Abstract
Background and aims: Hepatitis B virus (HBV) infection is a common cause of hepatocellular carcinoma (HCC) in the United States. This study evaluated the impact of surveillance and treatment on HBV-infected HCC patients and identified factors associated with survival. Methods: From 1981 to 2010, 166 hepatitis B surface antigen (HBsAg)-positive HCC patients were evaluated. Fifty-eight patients had HCC detected by surveillance, while 108 patients presented with HCC. Results: Compared to patients detected by surveillance, those presenting with HCC had more symptoms (65.7% vs 41.4%; P=.002), were more frequently outside of Milan criteria (73.7% vs 29.6%; P<.001), more often presented with diffuse tumors (23.2% vs 1.9%; P<.001), and had a shortened median survival time (9.5 months vs 18.7 months; P=.003). Patients who presented with diffuse tumors were younger and more often male (P=.002-.007), had a higher alpha-fetoprotein (AFP) level (P=.023), and had a median survival time of only 1.68 months. By multivariate analysis, factors that were significantly associated with mortality included diffuse tumors (hazard ratio [HR], 6.30; 95% confidence interval [CI], 3.14-12.66; P<.001), being outside of Milan criteria (HR, 2.02; 95% CI, 1.26-3.23; P=.005), albumin level (HR per 1 standard deviation decrease, 1.4; 95% CI, 1.15-1.72; P=.001), AFP level (HR per 1 log standard deviation increase, 1.38; 95% CI, 1.13-1.67; P=.001), and receiving liver transplantation versus other treatments (HR, 0.08-0.38; 95% CI, 0.03-0.87; P<.001 to P=.022). Conclusion: In the United States, HBV-related HCC is a common malignancy, especially among Asian immigrants. Identification of HBsAgpositive subjects and routine HCC surveillance are essential for improving survival in these patients.
Keywords: Hepatitis B virus, hepatocellular carcinoma, surveillance, liver transplantation, resection, locoregional therapy, survival
Hepatocellular carcinoma (HCC) is a common malignancy worldwide and is responsible for up to 600,000 deaths each year.1 HCC is the fifth and eighth most common cancer in men and women, respectively. More than 85% of HCC cases arise from the Asian and African continents, with China alone accounting for over 50% of all liver cancer cases worldwide. In the United States, the incidence of HCC more than doubled from 1975 through 2005.2 The largest increase in the proportion of HCC cases was in non-Hispanic whites, but Asian Americans had both the highest age-adjusted HCC incidence rate and the highest HCC mortality rate compared to all other ethnicities.3 Within the United States, the etiology of HCC differs among racial groups. Hepatitis C virus infection is the major risk factor in individuals of white or black ethnicity, while hepatitis B virus (HBV) infection remains the main risk factor among Asians.4 Up to 80% of HBV-related HCC cases arise in the setting of cirrhosis.5
The association between chronic HBV infection and the development of HCC has been well established for more than 4 decades.6 A report from Taiwan showed that the age-adjusted annual incidence of HCC was 474 cases per 100,000 individuals among men who were positive for hepatitis B surface antigen (HBsAg) compared to 6 cases per 100,000 individuals among HBsAg-negative men.7 In a previous report from the United States, high risks for both HBV infection and HCC were noted in Chinese men living in New York City.8 During the time period covered in this study, Chinese Americans comprised only 0.9% of the city’s population, yet 5.4% of all deaths from HCC occurred in this ethnic group. A subsequent report from Los Angeles, California showed that 80% of HBsAg-positive HCC cases occurred in Asian Americans, and the percentage of HCC cases that occur in this ethnic group remains consistently high to date.5
The survival rate after diagnosis of HBV-related HCC is poor and depends on both the extent of the tumor burden and the severity of the underlying chronic liver disease. In an early report assessing 211 cases of HCC from Hong Kong, predominately among HBV-infected patients, the median survival after HCC diagnosis was only 3.5 weeks.9 In this study, a majority of the patients already had clinical symptoms at presentation, either related to their tumor or due to hepatic decompensation. Prior to adoption of the Milan criteria for liver transplantation (LT), the median survival after treatment for HCC in HBsAg-positive patients was only 3 months.10 In the United States, the 1-year and 5-year survival rates after HCC diagnosis were less than 50% and 10%, respectively.3
Few studies on HBV-infected HCC patients have been conducted in the United States. The current paper describes findings from 166 patients with HBV-related HCC who presented to our clinic from 1981 to 2010. Within this time period, routine surveillance for HCC was initiated, and more treatments for HCC became available. Thus, we were able to observe the clinical course of HCC prior to the initiation of surveillance in our clinic, which occurred in 1991, and to follow the clinical course of patients who subsequently received definitive surgical and locoregional therapies.11 This study describes factors associated with survival in this population of HBV-infected HCC patients.
Methods
Patients
From 1981 to 2010, 166 HBsAg-positive patients with HCC were evaluated at the Liver Center of Huntington Medical Research Institutes in Pasadena, California. Of these patients, 108 were referred by their family physicians with a prior diagnosis of HCC; these patients comprised the no-surveillance group. In the remaining 58 patients, HCC was detected through surveillance using serum alpha-fetoprotein (AFP) testing and abdominal ultrasound examinations. In HBsAg-positive inactive carriers (defined as patients who were negative for hepatitis B e antigen [HBeAg], had an alanine aminotransferase [ALT] level ≤40 U/L, and had an HBV DNA level ≤ 10,000 copies/mL), surveillance tests were performed every 6—12 months. In patients with chronic hepatitis or cirrhosis, AFP testing and abdominal ultrasound examination were performed every 6 months. Since 1991, the minimal surveillance interval was 12 months.11 Inactive carriers were seen less frequently, while patients with chronic hepatitis and cirrhosis had return visits every 3—6 months; the HCC surveillance intervals coincided with scheduled clinic visits. All patients in the surveillance group had compensated liver disease at the time of HCC detection. If the serum AFP level was elevated above the upper limit of normal (10 ng/mL) or if a liver lesion was observed on ultrasound examination, further diagnostic studies were performed, including a computed tomography (CT) scan, magnetic resonance imaging (MRI), and/or a biopsy of the lesion. All HCC patients underwent baseline laboratory tests that measured platelet counts and levels of serum albumin, total bilirubin, alkaline phosphatase, aspartate aminotransferase (AST), ALT, AFP, and HBeAg. Virologic markers—including precore A1896 mutation, basal core promoter T1762/A1764 mutation, HBV genotype, and HBV DNA level—were measured as previously described.12
The number, location, and size of HCC lesions were determined by either CT scan or MRI, and patients were classified by the Milan criteria (single lesion ≤5 cm, maximum of 3 lesions with none >3 cm), by the University of California at San Francisco (UCSF) criteria (single lesion ≤6.5 cm, maximum of 3 lesions with none >4.5 cm, or a total tumor burden of ≤8 cm), and by the presence or absence of macrovascular invasion.13,14 Large infiltrating lesions without definite borders and tumors greater than or equal to 10 cm in diameter were categorized as diffuse HCC since both presentations have similar survival times.
