormone peptidase activities in the female rat: characterization by an assay based on high-performance liquid chromatography. Anal Biochem. 1982 Sep 1;125(1):41–49. doi: 10.1016/0003-2697(82)90380-3. [DOI] [PubMed] [Google Scholar]
- Almenoff J., Orlowski M. Membrane-bound kidney neutral metalloendopeptidase: interaction with synthetic substrates, natural peptides, and inhibitors. Biochemistry. 1983 Feb 1;22(3):590–599. doi: 10.1021/bi00272a011. [DOI] [PubMed] [Google Scholar]
- Bünning P., Riordan J. F. Activation of angiotensin converting enzyme by monovalent anions. Biochemistry. 1983 Jan 4;22(1):110–116. doi: 10.1021/bi00270a016. [DOI] [PubMed] [Google Scholar]
- Caldwell P. R., Seegal B. C., Hsu K. C., Das M., Soffer R. L. Angiotensin-converting enzyme: vascular endothelial localization. Science. 1976 Mar 12;191(4231):1050–1051. doi: 10.1126/science.175444. [DOI] [PubMed] [Google Scholar]
- Cascieri M. A., Bull H. G., Mumford R. A., Patchett A. A., Thornberry N. A., Liang T. Carboxyl-terminal tripeptidyl hydrolysis of substance P by purified rabbit lung angiotensin-converting enzyme and the potentiation of substance P activity in vivo by captopril and MK-422. Mol Pharmacol. 1984 Mar;25(2):287–293. [PubMed] [Google Scholar]
- Chan S. Y., Tang L. C. Immunoreactive LHRH-like factor in human seminal plasma. Arch Androl. 1983 Mar;10(1):29–32. doi: 10.3109/01485018308990166. [DOI] [PubMed] [Google Scholar]
- Defendini R., Zimmerman E. A., Weare J. A., Alhenc-Gelas F., Erdös E. G. Angiotensin-converting enzyme in epithelial and neuroepithelial cells. Neuroendocrinology. 1983 Jul;37(1):32–40. doi: 10.1159/000123512. [DOI] [PubMed] [Google Scholar]
- Erdos E. G., Yang H. Y. An enzyme in microsomal fraction of kidney that inactivates bradykinin. Life Sci. 1967 Mar 15;6(6):569–574. doi: 10.1016/0024-3205(67)90090-2. [DOI] [PubMed] [Google Scholar]
- Fernstrom M. H., Fernstrom J. D. Rapid measurement of free amino acids in serum and CSF using high-performance liquid chromatography. Life Sci. 1981 Nov 16;29(20):2119–2130. doi: 10.1016/0024-3205(81)90669-x. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Matsas R., Turner A. J., Kenny A. J. Kidney neutral endopeptidase and the hydrolysis of enkephalin by synaptic membranes show similar sensitivity to inhibitors. Biochem J. 1982 May 1;203(2):519–522. doi: 10.1042/bj2030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford J. T., Skidgel R. A., Erdös E. G., Hersh L. B. Human kidney "enkephalinase", a neutral metalloendopeptidase that cleaves active peptides. Biochemistry. 1983 Jun 21;22(13):3265–3271. doi: 10.1021/bi00282a035. [DOI] [PubMed] [Google Scholar]
- Gavras H., Gavras I., Brunner H. R. Angiotensin inhibitors for hypertension. Compr Ther. 1980 May;6(5):14–18. [PubMed] [Google Scholar]
- Gibbons J. M., Jr, Mitnick M., Chieffo V. In vitro biosynthesis of TSH- and LH-releasing factors by the human placenta. Am J Obstet Gynecol. 1975 Jan 1;121(1):127–131. doi: 10.1016/0002-9378(75)90989-8. [DOI] [PubMed] [Google Scholar]
- Hall E. R., Kato J., Erdös E. G., Robinson C. J., Oshima G. Angiotensin i-converting enzyme in the nephron. Life Sci. 1976 Jun 1;18(11):1299–1303. doi: 10.1016/0024-3205(76)90208-3. [DOI] [PubMed] [Google Scholar]
- Hersh L. B., Gafford J. T., Powers J. C., Tanaka T., Erdös E. G. Novel substrates for angiotensin I converting enzyme. Biochem Biophys Res Commun. 1983 Jan 27;110(2):654–659. doi: 10.1016/0006-291x(83)91199-3. [DOI] [PubMed] [Google Scholar]
