Synopsis
PD (Parkinson’s disease) is the most common neurodegenerative movement disorder. Mutations in LRRK2 (leucine-rich repeat kinase 2) gene are linked to the most common inherited and sporadic PD. Overexpression of LRRK2 and its mutants could induce mitochondrial-dependent neuronal apoptosis. However, the underlying mechanism remains elusive. We have identified several novel LRRK2 interacting proteins and showed that LRRK2 can interact with three components of the PTPC (permeability transition pore complex) including ANT (adenine nucleotide translocator), VDAC (voltage-dependent anion channel) and uMtCK [ubiquitous MtCK (mitochondrial creatine kinase)]. Those components have been reported to be involved in the permeability of mitochondrial membrane. We provide evidence that LRRK2 is likely to interact with uMtCK directly and expression of LRRK2 and its mutant form can suppress the processing of the immature form of uMtCK. LRRK2 expression keeps the uMtCK preprotein on the outer mitochondrial membrane instead of entering the mitochondria. In addition, the expression of both wild-type and mutant forms of LRRK2 promotes the interaction between ANT and VDAC, which plays a role in permeabilization transition pore opening. Finally, LRRK2-induced cell death can be suppressed by uMtCK. Our findings imply that LRRK2 can interact directly with uMtCK to block its entry into mitochondria and its subsequent processing, resulting in inhibition of mitochondrial energy channelling. Meanwhile, the decrease of uMtCK in mitochondria results in elevated interaction between ANT and VDAC and leads to neuronal apoptosis. Thus, our study provides the rational for clinical trials using creatine to treat PD and supports the notion of exploiting LRRK2 as a drug target for PD.
Keywords: apoptosis, leucine-rich repeat kinase 2 (LRRK2), mitochondria, Parkinson’s disease (PD), permeability transition pore complex (PTPC)
INTRODUCTION
PD (Parkinson’s disease) is a common progressive neurodegenerative disease. The primary hallmarks of PD are progressive loss of dopaminergic neurons and deposition of Lewy bodies in the substantia nigra. Although most PD occurs as a sporadic disease, approx. 5–15% of cases are due to genetic alterations.
In recent years, LRRK2 (leucine-rich repeat kinase 2) gene emerged as a novel and very important causative gene for familial parkinsonism. The LRRK2 gene encodes a large protein of 2527 aas (amino acids) with multiple domains, Roc (Ras of complex proteins), COR (C-terminal of Roc) domain, a leucine-rich repeat domain, a protein kinase catalytic domain [MAPKKK (mitogen-activated protein kinase kinase kinase)], a WD40 domain and an ANK (ankyrin) domain [1, 2]. LRRK2 belongs to the ROCO-protein superfamily and the kinase domain shares high homology with the MLK (mixed-lineage kinase) protein family that has been shown by our group and others to be important for neuronal apoptosis [3–5].
Many autosomal-dominantly inherited mutations found in almost every domain of LRRK2 cause late-onset PD [2, 6, 7] and various mutations exhibit relatively increased kinase activity compared with WT (wild-type) LRRK2, suggesting a gain-of-function mechanism [8, 9]. A single mutation (G2019S) in LRRK2 which increases its kinase activity and accounts for 5–6% of all autosomal-dominant PD patients and even 1% of sporadic late-onset diseases has attracted more attention [10–12]. Endogenous LRRK2 localizes throughout the cytosol and is particularly enriched on microtubules, membranous and vesicular structures [13]. In particular, a significant proportion (~10%) of LRRK2 is enriched in the mitochondrial fraction, but LRRK2 is only associated with the OM (outer membrane) and is absent from the intermembrane space of mitochondria [14].
MPT (mitochondrial membrane permeabilization transition) is a central, rate-limiting step of the mitochondrial (or intrinsic) pathway of apoptosis controlling the release of pro-apoptotic proteins such as Cyto C (cytochrome c), Smac/Diablo, HtrA2 (Omi), AIF (apoptosis-inducing factor) and DNaseG into the cytoplasm [15, 16]. The permeabilization of the mitochondrial membrane is mainly mediated by the PTPC (permeability transition pore complex). PTPC is a polyprotein complex formed at the mitochondrial membrane contact site and involves, at least, the IM (inner membrane) protein ANT (adenine nucleotide translocator), the OM protein VDAC (voltage-dependent anion channel), the matrix protein cyclophilin D, the cytosolic protein hexokinase and the intermembrane space protein MtCK (mitochondrial creatine kinase) [16].
The interaction between the IM protein ANT and the OM protein VDAC forms the contact sites of the mitochondrial membrane [17]. Changes in the conformation of ANT can affect the conformation and function of VDAC, and vice versa. There are two types of VDAC–ANT complexes: the hexokinase-VDAC–ANT complex and the VDAC–MtCK–ANT complex. When VDAC and ANT interact directly, VDAC has higher affinity for cytosolic protein hexokinase. The direct interaction between VDAC and ANT in the hexokinase-VDAC–ANT complex changes the complex structure to an unspecific PT (permeabilization transition) pore [18]. In the other complex, VDAC interacts indirectly with ANT through MtCK [19]. MtCK can inhibit the direct interaction between VDAC and ANT and change the VDAC structure into low affinity for hexokinase and therefore, suppress PT pore opening [18, 20].
Most cell types express both cytosolic and MtCK isoenzymes. There are two MtCK isoforms, sMtCK (sarcomeric MtCK) and uMtCK (ubiquitous MtCK). sMtCK is restricted to muscle and uMtCK is found in non-muscle tissues including the brain [21]. The mitochondrial and cytosolic CK (creatine kinase) isoenzymes catalyse the reversible transfer of high-energy phosphate between PCr (phosphocreatine) and ATP. ATP produced in the mitochondria can be stocked and transported in the form of the high-energy compound PCr that is easily diffusible and can replenish the cytosolic ATP pools. This PCr circuit plays an important role in cellular energy homoeostasis, especially in cells of high and fluctuating energy demand [22–24].