All HCC patients were referred to academic centers for surgical and/or locoregional therapies, which included LT, surgical resection, radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), or transcatheter arterial chemoembolization (TACE). If HCC patients received more than 1 treatment, the patient was assigned to a category based on the most definitive treatment; LT was considered to be the most definitive treatment, followed by resection, and then by locoregional therapies. HCC patients who did not receive the above treatments were offered chemotherapy or supportive care. The dates of diagnosis, initial treatment, tumor progression, last follow-up visit, and death were recorded for calculation of overall survival.
Statistical Analyses
For descriptive analyses, the P-values for comparing continuous variables by surveillance were computed using the Wilcoxon rank-sum test and the student t test. The chisquare test was used to compare categorical variables by surveillance. The variables for bilirubin, alkaline phosphatase, AST, ALT, HBV DNA, AFP, platelets, and creatinine were log10 transformed prior to analysis in order to improve normality/linearity. Unadjusted overall survival and disease-free survival curves by surveillance and treatment modalities were estimated using the Kaplan-Meier method and compared by using the log-rank test. To determine potential predictors of mortality and disease progression, univariate and multivariate analyses were performed using the Cox regression model. The backward stepwise procedure with significance defined as a P-value less than .25 was used as the retention criteria. Analyses were conducted using SAS version 9.2 (SAS Institute Inc.), and the level of significance was defined as a 2-sided P-value less than .05.
Results
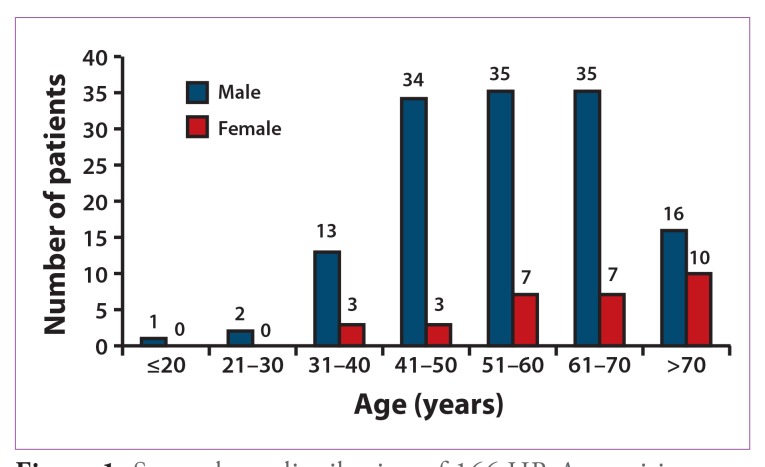
Between 1981 and 2010, 166 HBsAg-positive patients with HCC were evaluated in our clinic. Patients’ average age was 56.7 years (range, 6—86 years), and 81.9% of patients were male (Table 1). The sex and age distributions of the study participants are shown in Figure 1. A majority of male patients were 41—70 years of age; among female patients, one third of cases occurred in individuals over the age of 70 years. Indeed, male HCC patients presented at a younger age than females (55.4±13.4 years vs 62.6±13.2 years; P=.008). A total of 154 (92.8%) patients were Asian; of these patients, 150 (97.4%) were born outside of the United States. A family history of HBsAg positivity was reported in 50.7% of patients, and a family history of HCC was reported in 26.6% of patients. Cirrhosis occurred in 117 of 166 (70.5%) HCC patients. Baseline laboratory and virologic test results are shown in Table 1. A baseline AFP level greater than 10 ng/mL was observed in 111 of 156 (71.2%) patients; the mean AFP level in these patients was 51,716±223,684 ng/mL.
Table 1.
Baseline Characteristics of HBsAg-Positive Patients with Hepatocellular Carcinoma (HCC; N=166)
| Sex* | |
| Male | 136 (81.9%) |
| Female | 30 (18.1%) |
| Mean age (years)** | 56.7±13.6 |
| Male | 55.4±13.4 |
| Female | 62.6±13.2 |
| Race* | |
| White | 10 (6.0%) |
| African American | 2 (1.2%) |
| Asian | 154 (92.8%) |
| Chinese | 110 (66.3%) |
| Korean | 21 (12.7%) |
| Vietnamese | 10 (6.0%) |
| Filipino | 7 (4.2%) |
| Other | 6 (3.6%) |
| Birthplace* | |
| United States | 16 (9.6%) |
| Outside of the United States | 150 (90.4%) |
| Family history of HBsAg positivity (N=148)* | |
| Yes | 75 (50.7%) |
| No | 73 (49.3%) |
| Family history of HCC (N=154)* | |
| Yes | 41 (26.6%) |
| No | 113(73.4%) |
| Cirrhosis* | |
| Yes | 117(70.5%) |
| No | 49 (29.5%) |
| Mean albumin level (g/dL)** | 3.65±0.72 |
| Mean total bilirubin level (mg/dL)** | 1.9±3.0 |
| Mean alkaline phosphatase level (U/L)** | 168.7±168.5 |
| Mean AST level (U/L)** | 117.3±143.2 |
| Mean ALT level (U/L)** | 73.4±59.1 |
| Mean platelet count (x10,000/mm3)** | 188.4±122.6 |
| Mean creatinine level (mg/dL)** | 1.1 ± 0.6 |
| AFP level (N= 156) | |
| AFP ≤10 ng/mL* | 45 (28.8%) |
| AFP >10 ng/mL* | 111 (71.2%) |
| Mean AFP level in patients with an abnormal value (ng/mL)** | 51,716±223,684 |
| HBeAg status (N=155)* | |
| Positive | 42 (27%) |
| Negative | 113(73%) |
| Precore A1896 status (N=112)* | |
| Wild | 60 (53.6%) |
| Mutant | 52 (46.4%) |
| Basal core promoter T1762/A1764 status (N=102)* | |
| Wild | 23 (22.5%) |
| Mutant | 79 (77.5%) |
| Genotype (N=105)* | |
| A | 7 (6.7%) |
| B | 23(21.9%) |
| C | 72 (68.6%) |
| D | 1 (1.0%) |
| Mixed | 2(1.9%) |
| HBV DNA status (N=151) | |
| Positive* | 113(74.8%) |
| Negative* | 38 (25.2%) |
| HBV DNA level in patients who are positive (log10 copies/mL)** | 5.33±1.39 |
| Treatment* | |
| Liver transplantation | 16 (9.64%) |
| Resection | 31 (18.67%) |
| Locoregional treatment | 41 (24.70%) |
| Chemotherapy | 12(7.23%) |
| Supportive treatment | 66 (39.76%) |
*Number of patients (%).