- Horsthemke B., Bauer K. Substrate specificity of an adenohypophyseal endopeptidase capable of hydrolyzing luteinizing hormone-releasing hormone: preferential cleavage of peptide bones involving the carboxyl terminus of hydrophobic and basic amino acids. Biochemistry. 1982 Mar 2;21(5):1033–1036. doi: 10.1021/bi00534a032. [DOI] [PubMed] [Google Scholar]
- Horsthemke B., Knisatschek H., Rivier J., Sandow J., Bauer K. Degradation of luteinizing hormone - releasing hormone and analogs by adenohypophyseal peptidases. Biochem Biophys Res Commun. 1981 May 29;100(2):753–759. doi: 10.1016/s0006-291x(81)80239-2. [DOI] [PubMed] [Google Scholar]
- Inokuchi J., Nagamatsu A. Tripeptidyl carboxypeptidase activity of kininase II (angiotensin-converting enzyme). Biochim Biophys Acta. 1981 Dec 15;662(2):300–307. doi: 10.1016/0005-2744(81)90042-5. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Skidgel R. A., Gafford J. T., Erdös E. G. Enzymes in placental microvilli: angiotensin I converting enzyme, angiotensinase A, carboxypeptidase, and neutral endopeptidase ("enkephalinase"). Peptides. 1984 Jul-Aug;5(4):789–796. doi: 10.1016/0196-9781(84)90023-8. [DOI] [PubMed] [Google Scholar]
- Khodr G. S., Siler-Khodr T. M. Placental luteinizing hormone-releasing factor and its synthesis. Science. 1980 Jan 18;207(4428):315–317. doi: 10.1126/science.6985750. [DOI] [PubMed] [Google Scholar]
- Krause J. E., Advis J. P., McKelvy J. F. Characterization of the site of cleavage of luteinizing hormone-releasing hormone under conditions of measurement in which LHRH degradation undergoes physiologically related change. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1475–1481. doi: 10.1016/s0006-291x(82)80073-9. [DOI] [PubMed] [Google Scholar]
- Kuhl H., Sandow J., Krauss B., Taubert H. D. Enzyme kinetic studies and inhibition by oligopeptides of LH-RH degradation in rat hypothalamus and pituitary. Neuroendocrinology. 1979;28(5):339–348. doi: 10.1159/000122881. [DOI] [PubMed] [Google Scholar]
- Miyake A., Sakumoto T., Aono T., Kawamura Y., Maeda T., Kurachi K. Changes in luteinizing hormone-releasing hormone in human placenta throughout pregnancy. Obstet Gynecol. 1982 Oct;60(4):444–449. [PubMed] [Google Scholar]
- Oshima G., Gecse A., Erdös E. G. Angiotensin I-converting enzyme of the kidney cortex. Biochim Biophys Acta. 1974 May 20;350(1):26–37. doi: 10.1016/0005-2744(74)90199-5. [DOI] [PubMed] [Google Scholar]
- Poth M. M., Heath R. G., Ward M. Angiotensin-converting enzyme in human brain. J Neurochem. 1975 Jul;25(1):83–85. doi: 10.1111/j.1471-4159.1975.tb07698.x. [DOI] [PubMed] [Google Scholar]
- SKEGGS L. T., Jr, KAHN J. R., SHUMWAY N. P. The preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956 Mar 1;103(3):295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R., Holmquist B., Riordan J. F. Anion activation of angiotensin converting enzyme: dependence on nature of substrate. Biochemistry. 1983 Aug 2;22(16):3850–3857. doi: 10.1021/bi00285a021. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Fraser H. M., Cooper I., Rommerts F. F. Sertoli-Leydig cell communication via an LHRH-like factor. Nature. 1981 Apr 30;290(5809):785–787. doi: 10.1038/290785a0. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A., Engelbrecht S., Johnson A. R., Erdös E. G. Hydrolysis of substance p and neurotensin by converting enzyme and neutral endopeptidase. Peptides. 