It has been reported that overexpression of mutant LRRK2 in cultured neuron-induced apoptosis involves Cyto C release and caspase 3 activation [25]. However, the underlying mechanism remains elusive. The aim of our study was to investigate the mechanisms of LRRK2-induced mitochondria-dependent apoptosis. In the present study, we identified several novel LRRK2-binding proteins including ANT, VDAC and uMtCK and those proteins played a role in LRRK2 expression-induced cell death. We provide evidence that the expression of LRRK2 mutant could suppress the processing of uMtCK and keep the immature form of uMtCK from getting into mitochondria. In addition, LRRK2 can promote the interaction between ANT and VDAC. We propose a model in which LRRK2 prevents the processing of uMtCK and thus inhibits mitochondrial energy channelling. Meanwhile, the lack of uMtCK increases the interaction between ANT and VDAC and leads to the PT pore opening, release of Cyto C and neuronal apoptosis.
MATERIALS AND METHODS
Cell culture and transfection
HEK (human embryonic kidney)-293 cells and U2OS cells were cultured in DMEM (Dulbecco’s modified Eagle’s medium), supplemented with 10% fetal bovine serum (from PAA). PC12 cells were cultured and transfected as described previously [5]. Transfected cells were lysed 24–36 h after transfection in cell lysis buffer (10 mM Tris/HCl, pH 7.4, 1.0% Triton X-100, 0.5% Nonidet P-40, 150 mM NaCl, 20 mM NaF, 1.0 mM EDTA and 1.0 mM EGTA) supplemented with protease inhibitors.
Antibodies and chemicals
FLAG antibody, FLAG-agarose beads, HA (haemagglutinin) antibody, Hsp60 (heat-shock protein 60) antibody and Tom20 antibody were purchased from Sigma; Myc tag monoclonal antibody from MBL Ltd; and mouse GFP (green fluorescent protein) (clone B-2) antibody from Santa Cruz; Mitotracker from Invitrogen.
Plasmids
The LRRK2 gene was amplified from pDEST-LRRK2-WT [26] using primers 5′-CCGCTCGAGCCACCATGGACTACAAGGACGATGACGATAAGGCTAGTGGCAGCTGTCAG-3′ and 5′-CGGGGTACCTCAACAGATGTTCGTCTC-3′, and inserted into XhoI and KpnI sites of pcDNA3.1/Myc-His(−) B (Invitrogen). Flag-LRRK2-M (aa 671–1299) and Flag-LRRK2-C (1212–2527) were subcloned from this construct and then inserted into pcDNA3.1/Myc-His(−) B. The ANT1 gene was amplified from human cDNA library using primers 5′-CGGAATTCATGGGTGATCACGCTTGG-3′ and 5′-CCGCTCGAGTTAGACATATTTTTTGATCTCATC-3′ and inserted into EcoRI/XhoI sites of pcDNA3.1 HA-Myc His (C) (Invitrogen). The ANT2 gene was amplified from a human cDNA library using primers 5′-CGGATATCATGACAGATGCCGCTGTGTC-3′ and 5′-CCGCTCGAGTTATGTGTACTTCTTGATTTC-3′ and inserted into EcoRV/XhoI sites of pcDNA3.1 HA-Myc (A) (Invitrogen). The ANT3 gene was amplified from human cDNA library using primers 5′-CGGAATTCATGACGGAACAGGCCATCTC-3′ and 5′-CCGCTCGAGTTAGATCACCTTCTTGAGC-3′ and inserted into EcoRI/XhoI sites of pcDNA3.1 HA-Myc (C) (Invitrogen). pcDNA3-uMtCK-Myc is a gift from Ramin Homayouni [27]. FLAG fusion VDAC1 was subcloned from pCMV-HA-VDAC1 using primers 5′-CCGCTCGAGCCACCATGGACTACAAGGACGATGACGATAAGGCTGTGCCACCCA-3′ and 5′-CGGGATCCTGCTTGAAATTCCAG-3′ and inserted into XhoI/BamHI sites of pcDNA3.1/Myc-His (−) B (Invitrogen). N-terminal flag-tagged uMtCK was subcloned from pcDNA3-uMtCK-Myc using primers 5′-CGGGATCCATGGCTGGTCCCTT-3′ and 5′-CGGAATTCATGTTTGCTGTGGAC-3′ and then inserted into the BamHI/EcoRI site of pCMV-Flag vector. GST (glutathione transferase) fusion uMtCK was subcloned from pcDNA3-uMtCK-Myc using primers 5′-CCGGAATTCATGGCTGGTCCCTT-3′ and 5′-ACGCGTCGACATGTTTGCTGTGGAC-3′ and then inserted into the EcoRI/SalI site of the pGEX-4T-1 vector (Amersham Biosciences).
Purification of GST and GST–uMtCK fusion proteins
GST and GST–uMtCK were expressed in Escherichia coli BL21 and purified as previously described [28].
RNAi (RNA interference)
Oligonucleotides of siRNA (small interfering RNA) were synthesized in Invitrogen. Two duplex sequences were designed for each target gene. siRNA-ANT1: 5′-GATCCGCTGCCTACTTCGGAGTCTTTCAAGAGAAGACTCCGAAGTAGGCAGCTTTTTTG-3′ and 5′-AATTCAAAAAAGCTGCCTACTTCGGAGTCTTCTCTTGAAAGACTCCGAAGTAGGCAGCG-3′; si-RNA-ANT2: 5′-GATCCGCTGGAGCTGAAAGGGAATTTCAAGAGAATTCCCTTTCAGCTCCAGCTTTTTTG-3′ and 5′-AATTCAAAAAAGCTGGAGCTGAAAGGGAATTCTCTTGAAATTCCCTTTCAGCTCCAGCG-3′; siRNA-ANT3: 5′-GATCCGCAACCTTGCCAACGTCATTTCAAGAGAATGACGTTGGCAAGGTTGCTTTTTTG-3′ and 5′-AATTCAAAAAAGCAACCTTGCCAACGTCATTCTCTTGAAATGACGTTGGCAAGGTTGCG-3′; siRNA-VDAC1: 5′-GATCCGTGAATGACGGGACAGAGTTTCAAGAGAACTCTGTCCCGTCATTCACTTTTTTG-3′ and 5′-AATTCAAAAAAGTGAATGACGGGACAGAGTTCTCTTGAAACTCTGTCCCGTCATTCACG-3′. The oligonucleotides were annealed and cloned into RNAi-Ready pSIREN-RetroQ-DsRed (BD Biosciences).