**Mean±standard deviation.
AFP=alpha-fetoprotein; ALT=alanine aminotransferase; AST=aspartate aminotransferase; HBeAg=hepatitis B e antigen; HBsAg=hepatitis B surface antigen; HBV=hepatitis B virus.
Figure 1.
Sex and age distribution of 166 HBsAg-positive patients with hepatocellular carcinoma.
HBsAg=hepatitis B surface antigen.
HBeAg positivity occurred in 42 of 155 (27.1%) patients, and serum HBV DNA was detectable in 113 of 151 (74.8%) HCC patients; the mean HBV DNA level in these patients was 5.33±1.39 log10 copies/mL. Precore A1896 mutations were detected in 52 of 112 (46.4%) HCC patients, and basal core promoter T1762/A1764 mutations were present in 79 of 102 (77.5%) HCC patients. Sixteen of 166 (9.64%) HCC patients were treated by LT, 31 patients (18.7%) underwent surgical resection, and 41 patients (24.7%) received locoregional treatments.
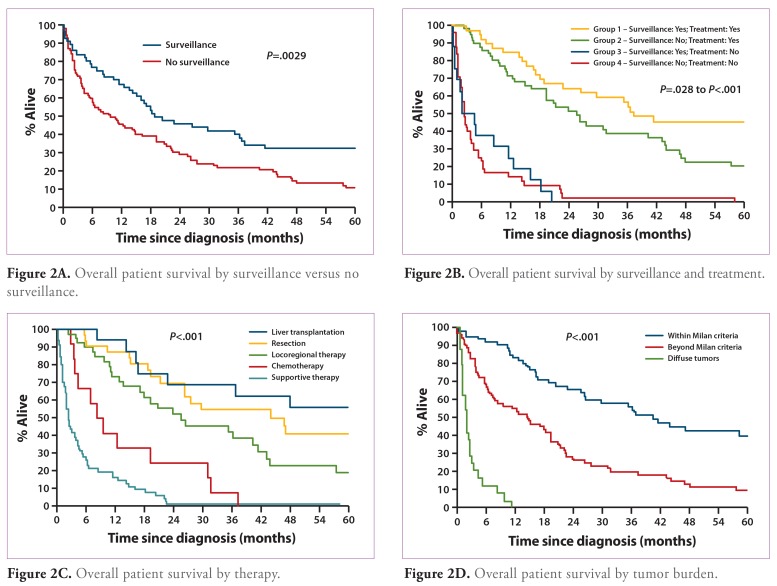
In 58 of 166 (34.9%) patients, HCC was detected by surveillance, while 108 (65.1%) patients presented with a prior diagnosis of HCC (no surveillance). In the surveillance patients, the mean time to development of HCC was 5.8±5.2 years (95% confidence interval [CI], 4.5—7.2 years). Compared to patients detected by surveillance, those who presented with a prior diagnosis of HCC exhibited more symptoms — such as fatigue, weight loss, and hepatomegaly (P= .002) — and had higher mean platelet counts and serum AST levels (P=.019 and P=.00l, respectively; Table 2). Serum AFP values were elevated above the normal value (>10 ng/mL) in 57.1% of the HCC patients detected by surveillance and in 79.6% of the patients who presented with HCC (P=.004). Among HCC patients with cirrhosis, 81% of those detected by surveillance had Child-Pugh class A cirrhosis and 13.8% had Child-Pugh class B cirrhosis; in the no-surveillance group, 56.1% of cirrhotic patients had Child-Pugh class A cirrhosis and 36.4% had Child-Pugh class B cirrhosis (P=.003). Also, more patients in the surveillance group had tumors within Milan criteria and UCSF criteria, while more patients in the no-surveillance group presented with diffuse tumors (P<.001 for all comparisons). More patients in the surveillance group received LT, while more patients who presented with HCC received supportive care only (P=.02). The 1-year, 2-year, and 3-year overall survival rates were significantly higher in the surveillance group compared to the no-surveillance group (P=.003; Table 2 and Figure 2A).
Table 2.
