Abstract
Analytes and biomarkers present in saliva may provide insight into individual differences in environmental chemical exposures, variation in reproductive hormones, therapeutic and illegal substance use, changes in stress-related physiology, and the immunologic footprints of infectious disease. The wealth of information provided by salivary analytes has the potential to enrich biobehavioral nursing research by enabling researchers to measure these individual differences in the clinic as well as in patients' and participants' everyday social worlds. In this paper we provide a roadmap for researchers new to this area who would like to learn more about integrating salivary biospecimens into the next generation of health research. In addition, we highlight best practices and strategies to avoid common pitfalls for researchers already engaged in this field.
Keywords: Salivary biomarkers, Nursing research, Best practices
Individuals continually adapt to their internal and external environments. These adaptations are reflected in the biology of health and illness. Research in nursing and other health science disciplines has been increasingly influenced by theoretical models that explain individual differences in behavior and disease risk as a function of interrelated biological, behavioral, and contextual forces (Szanton & Gill, 2010). Recent technical innovations have made it possible to assess the activity of many biological systems in straightforward, portable, and cost-efficient ways. Saliva, in particular, has received attention as a biospecimen. Compared to blood sampling, saliva collection is generally less burdensome for research participants (see Table 1); a single specimen can provide information about a range of physiologic systems, chemical exposures, and genetic variability relevant to basic biological function, health, and disease.
Table 1.
Perceived Advantages of Oral Fluids as a Research Specimen Compared to Serum.
| Minimally Invasive | Considered “acceptable and non-invasive” by research participants and patients |
| Collection is quick, non-painful, uncomplicated | |
| Safety | Reduces transmission of infectious disease by eliminating the potential for accidental needle sticks |
| CDC does not consider saliva a Class II biohazard unless visibly contaminated with blood | |
| Self-collection | Allows for community- and home-based collection |
| Enables specimen collection in special populations | |
| Economics | Eliminates the need for a health care intermediary (e.g., phlebotomist, nurse) |
| Resources for collection and processing samples are low cost and available | |
| Accuracy | Salivary levels of many analytes represent the “free unbound fraction” or biological active fraction in the general circulation |
Source: U. S. Department of Health and Human Services (2000).
Studies using salivary biomarkers have already helped to address important questions about health, development and disease risk across the lifespan. Findings include, for example, cortisol co-regulation between mother and infant (Neu, Laudenslager, & Robinson, 2009), associations between salivary cortisol and behavior patterns in older nursing home residents (Hodgson, Freedman, Granger, & Erno, 2004; Woods & Mentes, 2011), physiological differences between those with posttraumatic stress disorder and those without (Gill, Szanton, Taylor, Page, & Campbell, 2009), and salivary cotinine as an index of secondhand tobacco smoke exposure in inner-city children (Butz et al., 2011). The purpose of this review is to provide a roadmap for scientists new to the area who are interested in integrating oral fluids into the next generation of health research. In addition, we highlight best practices and strategies to avoid common pitfalls for researchers already engaged in this field. We direct those who seek more technical and exhaustive reviews of this area to Malamud and Niedbala (2007) and Hu, Loo, and Wong (2007),for further reading.
Saliva as a Biospecimen
Unlike blood, which is generally similar in composition throughout the body, saliva is a composite of oral fluids secreted from many different glands. Each secretory gland produces a fluid that differs in volume, composition, and constituents from the others (e.g., Veerman, Van Den Keijbus, Vissink, & Nieuw Amerongen, 1996). The source glands are located in the upper posterior area of the oral cavity (parotid gland area), lower area of the mouth between the cheek and jaw (submandibular gland area), and under the tongue (sublingual gland area; see Figure 1). There are also many minor secretory glands in the lip, cheek, tongue, and palate. In addition, a fraction of oral fluid, which leaks from the cleft area between teeth and gums, is comprised of blood products (i.e, serum) that results from mucosal injury or inflammation. The degree of the contribution from serum to oral fluid depends largely on the degree of mucosal or epithelial inflammation. Oral fluid, therefore, is a more precise term for the fluid collected from the mouth, a significant proportion of which is saliva; nonetheless, for clarity and simplicity, we refer to “salivary” biomarkers from this point forward.