Immunoprecipitation, Western immunoblotting and in vitro-binding assay
Immunoprecipitation, Western immunoblotting and in vitro-binding assay were carried out as previously described [4, 5].
Mitochondrial isolation protocol
For mitochondria isolation, 48 h after transfection, HEK-293 cells were washed twice with cold PBS and then harvested by scraping into buffer A [250 mM sucrose, 1 mM EDTA, 50 mM Tris/HCl, 1 mM DTT (dithiothreitol), 1 mM PMSF, 1 mM benzamidine, 0.28 unit/ml apotinin, 50 µg/ml leupeptin and 7 µg/ml pepstainA (pH 7.4)]. The cells were homogenized three times on ice and then centrifuged at 1000 g for 10 min. The supernatant was transferred to a new tube and centrifuged at 10 000 g for 20 min. The supernatant and pellet were crude fractions of cytosol and mitochondria respectively. To eliminate the contamination of peroxisome and lysosome, the pellet fraction was washed with buffer B [250 mM sucrose, 1 mM EGTA, 10 mM Tris/HCl, 1 mM DTT, 1 mM PMSF, 1 mM benzamidine, 0.28 unit/ml apotinin, 50 µg/ml leupeptin and 7 µg/ml pepstain A (pH 7.4)] and centrifuged at 10 000 g for 10 min. This resuspension and centrifugation was repeated three times and then the purified mitochondria were lysed in cell lysis buffer.
RESULTS
LRRK2 interacts with the three main PTPC members ANT, VDAC and uMtCK in vivo
Through co-IP (co-immunoprecipitation) using Flag-LRRK2 transgenic mouse brain homogenates [29] and yeast two-hybrid screens, we have identified many LRRK2 interacting proteins (C. Wang and Z. Xu, unpublished work). Three of them are ANT, VDAC and uMtCK, which are components of the PTPC. Since mutation of LRRK2 is likely to induce cell death through the mitochondria pathway, we went on to investigate the relationship between LRRK2 and these three proteins.
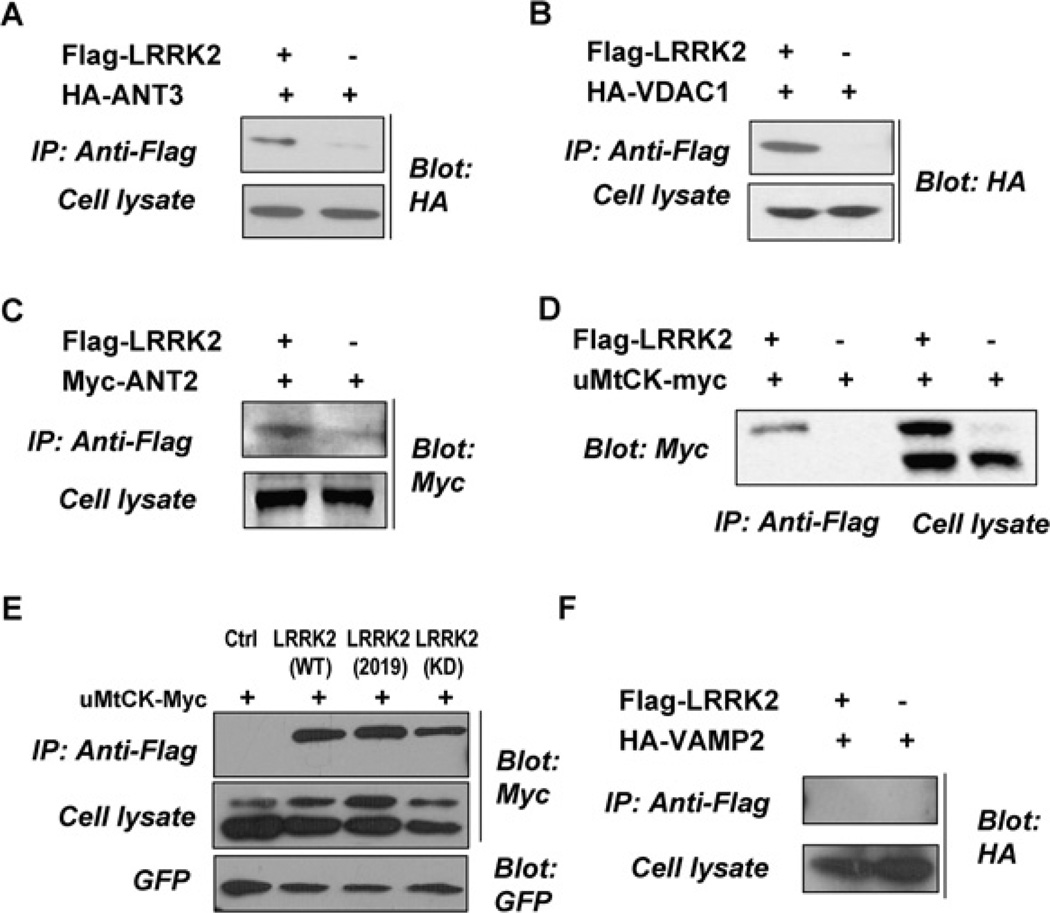
To confirm the interaction between LRRK2 and ANT, VDAC and uMtCK, Flag-tagged LRRK2 and various epitope-tagged VDAC1 and uMtCK and different isoforms of ANTs including ANT1, ANT2, ANT3 were transfected into HEK-293 cells, either alone or in combination. Cell lysates were immunoprecipitated with anti-Flag antibody and the immunocomplexes were probed for tagged VDAC1, uMtCK, ANT1, ANT2 and ANT3. VDAC1, uMtCK, ANT2 and ANT3 were detected in the LRRK2 immunocomplex derived from co-transfected cells but not in cells transfected alone (Figures 1A–1E). We also examined the interaction of LRRK2 with an unrelated protein, VAMP2 (vesicle-associated membrane protein 2) and found that there was no interaction between them (Figure 1F). It is interesting to note that when uMtCK was expressed alone, there was mainly one band present with the molecular mass of approx. 47 kDa and expression of LRRK2 resulted in an accumulation of HMW-uMtCK (higher molecular mass form of uMtCK) (Figure 1D). Thus, two bands (approx. molecular mass of 47 and 52 kDa) of uMtCK were observed. In addition, LRRK2 interacted only with HMW-uMtCK. We went on to investigate whether the kinase activity of LRRK2 would affect the presence of HMW-uMtCK and its interaction with uMtCK. As shown in Figure 1(E), expression of the disease-associated LRRK2 mutant LRRK2 2019S induced more accumulation of HMW-uMtCK than the wild-type LRRK2 and the kinase dead LRRK2. However, the kinase activity of LRRK2 did not seem to have an apparent effect on its interaction with HMW-uMtCK.