Characteristics of Patients with Hepatocellular Carcinoma Detected by Surveillance Versus No Surveillance
| Surveillance | No surveillance | P-value | |
|---|---|---|---|
| Total number of patients* | 58 (34.9%) | 108 (65.1%) | |
| Age (years)** | 57.45±13.78 | 56.31±13.57 | .610 |
| Gender* | |||
| Male | 51 (87.9%) | 85 (78.7%) | .141 |
| Female | 7(12.1%) | 23(21.3%) | |
| Symptoms at presentation* | .002 | ||
| Yes | 24(41.4%) | 71 (65.7%) | |
| No | 34 (58.6%) | 37 (34.3%) | |
| Mean albumin level (g/dL)** | 3.77±0.72 | 3.61±0.70 | .173 |
| Mean total bilirubin level (mg/dL)** | 1.6±3.10 | 2.0±2.9 | .270 |
| Mean alkaline phosphatase level (U/L)** | 129.6±84.4 | 189.9±197.1 | .055 |
| Mean AST level (U/L)** | 92.5±169.3 | 130.7±125.8 | .001 |
| Mean ALT level (U/L)** | 63.2±62.5 | 79.0±56.6 | .110 |
| Mean platelet count (x10,000/mm3)** | 159.6±99.0 | 206.9±132.9 | .019 |
| HBV DNA level (log10 copies/mL)** | 5.16±1.58 | 5.39±1.32 | .464 |
| AFP level (N=159) | .004 | ||
| AFP ≤10 ng/mL* | 24 (42.9%) | 21 (20.4%) | |
| AFP ≤10 ng/mL* | 32(57.1%) | 82 (79.6%) | |
| Mean AFP level in patients with an abnormal value (ng/mL)** | 47,806.4±237,509.3 | 53,299.9±219,393.3 | .083 |
| HBeAg status (N=155)* | .310 | ||
| Positive | 13 (22.4%) | 29 (29.9%) | |
| Negative | 45 (77.6%) | 68 (70.1%) | |
| Cirrhosis* | .502 | ||
| Yes | 39 (67.2%) | 78 (72.2%) | |
| No | 19 (32.8%) | 30 (27.8%) | |
| Child-Pugh score (N=165)* | .003 | ||
| A | 47(81.0%) | 60 (56.1%) | |
| B | 8 (13.8%) | 39 (36.4%) | |
| C | 3 (5.2%) | 8 (7.5%) | |
| Milan criteria (N=153)* | <.001 | ||
| Within | 38 (70.4%) | 26 (26.3%) | |
| Outside | 16 (29.6%) | 73 (73.7%) | |
| UCSF criteria (N=153)* | <.001 | ||
| Within | 45 (83.3%) | 30 (30.3%) | |
| Outside | 9 (16.7%) | 69 (69.7%) | |
| Diffuse tumor (N=153)* | <.001 | ||
| Yes | 1 (1.9%) | 23 (23.2%) | |
| No | 53 (98.1%) | 76 (76.8%) | |
| Treatments*† | .020 | ||
| LT and LT + other‡ | 11 (19.0%) | 5 (4.6%) | |
| Resection and resection + other’ | 13 (22.4%) | 17(15.7%) | |
| Locoregional treatment alone§ | 14 (24.1%) | 28 (25.9%) | |
| Chemotherapy alone | 3 (5.2%) | 9 (8.3%) | |
| Supportive treatment | 17 (29.3%) | 49 (45.4%) | |
| Recurrence after treatment (N=88)* | .031 | ||
| Yes | 14 (36.8%) | 30 (60%) | |
| No | 24 (63.2%) | 20 (40%) | |
| Survival | .003 | ||
| At 1 year | 68.00% | 45.90% | |
| At 2 years | 46.10% | 29.40% | |
| At 3 years | 38.60% | 22.30% | |
| Median survival (months) | 18.7 | 9.5 |
*Total number of patients.
**Mean±standard deviation.
† In patients who received LT, resection, and locoregional therapies.
‡ Any pre-LT treatment including resection, radiofrequency ablation, percutaneous ethanol injection, transcatheter arterial chemoembolization, or chemotherapy.
¶ Any pre—liver resection treatment including radio-frequency ablation, percutaneous ethanol injection, transcatheter arterial chemoembolization, or chemotherapy.
§ Single treatment or a combination of locoregional treatments including transcatheter arterial chemoembolization, radiofrequency ablation, and/or percutaneous ethanol injection.
AFP=alpha-fetoprotein; ALT=alanine aminotransferase; AST=aspartate aminotransferase; HBeAg=hepatitis B e antigen; HBV=hepatitis B virus; LT=liver transplantation; UCSF=University of California at San Francisco.
Figure 2A.
Overall patient survival by surveillance versus no surveillance.
A comparison of patient survival by surveillance and treatments is shown in Figure 2B. Group 1 included patients with HCC detected by surveillance who received treatment, group 2 included patients who presented with HCC (no surveillance) and received treatment, group 3 included patients with HCC detected by surveillance who did not receive treatment, and group 4 included patients who presented with HCC (no surveillance) and did not receive treatment. A significant increase in survival was noted when group 1 was compared to groups 2, 3, and 4 (P=.028 to P<.0001). An increase in survival was also noted when group 2 was compared to group 3 or 4 (P<.0001 for both comparisons). However, groups 3 and 4 had similarly poor survival rates.
Figure 2B.
Overall patient survival by surveillance and treatment.
Table 3 shows a comparison of HCC patients whose tumors were within Milan criteria, those whose tumors were beyond Milan criteria, and those who presented with diffuse tumors. Patients who presented with diffuse tumors were younger in age (P=.002); more frequently male (P=.007); had a lower mean albumin level (P=.018); and had higher levels of total bilirubin, alkaline phosphatase, AST, ALT, platelets, and AFP (P=.003 to P<.001). Compared to the other 2 groups, patients who presented with diffuse tumors had a higher mean HBV DNA level and were more frequently HBeAg-positive (P=.054 and P=.0l7, respectively). Also, more patients with diffuse tumors presented with clinical symptoms (P<.001), a majority of these patients received supportive care only (P<.001), and the median survival in this group was only 1.68 months (P<.001; Figure 2C). Figure 2D shows overall survival according to treatment modality for all 166 HCC patients. Eighty-eight of 166 patients (53%) received LT, resection, or locoregional therapies. Patients who received LT had the highest survival rate, followed by those who received resection, and then by those who received locoregional interventions (P<.001).
Table 3.