Figure 1.
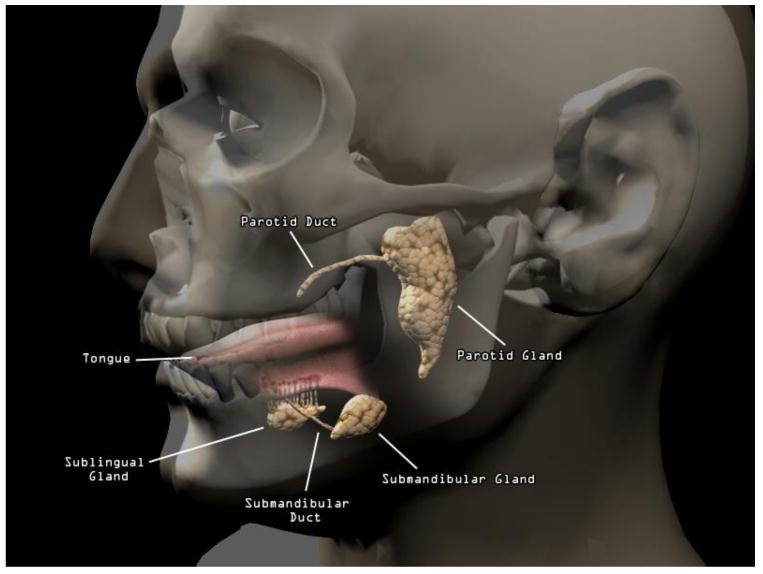
Anatomy of human salivary system.
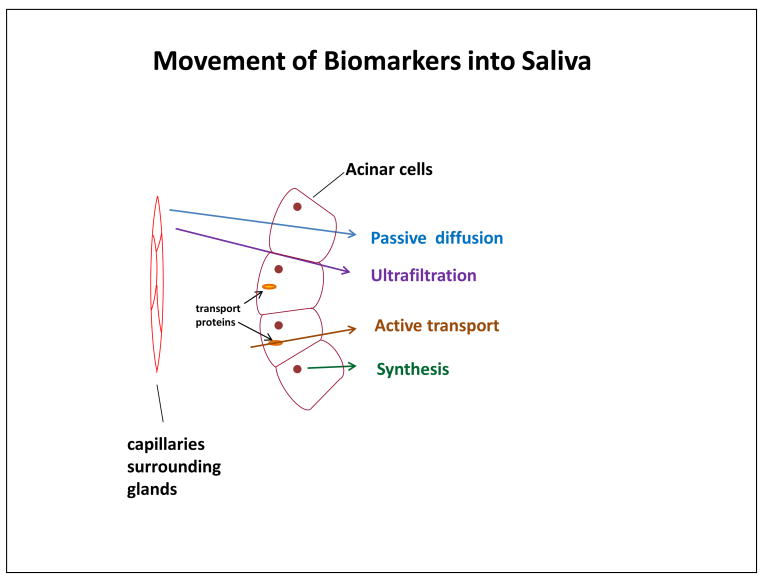
To interpret individual differences in a salivary analyte, it is important to understand how that analyte makes it way into a given saliva sample. Is it produced locally in the mouth? Diffused into saliva from blood? Many of the salivary analytes of interest in biobehavoral research are serum constituents (e.g., steroid hormones). Serum constituent analytes are transported into saliva either by filtration from the tight spaces between acinus or duct cells in the salivary glands or by diffusion through acinus or duct cell membranes (see Figure 2). In contrast, some analytes are synthesized, stored, and released from the granules within the secretory cells of the saliva glands (i.e., enzymes, mucins, cystatins, histatins). Still others are components of humoral immunity (e.g., antibodies, complement) or compounds (e.g., cytokines) secreted by neutrophils, macrophages, or lymphocytes present in the oral mucosa.