Figure 1. LRRK2 interacts with PTPC members ANT, VDAC and uMtCK in vivo.
HEK-293 cells were transfected with Flag-tagged WT LRRK2, LRRK2 2019S or a kinase dead form of LRRK2 and various epitope-tagged VDAC1, uMtCK, different isoforms of ANTs and VAMP2, either alone or in combination as indicated. Thirty-six hours later, aliquots of cell lysates were saved for the analysis of expressed tagged proteins. The remaining portions of the lysates were immunoprecipitated with an anti-Flag antibody. The immunocomplexes were analysed with anti-HA (A, B and F) and anti-Myc (C, D and E) antibodies.
LRRK2 interacts with PTPC members through its ANK–LRR domains
LRRK2 has several protein–protein interaction domains including armadillo, ANK, LRR (leucine-rich repeat) and WD40 that may serve as a scaffold for a multiprotein complex. This suggests that protein–protein interactions play important roles in the normal function of LRRK2. In order to identify which domains of LRRK2 interact with PTPC members, co-IP experiments with anti-Flag antibody were performed using cell lysates from HEK-293 cells transiently transfected with various epitope-tagged VDAC1, uMtCK, ANT1, ANT2, ANT3 either alone or together with two fragments of LRRK2: Flag-LRRK2-M (aa 671–1299) which contains the ANK-LRR domains or Flag-LRRK2-C (aa 1212–2527) which contains the ROC-COR-kinase-WD40 domains. Analysis of the immunoprecipitates revealed interactions between the LRRK2 ANK-LRR domains and the PTPC members (Figure 2A and Supplementary Figure S1 available at http://www.bioscirep.org/bsr/031/bsr0310429add.htm). On the other hand, ROC, COR, kinase and WD40 domains of LRRK2 were not involved in its interaction with PTPC members (Figure 2B). To determine whether the interactions between LRRK2 and ANT, VDAC1 and uMtCK are direct or not, we incubated purified GST and GST-fused uMtCK with Flag-LRRK2-M prepared in an in vitro transcription–translation system. Flag-LRRK2 binds to GST–uMtCK fusion protein but not GST (Figure 2C).
Figure 2. LRRK2 interacts with PTPC members through its ANK-LRR domains.
(A, B) PTPC members interact with the ANK-LRR domains of LRRK2 in vivo. HEK-293 cells were transfected with Flag-LRRK2-M (aa 671–1299) or Flag-LRRK2-C (aa 1212–2527) and various epitope-tagged PTPC members, either alone or in combination as indicated. At 36 h later, cell lysates were immunoprecipitated with an anti-Flag antibody. The immunocomplexes were analysed with an anti-HA antibody. (A) LRRK2 interacts with PTPC members through its ANK-LRR domains. (B) The Roc-COR-kinase-WD40 domains of LRRK2 do not interact with PTPC members. (C) ANK-LRR domains of LRRK2 interact with uMtCK in vitro. GST and GST–uMtCK fusion protein were expressed and purified (lower panel). They were incubated with 35S-labelled Flag-LRRK2-M prepared in an in vitro transcription–translation system for pull-down assays (upper panel). Radioactive proteins that bound to GST–uMtCK were visualized by SDS/PAGE/autoradiography (upper panel).
These observations indicate that uMtCK binds to the aa 671–1299 of LRRK2 and that this binding is likely to be direct. Unfortunately, different isoforms of ANT, VDAC1 and fragments of the middle part of LRRK2 were expressed in the inclusion body in bacteria. It is, therefore, hard to determine whether the interactions between LRRK2 and ANT or VDAC1 are direct or indirect.