Characteristics of Patients with Hepatocellular Carcinoma by Tumor Burden
| Within Milan criteria | Beyond Milan criteria | Diffuse tumors | P-value | |
|---|---|---|---|---|
| Total number of patients* | 64 (41.8%) | 65 (42.5%) | 24 (15.7%) | |
| Age (years)** | 59.7±11.7 | 56.0±14.7 | 49.0±11.6 | .002 |
| Gender* | .007 | |||
| Male | 46 (71.9%) | 57 (87.7%) | 22(91.7%) | |
| Female | 18 (28.1%) | 8 (12.3%) | 2 (8.3%) | |
| Symptoms at presentation* | <.001 | |||
| Yes | 23 (35.9%) | 41 (63.1%) | 21 (87.5%) | |
| No | 41 (64.1%) | 24 (36.9%) | 3 (12.5%) | |
| Mean albumin level (g/dL)** | 3.8±0.7 | 3.7±0.7 | 3.4±0.6 | .018 |
| Mean total bilirubin level (mg/dL)** | 1.0±0.7 | 2.0±2.5 | 4.2±6 | <.001 |
| Mean alkaline phosphatase level (U/L)** | 113.9±64.4 | 192.9±218.2 | 269.9±183.9 | <.001 |
| Mean AST level (U/L)** | 62±66.5 | 109.9±94.1 | 272.4±274.1 | <.001 |
| Mean ALT level (U/L)** | 54±32 | 82±74.2 | 108.4±61.7 | <.001 |
| Mean platelet count (x10,000/mm3)** | 148.6±73.1 | 199.4±114.4 | 283.8±193.1 | <.001 |
| HBV DNA level (log10 copies/mL)** | 3.2±2.8 | 4.2±2.6 | 4.9±1.9 | .054 |
| AFP level | .003 | |||
| AFP≤10ng/mL* | 26 (40.6%) | 15(25.4%) | 1 (4.3%) | |
| AFP>10ng/mL* | 38 (59.4%) | 44 (74.6%) | 22 (95.7%) | |
| Mean AFP level in patients with an abnormal value (ng/mL)** | 4,762.4± 14,999 | 49,595±209,302 | 146,437±197,517 | .023 |
| HBeAg status* | .017 | |||
| Positive | 21 (34.4%) | 9 (14.3%) | 7 (35%) | |
| Negative | 40 (65.6%) | 54 (85.7%) | 13 (65%) | |
| Cirrhosis* | .276 | |||
| Yes | 48 (75%) | 42 (64.6%) | 19 (79.2%) | |
| No | 16 (25%) | 23 (35.4%) | 5 (20.8%) | |
| Child-Pugh score* | .07 | |||
| A | 49 (76.6%) | 42 (64.6%) | 11 (45.8%) | |
| B | 13 (20.3%) | 19 (29.2%) | 10(41.7%) | |
| C | 2(3.1%) | 4 (6.2%) | 3 (12.5%) | |
| Treatments*† | <.001 | |||
| LT and LT + other‡ | 11 (17.2%) | 5 (7.7%) | 0 (0%) | |
| Resection and resection + other¶ | 19 (29.7%) | 11 (16.9%) | 0 (0%) | |
| Locoregional treatment alone§ | 23 (35.9%) | 15(23.1%) | 2 (8.3%) | |
| Chemotherapy alone | 0 (0%) | 10(15.4%) | 2 (8.3%) | |
| Supportive treatment | 11 (17.2%) | 24 (38.9%) | 20 (83.3%) | |
| Survival | ||||
| At 1 year | 83.50% | 54.70% | 0% | |
| At 2 years | 65.60% | 26.60% | 0% | |
| At 3 years | 53.90% | 20.00% | 0% | |
| Median survival (months) | 40 | 14 | 1.68 | <.001 |
*Total number of patients.
**Mean±standard deviation.
†In patients who received LT, resection, and locoregional therapies.
‡Any pre-LT treatment including resection, radiofrequency ablation, percutaneous ethanol injection, transcatheter arterial chemoembolization, or chemotherapy.
¶Any pre—liver resection treatment including radiofrequency ablation, percutaneous ethanol injection, transcatheter arterial chemoembolization, or chemotherapy.
§Single treatment or a combination of locoregional treatments including transcatheter arterial chemoembolization, radiofrequency ablation, and/or percutaneous ethanol injection.
AFP=alpha-fetoprotein; ALT=alanine aminotransferase; AST=aspartate aminotransferase; HBeAg=hepatitis B e antigen; HBV=hepatitis B virus; LT=liver transplantation.
Figure 2C.
Overall patient survival by therapy.
Figure 2D.
Overall patient survival by tumor burden.
Univariate and multivariate analyses of factors associated with increased mortality are shown in Table 4. On multivariate analysis, factors that were significantly associated with mortality were presentation with diffuse tumors (hazard ratio [HR], 6.3; 95% CI, 3.14-12.66; P<.001), being outside Milan criteria (HR, 2.02; 95% CI, 1.26-3.23; P=.005), albumin level (HR per 1 standard deviation decrease, 1.40; 95% CI, 1.15-1.72; P=.001), AFP level (HR per log 1 standard deviation increase, 1.38; 95% CI, 1.13-1.67; P=.001), and receiving LT versus resection, TACE, chemotherapy, or supportive treatments (HR, 0.08-0.38; 95% CI, 0.03-0.87; P<.001 to P=.022).
Table 4.
Univariate and Multivariate Analyses of Factors Associated with Mortality
| Hazard ratio | 95% confidence interval | P-value | |
|---|---|---|---|
| Univariate analysis | |||
| Age* | 0.99 | 0.98-1.01 | .246 |
| Sex (male vs female) | 1.49 | 0.92-2.42 | .107 |
| Symptoms at presentation | 2.24 | 1.56-3.22 | <.001 |
| HBeAg status | 1.19 | 0.80-1.77 | .384 |
| Albumin level (g/dL)** | 1.56 | 1.31-1.85 | <.001 |
| HBV DNA level (copies/mL) | 1.10 | 0.91-1.32 | .338 |
| Total bilirubin level (mg/dL)† | 1.85 | 1.55-2.19 | <.001 |
| Alkaline phosphatase level (U/L)† | 1.53 | 1.28-1.83 | <.001 |
| AST level (U/L)† | 2.22 | 1.84-2.67 | <.001 |
| ALT level (U/L)† | 1.55 | 1.30-1.86 | <.001 |
| Platelet count (x10,000/mm3)† | 1.13 | 0.92-1.39 | .252 |
| Creatinine level (mg/dL)† | 1.29 | 1.07-1.56 | .008 |
| AFP level (ng/mL)† | 1.80 | 1.51-2.16 | <.001 |
| AFP above upper limit of normal (>10 ng/mL) | 2.41 | 1.57-3.70 | <.001 |
| Cirrhosis | 1.22 | 0.83-1.79 | .312 |
| Surveillance | 0.57 | 0.40-0.83 | .003 |
| Beyond versus within Milan criteria | 2.44 | 1.62-3.67 | <.001 |
| Diffuse tumor versus within Milan criteria | 16.77 | 9.08-30.96 | <.001 |
| HCC treatment‡ | |||
| LT | 0.27 | 0.13-0.54 | <.001 |
| Resection | 0.40 | 0.25-0.64 | <.001 |
| RFA | 0.24 | 0.13-0.47 | <.001 |
| TACE | 0.57 | 0.38-0.84 | .004 |
| Chemotherapy | 0.86 | 0.56-1.30 | .466 |
| Supportive therapy | 6.62 | 4.47-9.79 | <.001 |
| Multivariate analysis | |||
| Sex (male vs female) | 1.45 | 0.90-2.64 | .112 |
| Albumin level (g/dL)** | 1.40 | 1.15-1.72 | .001 |
| ALT level (U/L)† | 1.16 | 0.095-1.41 | .148 |
| AFP level (ng/mL)† | 1.38 | 1.13-1.67 | .001 |
| Beyond versus within Milan criteria | 2.02 | 1.26-3.23 | .005 |
| Diffuse tumor versus within Milan criteria | 6.30 | 3.14-12.66 | <.001 |
| HCC treatment | |||
| LT versus resection | 0.38 | 0.17-0.87 | .022 |
| LT versus RFA | 0.83 | 0.25-2.80 | .765 |
| LT versus TACE | 0.28 | 0.12-0.64 | .002 |
| LT versus chemotherapy | 0.18 | 0.07-0.46 | <.001 |
| LT versus supportive therapy | 0.08 | 0.03-0.18 | <.001 |
*Per 1 year increase.