Figure 2.
Illustration of routes by which biomarkers make their way into saliva.
The rate of saliva secretion can significantly influence levels of salivary analytes produced locally in the mouth (e.g., alpha-amylase [sAA], secretory immunoglobulin A [IgA]) as well as those that migrate into saliva from blood by filtration (e.g., dehydroepiandrosterone-sulfate and other conjugated steroids; e.g., Kugler, Hess, & Haake, 1992). Saliva secretion is influenced by many factors, including the day–night cycle, autonomic nervous system activity, chewing, taste and smell, as well as medications, treatments and medical conditions that affect saliva gland function (e.g., radiation therapy, Sjorgen's syndrome). When an analyte is influenced by salivary flow rate, estimates of the measured concentration (e.g., pg/mL) or activity (e.g., U/mL) of the analyte may be diluted. Investigators must, therefore, record the volume (mL) of fluid produced over the time interval (min) during which the specimen was collected. Sample volumes can be measured directly or can be indirectly estimated by sample weight. The correction procedure to account for flow rate involves multiplying the concentration (e.g., pg/mL) or units of activity (e.g., U/mL) by the recorded mL/min term. The computation results in the cancelation of the “mL” terms, and the index produced is output as a function of time (e.g., U/min, pg/min).
Sample Collection
Saliva specimens should be handled with universal precautions when used in research and diagnostic applications. Even among healthy individuals, more than 250 species of bacteria are present in saliva (Paster et al., 2001). During upper respiratory infections (e.g., influenza), saliva is highly likely to contain agents of disease (Scannapieco, Papandonatos, & Dunford, 1998). Many infectious agents (e.g., hepatitis B, Epstein-Barr, cytomegalovirus, viral meningitis), both local and systemic, are shed in saliva.
Investigators often collect saliva by placing absorbent materials (e.g., small cotton or foam swabs) in the mouth for 2-3 min (e.g., Dabbs, 1991; Haeckel & Bucklitsch, 1987). Fluid is subsequently recovered from the collection material by centrifugation or compression (e.g., Gunnar, Mangelsdorf, Larson, & Hertsgaard, 1989). Collecting saliva with absorbent materials is popular because it is efficient, convenient, and straightforward for research participants to self-collect with minimal training. However, several aspects of the collection protocol may impact sample integrity and measurement reliability and validity. For example, where a swab is placed in the mouth during collection has the potential to affect the measured levels or activity of salivary analytes secreted locally in the mouth (e.g., Harmon, Towe, Fortunato, & Granger, 2008). Moreover, the maximum absorbent capacity of a swab may bias estimates of salivary flow if the material is left in the participant's mouth long after it is fully saturated (e.g., Beltzer et al., 2010). Standardizing swab placement instructions and monitoring participant compliance can minimize these threats to measurement validity.
There are some cases when investigators should avoid using absorbent material to collect saliva. For example, when the sample volume is small, the specimen can be diffusely distributed in the absorbent material, making it difficult to recover the sample for analysis (Harmon, Hibel, Rumyansteva, & Granger, 2007). In addition, the process of absorbing oral fluid with cotton and other materials may interfere with the assay of some analytes. Investigators should confer with their laboratory to ensure they choose appropriate sample collection methods before beginning their study (Groschl & Rauh, 2006; Shirtcliff, Granger, Schwartz, & Curran, 2001).
In early studies, investigators often stimulated saliva flow using techniques that involved tasting various substances (e.g., gums, waxes, sugar crystals, powdered drink mixes). However, some efforts to stimulate salivary flow may alter the composition of oral fluids (e.g., Schwartz, Granger, Susman, Gunnar, & Laird, 1998). Saliva is water-like in composition and has minimal buffering capacity; therefore, its acidity (pH) can change very quickly in response to substances placed in the mouth. Oral stimulants may also indirectly influence measured levels of analytes that may be diluted by saliva flow rate (e.g., SIgA; dehydroepiandrosterone-sulfate, DHEA-S; neuropeptide Y, NPY; vasoactive intestinal peptide, VIP). For these reasons, we advise researchers to avoid these techniques.