Expression of LRRK2 inhibits the processing of the uMtCK precursor and its entry into mitochondria
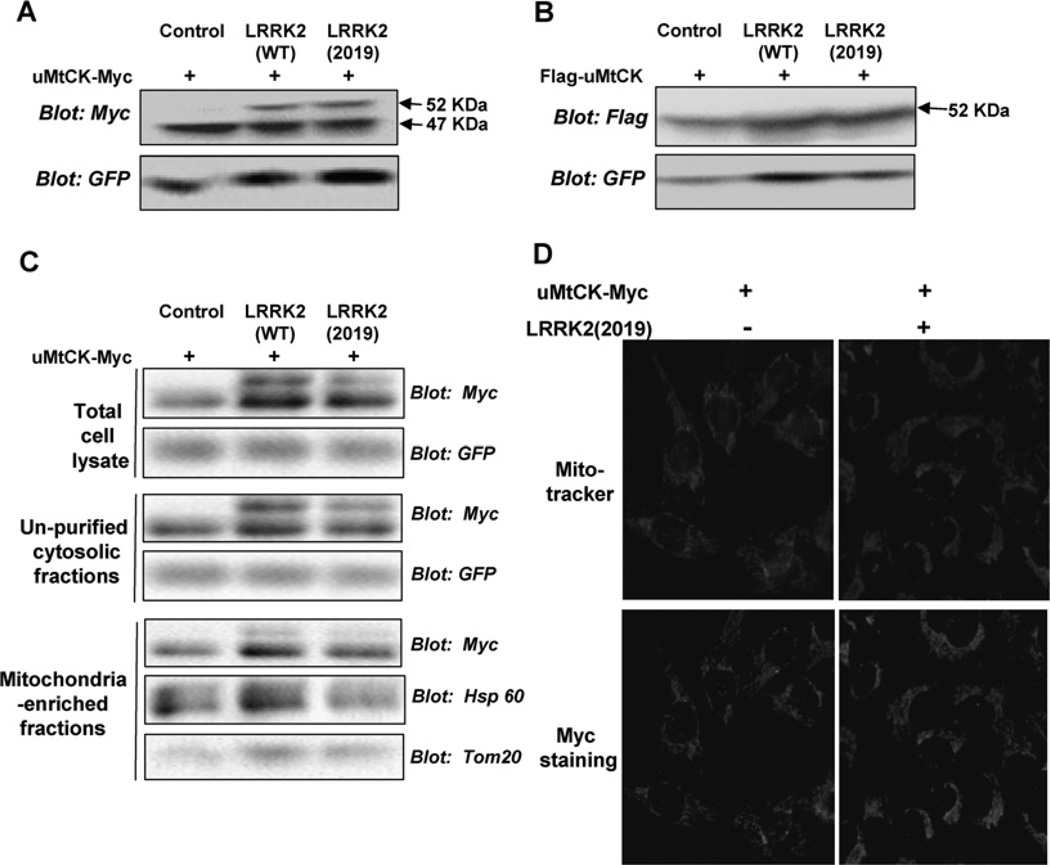
Since the expression of LRRK2 induced an accumulation of HMW-uMtCK and only the HMW-uMtCK interacted with LRRK2, we then compared the effect of wt LRRK2 with the LRRK2 mutant on uMtCK by co-transfecting LRRK2-WT or LRRK2-2019S with uMtCK-Myc into HEK-293 cells. Both LRRK2-WT and LRRK2-2019S led to the accumulation of HMW-uMtCK and it seems that the mutant form of LRRK2 induced more accumulation of HMW-uMtCK (Figure 3A).
Figure 3. LRRK2 expression leads to the accumulation of an unprocessed immature form of uMtCK.
(A) pcDNA3-uMtCK-Myc was transfected either alone or together with LRRK2 WT or LRRK2 2019S into HEK-293 cells. At 48 h later, cell lysates were analysed with an anti-Myc antibody. Expression of both LRRK2 WT and LRRK2 2019 leads to the accumulation of a higher-molecular-mass form of uMtCK-Myc. (B) pCMV-Flag-uMtCK was transfected alone or together with LRRK2 WT or LRRK2 2019S into HEK-293 cells. Forty-eight hours later, cell lysates were analysed using an anti-Flag antibody. Only the 52 kDa Flag-uMtCK bands were observed. (C) HEK-293 cells were transfected with pcDNA3-uMtCK-Myc either alone or together with LRRK2 WT or LRRK2 2019S. Forty-eight hours later, differential centrifugation was performed for the cell lysates. The total cell lysates, crude cytosolic fractions and mitochondria-enriched fractions were analysed with antibodies specific for Myc, GFP, Hsp60 and Tom20. (D) U2OS cells were transfected as in (A). MitoTracker Red dye was used to detect mitochondria. Location of uMtCK-Myc was shown by immunostaining with Myc antibody.
The HMW-uMtCK might be derived from some kind of posttranslational modification or the immature unprocessed uMtCK preprotein. Some common modifications such as phosphorylation and ubiquitination were excluded first by a series of experiments including a phosphorylation and ubiquitination assay (results not shown).
uMtCK is localized in the intermembrane space of mitochondria. It is synthesized in the cytoplasm as a precursor of 416 aa, including a 38 aa N-terminal signal sequence responsible for mitochondrial targeting and import. The transit peptide is cleaved off after leading the protein across the outer mitochondrial membrane [30]. The upper bands were approx. 5 kDa larger than the lower bands, consistent with the calculated molecular mass of the N-terminal signal sequence. Therefore the 52 kDa HMW-uMtCK induced by the expression of LRRK2 is likely to be the unprocessed form of uMtCK.
In the above experiments, we used the uMtCK-Myc construct in which a Myc tag was put at the C-terminal of uMtCK encoding sequence. Therefore, the fusion protein of 47 kDa can still be detected by an anti-Myc antibody even if the N-terminal signal sequence was cleaved after mitochondrial translocation. To verify whether the 52 kDa HMW-uMtCK is the unprocessed uMtCK precursor, pCMV-Flag-uMtCK was constructed in which a Flag tag was put at the N-terminal of the uMtCK encoding sequence. The same experiment was carried out with Flag-uMtCK as that with uMtCK-Myc. If the HMW-uMtCK is indeed the unprocessed preprotein, it should be detectable by an anti-Flag antibody while the matured form of uMtCK should not be. As shown in Figure 3(B), the HMW-uMtCK, but not the 47 kDa band, was recognized with an anti-Flag antibody as expected. However, it was interesting to note that HMW-uMtCK bands were observed whether LRRK2 was co-tranfected or not, although the amount was much more from cells co-transfected with LRRK2. It is likely that the Flag-tag placed in front of the N-terminal of uMtCK would affect the normal mitochondrial targeting function of the signal sequence.
To inspect whether LRRK2 could affect the location of uMtCK or not, uMtCK-Myc was transfected either alone or together with LRRK2-2019S into U2OS cells. Mito Tracker Red dyes were used to detect mitochondria. Immunostaining of those cells for uMtCK-Myc shows that almost all of the uMtCK-Myc co-localized with the mitochondria (Figure 3D). This indicates that LRRK2 did not affect the association of uMtCK with mitochondria very apparently.
To examine whether the LRRK2-induced accumulation of HMW-uMtCK can translocate intomitochondria or not, a subcellular fractionation experiment was carried out. uMtCK-Myc was transfected either alone or together with LRRK2-WT or LRRK2-2019S. Cell lysates from these cells were separated into fractions enriched in cytoplasmic and mitochondrial proteins and monitored by relevant marker proteins. Analysis of uMtCK revealed that the lower-mass form, the matured form of uMtCK, was enriched in the mitochondrial fraction. However, HMW-uMtCK was present in the total cell lysates, but not in the enriched and purified mitochondrial fraction, indicating that it was not tightly associated with the mitochondria (Figure 3C).