**Per 1 standard deviation decrease.
†Per log 1 standard deviation increase.
‡Compared to other treatments.
AFP=alpha-fetoprotein; ALT=alanine aminotransferase; AST=aspartate aminotransferase; HBeAg=hepatitis B e antigen; HBV=hepatitis B virus; HCC=hepatocellular carcinoma; LT=liver transplantation; RFA=radiofrequency ablation; TACE=transcatheter arterial chemoembolization.
Discussion
Approximately 400 million people are chronically infected with HBV, and this virus is the most common cause of HCC worldwide.15 Case-control studies have shown that HBV carriers have a 5-15-fold increased risk for HCC compared to uninfected individuals.16 In the United States, HBV infection accounts for up to 15% of all HCC cases; reports from the United States show that 41-84% of HBV-related HCC cases occur in Asians, 6—22% occur in whites, and up to 14% occur in African Americans.4,5 Among the Asian HBsAg-positive HCC patients in this study, 97% were born outside of the United States, where initial HBV infection may have taken place either at birth (perinatal transmission from HBsAg-positive carrier mothers) or during early childhood.2 The observation that 51% of the Asian HCC patients in this study had HBsAg-positive family members supports the role of intrafamilial spread of HBV17 Moreover, up to 27% of HCC patients in this study had blood relatives with HCC, which suggests that a genetic predisposition to HCC may exist within families.5,17 In a case-control study from the United States, individuals with chronic HBV infection and a first-degree relative with liver cancer had a significantly higher risk of developing HCC.18
Virologic factors have been associated with progression to HCC. The basal core promoter T1762/A1764 mutation and genotype C were frequently detected in HBV-related HCC cases, and both have been verified as independent risk factors for HCC development.12,19,20 The most important factor associated with progression to HCC is serum HBV DNA level. In a prospective study of over 3,600 Taiwanese men, a linear relationship was observed between the titer of HBV DNA—from 104 copies/mL to more than 108 copies/mL—and increasing frequency of HCC development.21 Moreover, suppression of HBV DNA levels by antiviral therapy significantly decreased the occurrence of HCC.22 Among the HCC patients reported in this study, 77.5% had basal core promoter mutations, 68.6% were genotype C, and 74.8% had measurable levels of serum HBV DNA.
Guidelines from the American Association for the Study of Liver Diseases (AASLD) recommend that initial HCC surveillance should be conducted in males over the age of 40 years and in females over the age of 50 years.23 If these criteria were applied to the HBV-infected HCC patients reported in this study, 11% of males and 20% of females would have been excluded from HCC surveillance (Figure 1). However, if the age limit for HCC surveillance were lowered to 30 years for both males and females, then the number of HCC patients excluded from surveillance would be reduced to 2% for males and 0% for females. In addition, lowering the age limit for HCC surveillance to below 40 years would ensure that surveillance includes the subgroup of younger noncirrhotic patients who may have a propensity to develop HCC.24
During the past 2 decades, AFP testing and ultrasound examination have been used as routine surveillance tests for HCC detection. Serum AFP testing has low sensitivity and specificity, since AFP levels may be elevated as a result of either liver injury or HCC, and use of AFP testing has been discouraged in recent AASLD guidelines.23 However, the AFP test is relatively inexpensive and readily available. Also, AFP levels are elevated in up to 70% of HCC cases; in these patients, this test is a valuable tool for evaluating treatment efficacy and detecting HCC recurrence. At present, AFP testing is recommended for HCC surveillance in Asian American patients with chronic HBV infection.25
Ultrasound examination is operator-dependent and, in general, may not be as accurate in a community practice setting as in an academic center. However, the cost of ultrasound examination is considerably less than that of more accurate imaging techniques such as CT scans or MRI. A recent report on HCC surveillance in a community hospital showed that the smallest HCC detected using ultrasound equipment in 1991 was 1 cm in diameter, which is comparable to the accuracy of more recently acquired ultrasound scanners.17 Therefore, adequate training of persons performing ultrasound examinations is of utmost importance.
The goal of HCC surveillance is to detect small tumors in patients with preserved liver function. There are few reports on HCC surveillance in the United States. Previously, we conducted a prospective surveillance program in patients with chronic viral hepatitis in which we performed AFP testing at 6-12-month intervals and abdominal ultrasound examinations at least once during each 12-month period.11 During a mean follow-up period of 35 months, 31 patients developed HCC: 18 patients had 1 lesion (with a mean diameter of 3.5 cm), 6 patients had 2 or more lesions, and 7 patients had diffuse lesions. During the time period of the study, 4 patients received surgical resection, 8 patients underwent LT, and 9 patients received either PEI or TACE. Neither the Barcelona criteria for resection nor the Milan criteria for LT were established as the standard of care at the time this study was conducted. After a mean of 16.7 months, 77% of patients had died, which raised the issue that treatment after HCC detection may have been inadequate.