Collecting whole saliva by “passive drool” (Granger, Kivlighan, Fortunato, et al., 2007) is an alternative collection approach that minimizes many of the threats to validity described above. Briefly, participants imagine they are chewing a favorite food, slowly moving their jaws in a chewing motion and allowing oral fluid to pool in their mouth. Next, they gently force the specimen through a short plastic drinking straw into a vial. This procedure has a number of advantages over the previously described procedure: (1) a large sample volume may be collected relatively quickly (3–5 min); (2) target collection volume may be confirmed by visual inspection in the field; (3) the fluid collected is a pooled specimen mixture of the output from all salivary glands; (4) the procedure does not introduce interference related to stimulating or absorbing saliva; and (5) collection materials are very low cost.
Blood Leakage into Oral Fluid
Blood poses a threat to the validity of salivary analyte measurements because most serum constituents are present in serum in much higher levels (10–100 fold) than in saliva (Granger, Cicchetti et al., 2007). Specifically, to reflect a valid measure of systemic (vs. oral) biological activity, analyte levels in saliva must be highly correlated with levels measured in serum. This serum–saliva association depends, in part, on circulating molecules being appropriately and consistently transported into saliva (Malamud & Tabak, 1993). When the integrity of diffusion or filtration is compromised (e.g., through blood leakage directly into salivary fluid), the level of the serological marker in saliva will be affected. Blood leakage is more common among individuals with poor oral health (i.e., open sores, periodontal disease, gingivitis) and certain infectious diseases (e.g., HIV; Arendorf, Bredekamp, Cloete, & Sauer, 1998) as well as tobacco users (Reibel, 2003). Samples visibly contaminated with blood exhibit varying degrees of yellow-brownish hue. There are methods to categorize blood contamination in saliva based on visual inspection that may be useful in some populations (e.g., see Kivlighan et al., 2004).
Particulate Matter and Interfering Substances
As noted above, items placed in the mouth can influence the integrity of saliva samples. Food residue in the oral cavity may introduce particulate matter, change salivary pH or composition, and/or contain substances (e.g., bovine hormones, active ingredients in medications, enzymes) that cross-react with assays (Granger, Hibel, Fortunato, & Kapelewski, 2009; Magnano, Diamond, & Gardner, 1989; Schwartz et al., 1998). Accordingly, research participants should not eat or drink for 20 min prior to sample donation (Granger, Kivlighan, Fortunato, et al., 2007). In the event that they do eat in this time window, participants should rinse their mouth with water. Because this rinsing process may dilute saliva, thereby artificially lowering estimates of measured analytes, participants should wait at least 10 min after rinsing before providing a specimen. Access to food and drink should be carefully planned and scheduled when study designs involve repeated sample collections over long time periods.
Sample Handling, Transport, and Storage
Typically, once specimens are collected, they should be kept cold or frozen. Refrigeration prevents degradation of some salivary analytes and restricts the activity of protolytic enzymes and growth of bacteria. Our conservative recommendation is that samples should be kept frozen. At minimum, samples should be kept cold (on ice or refrigerated) and frozen later in the day of collection. Repeated freeze–thaw cycles should be avoided. Some analytes, including DHEA, estradiol, and progesterone, are very sensitive to freeze–thaw, whereas others, including DNA, cortisol, testosterone, and salivary alpha amylase are robust (can withstand up to at least three freeze–thaw cycles; Granger, Schwartz, Booth, Curran, & Zakaria, 1999; Granger, Shirtcliff, Booth, Kivlighan, & Schwartz, 2004; O'Donnell, Kammerer, O'Reilly, Taylor, & Glover, 2009). Freeze–thaw cycles should be considered in the context of plans to aliquot and archive frozen samples for future assays. It should also be noted that some salivary analytes (e.g., neuropeptides such as oxytocin) may require that specimens be collected into pre-chilled storage vials and frozen within minutes of collection (Carter et al., 2007) or treated with neuropeptidase inhibitors (e.g., ethylenediaminetetraacetic acid [EDTA], aprotinin) to minimize degradation (Dawidson, Blom, Lundeberg, Theodorsson, & Angmar-Mansson, 1997; Santavirta et al., 1997). For large-scale national surveys, investigators working in remote areas (Clingerman & Brown, 2012), or patients collecting samples at home (Raff, Homar, & Skoner, 2003), freezing and shipping these frozen samples can be logistically complex and cost prohibitive. In such circumstances, investigators should document the impact of the handling and storage conditions by pilot work or should consider alternative biospecimens.