Taken together, the HMW-uMtCK induced by LRRK2 is indeed the unprocessed uMtCK preprotein. Our results indicate that the expression of LRRK2 not only interrupts the processing of the uMtCK precuror protein but also prevents it from getting into the mitochondria.
LRRK2 induces the interaction between ANT and VDAC and cell death
It has been reported previously that direct interaction between VDAC and ANT is required for PT pore opening and uMtCK can inhibit the interaction between them [18, 20]. Since LRRK2 can inhibit the entry of uMtCK into mitochondria, we postulated that LRRK2 might enhance the interaction between ANT and VDAC and induce cell death through the induction of PT pore opening.
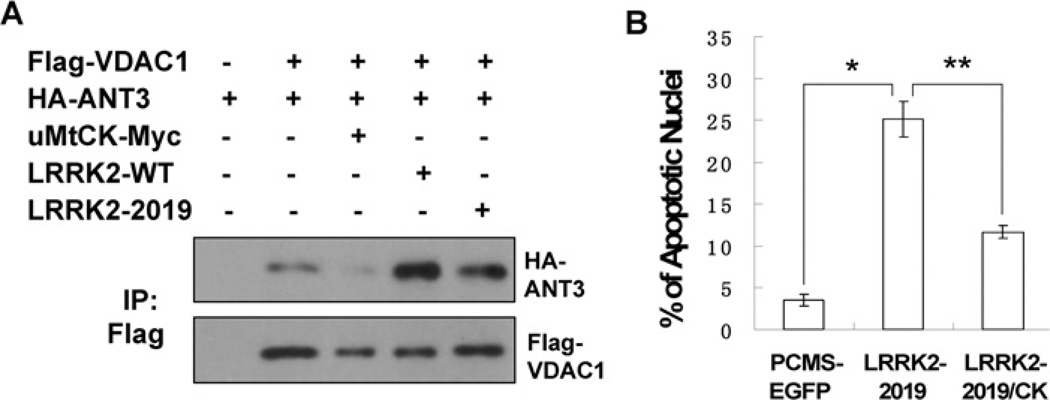
To test our hypothesis, Flag-VDAC1 and HA-ANT3 were co-transfected into cultured HEK-293 cells with or without different forms of LRRK2 or uMtCK. The cell lysates were immunoprecipitated with an anti-Flag antibody and the interaction between ANT and VDAC was analysed. As shown in Figure 4, VDAC and ANT could interact with each other. In agreement with previous reports, the interaction between ANT and VDAC was suppressed when uMtCK was co-transfected. However, the expression of LRRK2 WT or LRRK2 2019 substantially increased the interaction between ANT and VDAC (Figure 4A). Since uMtCK can inhibit the interaction between ANT and VDAC, we speculated that it might suppress LRRK2-induced cell death. To test our hypothesis, LRRK2-2019S in pCMS-EGFP (where EGFP is enhanced green fluorescent protein) vector was transfected into neuronally differentiated PC12 cells. Counts of intact GFP-positive cells revealed a decline in numbers within 4 days for LRRK2-2019S-transfected cultures but not for empty vector and kinase-inactive controls, indicating that the expression of LRRK2 induced cell death (results not shown). We then transfected LRRK2-2019S either alone or together with uMtCK into neuronally differentiated PC12 cells. We verified the ability of LRRK2-2019S to induce cell death by staining nuclei of transfected cells with Hoechst 33342. Approx. 25% of nuclei assayed 48 h after transfection with LRRK2 showed apoptotic morphology, compared with approx. 5% in control cultures (Figure 4B). Co-expression of uMtCK could significantly suppress LRRK2-2019S-induced cell death. This suggests that uMtCK can play a protective role in LRRK2-induced cell death.
Figure 4. LRRK2 promotes the interaction between ANT and VDAC and uMtCK can suppress LRRK2-induced apoptosis.
(A) Flag-VDAC1, HA-ANT3, uMtCK-Myc, LRRK2-WT and LRRK2-2019S were transected into HEK-293 cells either alone or in combination as indicated. Thirty-six hours later, the cell lysates were immunoprecipatated with an anti-Flag antibody. The immunocomplexes were analysed with anti-HA and anti-Flag antibodies. (B) pCMS-EGFP, pCMS-EGFP-LRRK2-2019 was transfected either alone or together with uMtCK-Myc into neuronally differentiated PC12 cells. Forty-eight hours later, the cell nuclei were stained with Hoechst 33342. The percentage of transfected cells exhibiting apoptotic morphology was calculated. Values represent means ± S.E.M. for three replicate cultures. *P<0.008 and **P<0.01.
DISCUSSION
We and others have found that LRRK2 localizes on the mitochondria [14] and LRRK2 expression induces cell death through Cyto C release [25]. Mitochondrial membrane PT which leads to the release of Cyto C plays a critical role in the mitochondrial (or intrinsic) pathway of apoptosis. The direct interaction between VDAC and ANT has been reported to be important for PT pore opening. In the present study, we have identified and confirmed several novel LRRK2-interacting proteins including VDAC, ANT and uMtCK. VDAC, ANT and uMtCK interacted with the middle part of LRRK2, aa 671–1299, which contains the ANK-LRR protein–protein interaction domains and the interaction between LRRK2 and uMtCK was likely to be direct. We confirmed that the expression of LRRK2 in neuronal differentiated PC12 cells could induce cell death. The cell death induced could be partially suppressed by the knock-down of different isoforms of ANT and VDAC expression although it was not statistically very significant (Supplementary Figure S2 available at http://www.bioscirep.org/bsr/031/bsr0310429add.htm). This is probably due to the possibility that different isoforms of ANT could compensate for each other or that the remaining ANT and VDAC in the cells were still able to induce cell death. Another possibility is that LRRK2 could induce cell death through another pathway.