1984 Jul-Aug;5(4):769–776. doi: 10.1016/0196-9781(84)90020-2. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A., Johnson A. R., Erdös E. G. Hydrolysis of opioid hexapeptides by carboxypeptidase N. Presence of carboxypeptidase in cell membranes. Biochem Pharmacol. 1984 Nov 1;33(21):3471–3478. doi: 10.1016/0006-2952(84)90122-9. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson M. A., Flouret G., Nakamura S., Gulczynski B., Carone F. A. In vivo metabolism of tritiated LHRH by the whole kidney and individual tubules of rats. Am J Physiol. 1983 Jun;244(6):F628–F632. doi: 10.1152/ajprenal.1983.244.6.F628. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson M. A., Flouret G., Peterson D. R. Handling of luteinizing hormone-releasing hormone by renal proximal tubular segments in vitro. Am J Physiol. 1981 Aug;241(2):F117–F122. doi: 10.1152/ajprenal.1981.241.2.F117. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson M. A., Yang D. C., Lipkowski A., McCartney L., Peterson D., Flouret G. An approach to the elucidation of metabolic breakdown products of the luteinizing hormone-releasing hormone. J Med Chem. 1981 Jun;24(6):688–692. doi: 10.1021/jm00138a009. [DOI] [PubMed] [Google Scholar]
- Stewart T. A., Weare J. A., Erdös E. G. Purification and characterization of human converting enzyme (kininase II). Peptides. 1981 Summer;2(2):145–152. doi: 10.1016/s0196-9781(81)80027-7. [DOI] [PubMed] [Google Scholar]
- Tan L., Rousseau P. The chemical identity of the immunoreactive LHRH-like peptide biosynthesized in the human placenta. Biochem Biophys Res Commun. 1982 Dec 15;109(3):1061–1071. doi: 10.1016/0006-291x(82)92047-2. [DOI] [PubMed] [Google Scholar]
- Weare J. A. Activation/inactivation of human angiotensin I converting enzyme following chemical modifications of amino groups near the active site. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1319–1326. doi: 10.1016/0006-291x(82)91394-8. [DOI] [PubMed] [Google Scholar]
- Weare J. A., Gafford J. T., Lu H. S., Erdös E. G. Purification of human kidney angiotensin I converting enzyme using reverse-immunoadsorption chromatography. Anal Biochem. 1982 Jul 1;123(2):310–319. doi: 10.1016/0003-2697(82)90451-1. [DOI] [PubMed] [Google Scholar]
- Westall F., Hesser H. Fifteen-minute acid hydrolysis of peptides. Anal Biochem. 1974 Oct;61(2):610–613. doi: 10.1016/0003-2697(74)90426-6. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Erdös E. G., Levin Y. A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochim Biophys Acta. 1970 Aug 21;214(2):374–376. doi: 10.1016/0005-2795(70)90017-6. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Erdös E. G., Levin Y. Characterization of a dipeptide hydrolase (kininase II: angiotensin I converting enzyme). J Pharmacol Exp Ther. 1971 Apr;177(1):291–300. [PubMed] [Google Scholar]
- Yang H. Y., Erdös E. G. Second kininase in human blood plasma. Nature. 1967 Sep 23;215(5108):1402–1403. doi: 10.1038/2151402a0. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Neff N. H. Distribution and properties of angiotensin converting enzyme of rat brain. J Neurochem. 1972 Oct;19(10):2443–2450. doi: 10.1111/j.1471-4159.1972.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Yokosawa H., Endo S., Ogura Y., Ishii S. A new feature of angiotensin-converting enzyme in the brain: hydrolysis of substance P. Biochem Biophys Res Commun. 1983 Oct 31;116(2):735–742. doi: 10.1016/0006-291x(83)90586-7. [DOI] [PubMed] [Google Scholar]