Medications
As noted above, many drugs, including diuretics, hypotensives, antipsychotics, antihistamines, barbiturates, hallucinogens, cannabis, and alcohol can indirectly affect some analytes by reducing salivary flow (e.g., Rees, 1992; Sreebny & Schwartz, 1997). Further, the condition for which the medication is prescribed or taken may itself directly influence analyte levels or activity (Granger et al., 2009). Few behaviorally oriented studies involving salivary analytes comprehensively document medication usage. Further, a lack of normative data coupled with wide individual variation in salivary analyte levels makes it challenging to identify improbable values due to medication use (unless the value is not physiologically plausible). Medications that are applied intranasally, inhaled, or applied as oral topicals (e.g., teething gels) are of particular concern. Residue in the oral cavity left by these substances may change saliva composition and/or interfere with antibody–antigen binding in immunoassays. We recommend that investigators record the name, dosage, and schedule of all medications taken (prescription and nonprescription) in the last 48 hr and use this information to statistically evaluate the possibility that medication use explains analyte–outcome relationships.
Assays for Salivary Analytes
Immunoassays are the main laboratory techniques employed to assess levels and activity of salivary analytes. Saliva samples are typically subjected to one freeze–thaw cycle and centrifuged prior to immunoassay to break apart the mucins that make saliva viscous and sticky. This process also removes particulate that might interfere with accurate liquid handling when the specimens are pipetted into microtiter wells. Most immunoassays involve three steps (see Chard, 1990). Antibodies prepared against a specific salivary analyte are coated to the bottom of a microtiter plate well; these antibodies are used to capture the target molecules. Alternatively, antigens may be coated to the wells to capture antibodies present in the sample. The majority of modern assays employ a labeling design known as enzyme immunoassay (EIA), which uses enzymes coupled to antigens or antibodies (i.e., the enzyme conjugate). To measure salivary cortisol, for instance, antibodies to cortisol are fixed to the plastic surface of a microtiter well. The specimen and a cortisol–enzyme conjugate (cortisol-HRP) are added into the well and incubated. During the incubation, cortisol from the sample and the cortisol–enzyme conjugate compete for available antibody-binding sites. The wells are then rinsed to remove unbound material. Next, a substrate is added that reacts with the enzyme conjugate (HRP) to produce a color. The degree of color in each reaction well is measured in units of optical density (OD). The more cortisol in the sample, the lower the amount of cortisol–conjugate that is bound to the plate and the lower the OD in that reaction well. To determine concentrations of cortisol in the unknown samples, samples with known concentrations of cortisol (standards) are analyzed as part of each assay. Results from the standards are used to establish a calibration curve from which concentration/volume units can be interpolated from OD.
Operationalizing Individual Differences
The “basal level” of an analyte is the level or activity that represents the “stable state” of the host during a resting period. One approach to assessing basal levels is to sample early in the morning before the events of the day are able to contribute variation (see Booth, Johnson, Granger, Crouter, & McHale, 2003). However, moment-to-moment variability differs across salivary analytes depending on a number of factors including inherent or pulsitile variation in the production/release of the analytes, rate of their metabolism/degradation, and their sensitivity to, and regulation by, environmental influences. Therefore, a single time-point measure of a salivary analyte (other than invariant genetic polymorphisms) is unlikely to yield meaningful insight into an individual's true basal level. Sampling at the same time of day across a number of days, then aggregating (by averaging assay results or physically pooling specimens) across days can help enhance the reliability of these estimates.