We went on to explore further the underlying mechanism in which LRRK2 induces cell death. We found that the expression of LRRK2, especially the mutated form of LRRK2 (2019S), led to the accumulation of the 52 kDa HMW-uMtCK that was found to be the unprocessed form of uMtCK later. Although LRRK2 did not affect the association of uMtCK with mitochondria, it inhibited the translocation of the 52 kDa HMW-uMtCK into mitochondria. Our results indicate that the expression of LRRK2 can not only interrupt the processing of uMtCK precursor protein but also prevents it from getting into the intermembrane space of mitochondria. Since uMtCK can suppress the ANT–VDAC interaction [18, 20] and LRRK2 can interact directly with and stabilize uMtCK preprotein on the OM of mitochondria, we speculated and confirmed that the expression of LRRK2 could induce the interaction between ANT and VDAC.
Creatine is a guanidine compound that plays a key role in energy buffering within the cell. Creatine and CK are thought to be particularly important in tissues with high-energy requirements such as the brain [31]. Creatine has been shown to exert neuroprotective effects in an MPTP (1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine) mouse model of PD [32]. In addition, National Institutes of Health has lunched a large-scale Phase III clinical trial to learn whether creatine can slow the progression of PD. However, it is still uncertain about its mechanism and how it might work against PD [33]. Our present study provides rationality in support of the clinical trial using creatine to treat PD and will expedite the development of therapies that exploit LRRK2 as a target.
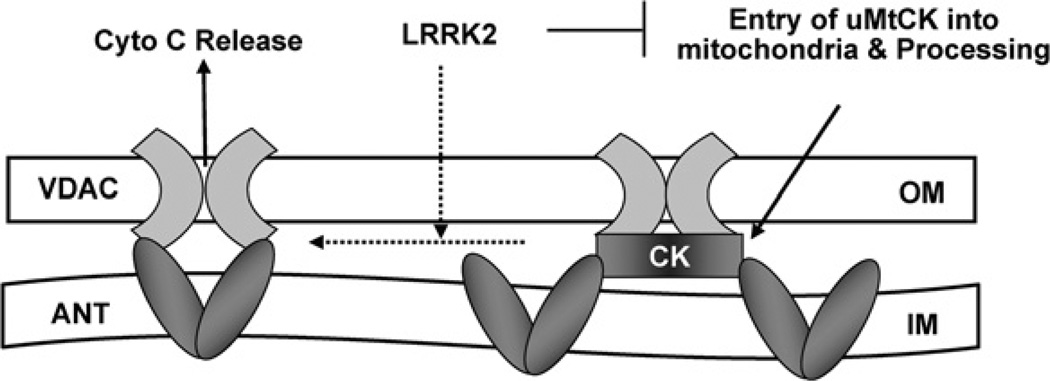
In conclusion, as depicted in Figure 5, our study implies that exogenously expressed LRRK2 can interact directly with uMtCK to block its entry into mitochondria and its processing and thus inhibit the mitochondrial energy channelling. Meanwhile, the lack of uMtCK in mitochondria leads to increased interaction between ANT and VDAC, and subsequent PT pore opening, release of Cyto C and neuronal apoptosis.
Figure 5. Schematic diagram showing the potential mechanism for LRRK2-induced cell death.
Overexpressed LRRK2 can bind to uMtCK directly and prevent it from entry into mitochondria for processing and thus inhibit mitochondrial energy channelling. Meanwhile, the lack of uMtCK in mitochondria leads to an increased interaction between ANT and VDAC, and subsequent PT pore opening, release of Cyto C and neuronal apoptosis.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Harvey and Dr Cookson for providing us with the constructs used in the present study.
FUNDING
This work was supported by the MOST of China, “973” program [grant number2007CB947202/2006CB500701], “863” program [grant number2006AA02Z173/2009DFA32450], NSF (China) [grant number 30725007/30670663] and Chinese Academy of Sciences [Bairen plan and grant number KSCX1-YW-R-62/84] (to Z.X.).
Abbreviations used
- aa
amino acids
- ANK
ankyrin
- ANT
adenine nucleotide translocator
- anti-HA
anti-haemagglutinin
- CK
creatine kinase
- co-IP
co-immunoprecipitation
- COR
C-terminal of Roc
- Cyto C
cytochrome c
- DTT
dithiothreitol
- EGFP
enhanced green fluorescent protein
- GFP
green fluorescent protein
- GST
glutathione transferase
- HA
haemagglutinin
- HEK
human embryonic kidney
- HMW-uMtCK
higher-molecular-mass form of uMtCK
- Hsp60
heat-shock protein 60
- IM
inner membrane
- LRR
leucine-rich repeat
- LRRK2
leucine-rich repeat kinase 2
- MLK
mixed-lineage kinase
- MtCK
mitochondrial creatine kinase
- OM
outer membrane
- PCr
phosphocreatine
- PD
Parkinson’s disease
- PTPC
permeability transition pore complex
- RNAi
RNA interference
- Roc
Ras of complex proteins
- siRNA
small interfering RNA
- sMtCK
sarcomeric MtCK
- uMtCK
ubiquitous MtCK
- VAMP2
vesicle-associated membrane protein 2
- VDAC
voltage-dependent anion channel
- WT
wild-type
Footnotes
AUTHOR CONTRIBUTION
Jie Cui designed and carried out most of the experiments, and drafted the manuscript. Mei Yu and Jingwen Niu performed some of the experiments. Zhenyu Yue provided the list of LRRK2 interacting proteins. Zhiheng Xu designed the study and drafted the manuscript. All authors read and approved the manuscript.