Other reports have supported this observation. In a randomized controlled trial of HCC surveillance in HBsAgpositive patients in China, researchers used AFP testing to identify patients with early-stage HCC, which was defined as subclinical disease and tumors less than 5 cm in diameter.26 No reduction in overall mortality was observed compared to HCC patients who had not undergone surveillance, which was thought to be because therapy for patients detected by surveillance was ineffective. Another surveillance program in Chinese HBV carriers detected small HCC tumors; the mean tumor size was 3.02 cm.27 In this report, the surgical resection rate was low, and no clinical benefit was demonstrated with early HCC detection. However, in a large randomized controlled trial of HCC surveillance conducted in Shanghai, China, biannual AFP testing and abdominal ultrasound examination identified patients with subclinical and small HCC tumors who were able to receive either resection or loco regional therapies.28 The 1-year, 3-year, and 5-year survival rates were significantly higher in the surveillance group, and the HCC mortality rate decreased by 37%. Similarly, in a recent report on surveillance for HCC in Asian American patients infected with HBV, more patients with smaller tumor burdens were identified who were able to receive treatments, which significantly improved survival.17 In the current study, HCC patients detected by surveillance were more likely to be within Milan and UCSF criteria, more likely to receive HCC therapies, and had improved survival rates compared to patients who presented with HCC (no surveillance). Thus, detection of HCC by surveillance must be followed by referral to medical facilities that can provide surgical and locoregional treatments for HCC.
LT offers a potentially curative treatment for HCC, and survival rates of over 70% after 3—4 years of follow-up have been reported.13,29 For patients with chronic HBV infection, LT offers the best possible treatment modality since this treatment removes cirrhotic livers containing HBV-infected cells and potentially premalignant hepatocytes. Moreover, the use of hepatitis B immune globulin and antiviral agents following LT prevents HBV reinfection in over 90% of transplant recipients.29,30 However, because of organ shortages, LT is limited to a small percentage of HCC patients.
Surgical resection has remained a viable treatment option in noncirrhotic patients with HCC and in patients with well-compensated liver disease.31 A recent report from Hong Kong involving a predominantly HBV-infected population showed that the 5-year overall survival and disease-free survival rates after HCC resection were 55% and 35%, respectively.32 Recently, percutaneous RFA in patients with lesions no larger than 3 cm in diameter resulted in 1-year, 3-year, and 5-year survival rates of 95%, 69%, and 58%, respectively.33 In a randomized controlled trial in Hong Kong, the 1-year, 2-year, and 3-year survival rates in patients who received TACE were 57%, 31%, and 26%, respectively; these rates were significantly higher than survival rates in patients who did not receive treatment (32%, 11%, and 3%, respectively).34 However, HCC recurrence is the main factor associated with survival after treatment.5,29,32,35,36 In the current study, tumors recurred in 44 of the 88 (50%) HCC patients who received either surgical or locoregional treatments.
There are limitations to the current study, one of which is that a majority of the HBV-infected HCC patients in this study were Asian; therefore, it was not possible to evaluate non-Asian HBsAg-positive HCC patients. In addition, this was a single-center study, which may have introduced patient selection and treatment biases. However, the HCC patients in this study were of heterogeneous ethnic origins, they were routinely referred to academic centers, and they had access to the most up-to-date surgical and locoregional therapies.
Another limitation of this study is that our HCC sample size may be too small for analyzing the effectiveness of surveillance on survival. However, compared to a large, population-based, surveillance study, our findings—which show prolonged survival in HCC patients treated in a community clinic—may be more relevant to physicians who provide day-to-day care for HBsAg-positive patients, and these findings provide further evidence supporting HCC surveillance in high-risk individuals.28
Another limitation of the current study is that we did not account for lead-time bias. However, our earlier report on HCC surveillance in HBsAg-positive patients did include an adjusted lead-time bias interval, and this previous report showed that surveillance identified patients with smaller tumor burdens who had significantly prolonged survival after receiving definitive therapies.17 In a large HCC surveillance trial of HBsAg-positive patients in China, lead-time bias was not included in the analysis of survival.28
Finally, during the first 15 years of the study period, fewer therapeutic options for HCC were available, even when early HCC was detected by surveillance. During the latter part of the study period, more effective treatment options become available, and we were able to demonstrate that both surveillance and definitive treatments are necessary for patient survival (Figure 2B).
Conclusion
HCC in HBsAg-positive patients remains an important health issue, especially in Asian Americans. Since a majority of HCC patients are born outside of the United States, HCC will continue to be prevalent among immigrants from countries where HBV is endemic. Routine surveillance for HCC must be advocated for all HBsAg-positive patients; in order to improve survival, effective treatment modalities must also be available after HCC detection. Thus, referral of HCC patients to centers where surgical and locoregional therapies are available is strongly recommended.
Footnotes
Ms. Daniela Markovic performed the statistical analysis for the study. Onyx Pharmaceuticals provided partial funding toward the cost for statistical analysis. This manuscript is dedicated to the memory of William G. Corey, MD.