Most salivary biomarker/analyte studies have involved a reactivity/regulation paradigm; this approach uses repeated samples to evaluate time-dependent changes in analytes (e.g., cortisol, sAA) in response to (or in anticipation of) a discrete event (see Figure 3). The number of samples that should be collected depends on the research question and logistical and practical issues (e.g., participant's tolerance for sampling burden). The optimal design for the measurement of salivary cortisol and sAA reactivity and regulation involves a pre-pre-[task]-post-post-post-post sampling scheme, with samples collected on arrival to the lab (after consent) immediately before the task, then again immediately, 10, 20 and approximately 40 min postchallenge (Granger, Kivlighan, El-Sheikh, Gordis, & Stroud, 2007; Kudelka, Schommer, Hellhammer, & Kirschbaum, 2004). Although some studies have yielded consistent mean-level differences in patterns of cortisol response following a stressful or novel event, there are more often significant individual differences in reactivity and regulation. Some individuals exhibit unexpected patterns of change (or no change) as well as continuously increasing or decreasing analyte levels over time.
Figure 3.
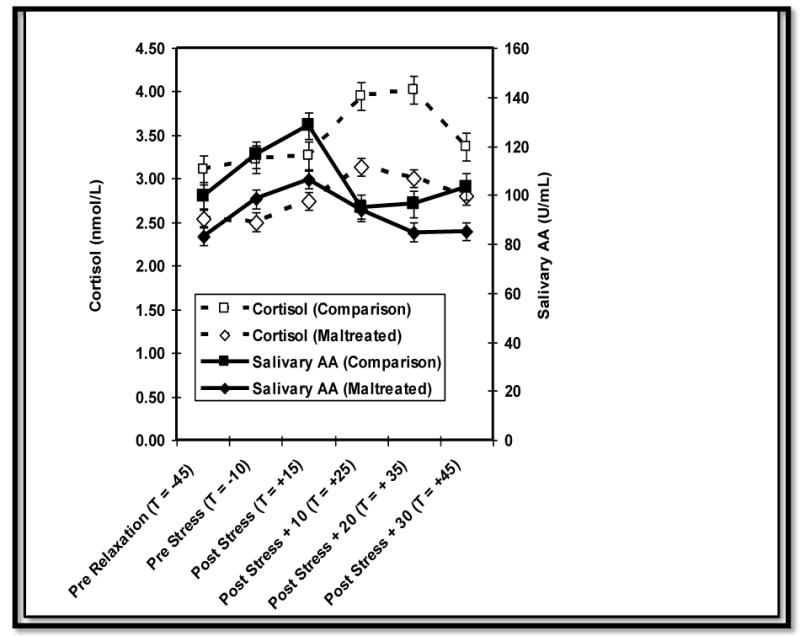
Cortisol and amylase (sAA) reactivity in response to a stressor among maltreated and comparison children. Reprinted from Gordis, Granger, Susman, & Trickett, 2008, with permission from Elsevier.
Enhancing Compliance and Accuracy of Repeated Measures
Obtaining repeated saliva samples can be a time-consuming and difficult task. Major challenges to obtaining reliable samples from individuals include ensuring that participants a) remember to collect the samples and b) collect the samples at the specified time. Reminders can be effective at increasing the likelihood of individuals' compliance and accuracy in saliva sampling.
One common method of reminding participants to collect samples over the course of one or several days is to call them at each of the scheduled sampling times. However, calling participants individually multiple times is time and labor intensive and may ultimately be unfeasible in studies with large numbers of participants or sampling instances. Another method of reminders that presents less of a burden is providing individuals with a multi-alarm wristwatch set to go off at programmed intervals based on the sampling protocol. While this method greatly reduces the burden and time involved in reminding individuals of each sampling instance over the course of several days, the costs may be excessive in some circumstances.