REFERENCES
- 1.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, Van Der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;4:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;4:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Marin I. The Parkinson disease gene LRRK2: evolutionary and structural insights. Mol. Biol. Evol. 2006;12:2423–2433. doi: 10.1093/molbev/msl114. [DOI] [PubMed] [Google Scholar]
- 4.Xu Z, Maroney AC, Dobrzanski P, Kukekov NV, Greene LA. The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis. Mol. Cell. Biol. 2001;14:4713–4724. doi: 10.1128/MCB.21.14.4713-4724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z, Kukekov NV, Greene LA. POSH acts as a scaffold for a multiprotein complex that mediates JNK activation in apoptosis. EMBO J. 2003;2:252–261. doi: 10.1093/emboj/cdg021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mata IF, Kachergus JM, Taylor JP, Lincoln S, Aasly J, Lynch T, Hulihan MM, Cobb SA, Wu RM, Lu CS, et al. Lrrk2 pathogenic substitutions in Parkinson’s disease. Neurogenetics. 2005;4:171–177. doi: 10.1007/s10048-005-0005-1. [DOI] [PubMed] [Google Scholar]
- 7.Taylor JP, Mata IF, Farrer MJ. LRRK2: a common pathway for parkinsonism, pathogenesis and prevention? Trends Mol. Med. 2006;2:76–82. doi: 10.1016/j.molmed.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat. Neurosci. 2006;10:1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- 9.West AB, Moore DJ, Choi C, Andrabi SA, Li X, Dikeman D, Biskup S, Zhang Z, Lim KL, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum. Mol. Genet. 2007;2:223–232. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- 10.Nichols WC, Pankratz N, Hernandez D, Paisan-Ruiz C, Jain S, Halter CA, Michaels VE, Reed T, Rudolph A, Shults CW, et al. Genetic screening for a single common LRRK2 mutation in familial Parkinson’s disease. Lancet. 2005;365:410–412. doi: 10.1016/S0140-6736(05)17828-3. [DOI] [PubMed] [Google Scholar]
- 11.Di Fonzo A, Rohe CF, Ferreira J, Chien HF, Vacca L, Stocchi F, Guedes L, Fabrizio E, Manfredi M, Vanacore N, et al. A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson’s disease. Lancet. 2005;365:412–415. doi: 10.1016/S0140-6736(05)17829-5. [DOI] [PubMed] [Google Scholar]
- 12.Gilks WP, Abou-Sleiman PM, Gandhi S, Jain S, Singleton A, Lees AJ, Shaw K, Bhatia KP, Bonifati V, Quinn NP, et al. A common LRRK2 mutation in idiopathic Parkinson’s disease. Lancet. 2005;365:415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- 13.Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, et al. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann. Neurol. 2006;5:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 14.West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl. Acad. Sci. U.S.A. 2005;46:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 16.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;22:2922–2933. [Google Scholar]
- 17.Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Brdiczka D, Remy R, Xie ZH, Reed JC, Kroemer G. The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J. Exp. Med. 1998;8:1261–1271. doi: 10.1084/jem.187.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vyssokikh M, Brdiczka D. VDAC and peripheral channelling complexes in health and disease. Mol. Cell. Biochem. 2004;256–257:117–126. doi: 10.1023/b:mcbi.0000009863.69249.d9. [DOI] [PubMed] [Google Scholar]
- 19.Schlattner U, Dolder M, Wallimann T, Tokarska-Schlattner M. Mitochondrial creatine kinase and mitochondrial outer membrane porin show a direct interaction that is modulated by calcium. J. Biol. Chem. 2001;51:48027–48030. doi: 10.1074/jbc.M106524200. [DOI] [PubMed] [Google Scholar]
- 20.Beutner G, Ruck A, Riede B, Welte W, Brdiczka D. Complexes between kinases, mitochondrial porin and adenylate translocator in rat brain resemble the permeability transition pore. FEBS Lett. 1996;396:189–195. doi: 10.1016/0014-5793(96)01092-7. [DOI] [PubMed] [Google Scholar]
- 21.Payne RM, Haas RC, Strauss AW. Structural characterization and tissue-specific expression of the mRNAs encoding isoenzymes from two rat mitochondrial creatine kinase genes. Biochim. Biophys. Acta. 1991;3:352–361. doi: 10.1016/0167-4781(91)90176-m. [DOI] [PubMed] [Google Scholar]
- 22.Bessman SP, Geiger PJ. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981;211:448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- 23.Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dzeja PP, Terzic A. Phosphotransfer networks and cellular energetics. J. Exp. Biol. 2003;206:2039–2047. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- 25.Iaccarino C, Crosio C, Vitale C, Sanna G, Carri MT, Barone P. Apoptotic mechanisms in mutant LRRK2-mediated cell death. Hum. Mol. Genet. 2007;11:1319–1326. doi: 10.1093/hmg/ddm080. [DOI] [PubMed] [Google Scholar]
- 26.Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, Van Der Brug MP, Beilina A, Blackinton J, Thomas KJ, et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol. Dis. 2006;2:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Burklen T, Yuan X, Schlattner U, Desiderio DM, Wallimann T, Homayouni R. Stabilization of ubiquitous mitochondrial creatine kinase preprotein by APP family proteins. Mol. Cell. Neurosci. 2006;2:263–272. doi: 10.1016/j.mcn.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Yu Y, Maggi LB, Jr, Brady SN, Apicelli AJ, Dai MS, Lu H, Weber JD. Nucleophosmin is essential for ribosomal protein L5 nuclear export. Mol. Cell. Biol. 2006;10:3798–3809. doi: 10.1128/MCB.26.10.3798-3809.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Tan YC, Poulose S, Olanow CW, Huang XY, Yue Z. Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson’s disease R1441C/G mutants. J. Neurochem. 2007;1:238–247. doi: 10.1111/j.1471-4159.2007.04743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin W, Khuchua Z, Cheng J, Boero J, Payne RM, Strauss AW. Molecular characterization of the creatine kinases and some historical perspectives. Mol. Cell. Biochem. 1998;184:153–167. [PubMed] [Google Scholar]
- 31.Burklen TS, Schlattner U, Homayouni R, Gough K, Rak M, Szeghalmi A, Wallimann T. The creatine kinase/creatine connection to Alzheimer’s disease: CK-inactivation, APP–CK complexes and focal creatine deposits. J. Biomed. Biotechnol. 2006;3:35936. doi: 10.1155/JBB/2006/35936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Calingasan NY, Wille EJ, Cormier K, Smith K, Ferrante RJ, Beal MF. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson’s and Huntington’s diseases. J. Neurochem. 2009;5:1427–1439. doi: 10.1111/j.1471-4159.2009.06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Couzin J. Clinical research. Testing a novel strategy against Parkinson’s disease. Science. 2007;318:1778. doi: 10.1126/science.315.5820.1778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.