References
- 1.Ferenci P, Fried M, Labrecque D, et al. World Gastroenterology Organisation Guidelines and Publications Committee. World Gastroenterology Organisation Guideline. Hepatocellular carcinoma (HCC): a global perspective. J Gastrointestin Liver Dis. 2010;19:311–317. [PubMed] [Google Scholar]
- 2.El-Serag HB, Lau M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167:1983–1989. doi: 10.1001/archinte.167.18.1983. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Bisceglie AM, Lyra AC, Schwartz M, et al. Liver Cancer Network. Hepatitis C-related hepatocellular carcinoma in the United States: influence of ethnic status. Am J Gastroenterol. 2003;98:2060–2063. doi: 10.1111/j.1572-0241.2003.t01-1-07641.x. [DOI] [PubMed] [Google Scholar]
- 5.Barazani Y, Hiatt JR, Tong MJ. Busuttil RW Chronic viral hepatitis and hepatocellular carcinoma. World J Surg. 2007;31:1243–1248. doi: 10.1007/s00268-007-9041-3. [DOI] [PubMed] [Google Scholar]
- 6.Tong MJ, Sun SC, Schaeffer BT, Chang NK, Lo KJ, Peters RL. Hepatitis-associated antigen and hepatocellular carcinoma in Taiwan. Ann Intern Med. 1971;75:687–691. doi: 10.7326/0003-4819-75-5-687. [DOI] [PubMed] [Google Scholar]
- 7.Beasley RP, Lin C-C, Hwang L-Y, Chien C-S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan lancet. 1981;318:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 8.Szmuness W, Stevens CE, Ikram H, Much MI, Harley EJ, Hollinger B. Prevalence of hepatitis B virus infection and hepatocellular carcinoma in Chinese-Americans. J Infect Dis. 1978;137:822–829. doi: 10.1093/infdis/137.6.822. [DOI] [PubMed] [Google Scholar]
- 9.Lai CL, Wu PC, Lam KC, Todd D. Histologic prognostic indicators in hepatocellular carcinoma. Cancer. 1979;44:1677–1683. doi: 10.1002/1097-0142(197911)44:5<1677::aid-cncr2820440522>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Hwang SJ, Tong MJ, Lai PP, et al. Evaluation of hepatitis B and C viral markers: clinical significance in Asian and Caucasian patients with hepatocellular carcinoma in the United States of America. J Gastroenterol Hepatol. 1996;11:949–954. [PubMed] [Google Scholar]
- 11.Tong MJ, Blatt LM. Kao VW Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16:553–559. doi: 10.1046/j.1440-1746.2001.02470.x. [DOI] [PubMed] [Google Scholar]
- 12.Tong MJ, Blatt LM, Kao JH, Cheng JT, Corey WG. Precore/basal core promoter mutants and hepatitis B viral DNA levels as predictors for liver deaths and hepatocellular carcinoma. World J Gastroenterol. 2006;12:6620–6626. doi: 10.3748/wjg.v12.i41.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 14.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 15.McClune AC, Tong MJ. Chronic hepatitis B and hepatocellular carcinoma. Clin Liver Dis. 2010;14:461–476. doi: 10.1016/j.cld.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 16.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 17.Tong MJ, Sun HE, Hsien C, Lu DS. Surveillance for hepatocellular carcinoma improves survival in Asian-American patients with hepatitis B: results from a community-based clinic. Dig Dis Sci. 2010;55:826–835. doi: 10.1007/s10620-009-1059-y. [DOI] [PubMed] [Google Scholar]
- 18.Hassan MM, Spitz MR, Thomas MB, et al. The association of family history of liver cancer with hepatocellular carcinoma: a case-control study in the United States. J Hepatol. 2009;50:334–341. doi: 10.1016/j.jhep.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kao JH, Chen PJ, Lai MY, Chen DS. Basal core promoter mutations of hepatitis B virus increase the risk of hepatocellular carcinoma in hepatitis B carriers. Gastroenterology. 2003;124:327–334. doi: 10.1053/gast.2003.50053. [DOI] [PubMed] [Google Scholar]
- 20.Yang HI, Yeh SH, Chen PJ, et al. REVEAL-HBV Study Group. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:1134–1143. doi: 10.1093/jnci/djn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C-J, Iloeje U, Yang H-I. Serum hepatitis B virus DNA as a predictor of the development of cirrhosis and hepatocellular carcinoma. Curr Hepat Rep. 2007;6:9–16. [Google Scholar]
- 22.Liaw Y-F, Sung JJ, Chow WC, et al. Cirrhosis Asian Lamivudine Multicentre Study Group. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 23.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Luan W, Villanueva GA, et al. Clinical prognostic variables in young patients (under 40 years) with hepatitis B virus-associated hepatocellular carcinoma. J Dig Dis. 2012;13:214–218. doi: 10.1111/j.1751-2980.2012.00577.x. [DOI] [PubMed] [Google Scholar]
- 25.Tong MJ, Pan CQ, Hann HW, et al. The management of chronic hepatitis B in Asian Americans. Dig Dis Sci. 2011;56:3143–3162. doi: 10.1007/s10620-011-1841-5. [DOI] [PubMed] [Google Scholar]
- 26.Chen JG, Parkin DM, Chen QG, et al. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204–209. doi: 10.1258/096914103771773320. [DOI] [PubMed] [Google Scholar]
- 27.Mok TS, Yeo W, Yu S, et al. An intensive surveillance program detected a high incidence of hepatocellular carcinoma among hepatitis B virus carriers with abnormal alpha-fetoprotein levels or abdominal ultrasonography results. J Clin Oncol. 2005;23:8041–8047. doi: 10.1200/JCO.2005.01.9927. [DOI] [PubMed] [Google Scholar]
- 28.Zhang BH, Yang BH. Tang ZY Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 29.Han SH, Reddy KR, Keeffe EB, et al. NIH HBV OLT Study Group. Clinical outcomes of liver transplantation for HBV-related hepatocellular carcinoma: data from the NIH HBV OLT study. Clin Transplant. 2011;25:E152–E162. doi: 10.1111/j.1399-0012.2010.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bzowej N, Han S, Degertekin B, et al. National Institutes of Health Hepatitis B Virus Orthotopic Liver Transplantation Study Group. Liver transplantation outcomes among Caucasians, Asian Americans, and African Americans with hepatitis B. Liver Transpl. 2009;15:1010–1020. doi: 10.1002/lt.21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffy JP, Hiatt JR, Busuttil RW. Surgical resection of hepatocellular carcinoma. Cancer J. 2008;14:100–110. doi: 10.1097/PPO.0b013e31816a5c1f. [DOI] [PubMed] [Google Scholar]
- 32.Fan ST, Mau Lo C, Poon RT, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253:745–758. doi: 10.1097/SLA.0b013e3182111195. [DOI] [PubMed] [Google Scholar]
- 33.Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684–692. doi: 10.1007/s00330-006-0461-5. [DOI] [PubMed] [Google Scholar]
- 34.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 35.Chang CH, Chau GY, Lui WY, Tsay SH, King KL, Wu CW. Long-term results of hepatic resection for hepatocellular carcinoma originating from the noncirrhotic liver. Arch Surg. 2004;139:320–325. doi: 10.1001/archsurg.139.3.320. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78. doi: 10.1186/1471-230X-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]