Emerging technologies can provide cost-effective methods of reminding participants to collect samples at the appropriate times. One such nascent mobile technology is that of text messaging (short message service [SMS]). SMS can be used across a wide range of smartphones or featurephones as well as across a variety of cellular phone service providers and platforms. SMS reminders have been shown to be feasible as a cost-effective and efficient method of increasing individuals' compliance with requested actions in changing and managing health behavior and obtaining entries in diary studies (Anhøj & Møldrup, 2004; Berkman, Falk, & Lieberman, 2011; Krishna, Boren, & Balas, 2009). Additionally, investigators have used low-cost services such as Frontline SMS (www.frontlinesms.org) and RedOxygen (www.redoxygen.com) effectively to manage the dissemination and tracking of SMS to and from individuals. For example, SMS reminders can be programmed in bulk to be sent out at specified times using these services; individuals receiving these SMS reminders can then send a short message back verifying completion of the sampling procedure and these responses can be channeled into an online database. SMS reminders may thus not only prove to be more cost-effective and less burdensome than other methods, they also have the advantage of being able to track participant responses and record each sampling completion time.
Analytic Methods of Addressing Individual Differences
An important component of variability in salivary analyte levels, both within and between individuals, is the diurnal rhythm (e.g., Gunnar & Vasquez, 2001; Smyth et al., 1997). Most salivary hormone levels (e.g., cortisol) are high in the morning, decline before noon, and then decline more slowly in the afternoon and evening hours (Nelson, 2005). By contrast, levels of sAA show the opposite pattern with low levels in the morning and higher levels in the afternoon (Nater, Rohleder, Schlotz, Ehlert, & Kirschbaum, 2007). The nonlinear nature of these patterns requires multiple sampling time points to create adequate statistical models. A typical sampling design for salivary cortisol involves sampling immediately upon waking, 30 min postwaking, midday (around noon), in the late afternoon, and immediately prior to bed (Hellhammer et al., 2007).
Investigators have used many analytical techniques to model individual differences in diurnal rhythm including calculating mean levels, evaluating the awakening response, and calculating summary measures of analytes over time (e.g., area under the curve [Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003]). Several techniques are available for the analysis of repeated measures of a salivary analyte across the day or surrounding a discrete (stressful) event. One approach is a form of structural equation modeling, namely growth curve modeling (McArdle & Bell, 2000), which has recently gained popularity for a number of reasons. Briefly, growth models include latent (unmeasured) factors that represent baseline levels of a salivary analyte and capture specific aspects of individual trajectories over time. Correlates and predictors of change can be evaluated, the impact of error or noise in measured values is minimized, and the presence of individual differences in the diurnal rhythm can be statistically tested (e.g., McArdle & Bell, 2000; McArdle & Nesselroade, 1994). With a genetically informative design (e.g., twin studies), structural equation modeling can be used to answer questions about the etiology of individual differences in analyte levels over time (e.g., De Geus, Kupper, Boomsma, & Snieder, 2007; Out, Bakermans-Kranenburg, Granger, Cobbaert, & Van IJzendoorn, 2011). For example, the existence of stable genetic or environmental influences affecting analyte levels across time and in different conditions can be examined, as well as the presence of new genetic or environmental influences that affect analyte levels only during a specific time or condition.
Multilevel modeling is another analytic approach that is suitable for studies in which multiple samples are collected for each individual across the day or before/after a discrete event (Bryk & Raudenbush, 1992; Hox, 2002; Kreft & De Leeuw, 1998; Twisk, 2006). These repeated measurements are said to be “nested” within the individual. A two-level model can then be tested: the separate measurements of salivary analyte are the lower level, while the individual is the higher level (additional levels can also be specified, for example, when patients are clustered within providers). To account for this hierarchical structure, a random intercept is added to the analysis, allowing the intercepts to differ between individuals. By calculating an intraclass correlation, it can be estimated to what extent the variance in analyte levels are due to differences between individuals (indicative of more stable, trait-like variance) or within individuals (state-like variance, e.g. when levels of cortisol fluctuate across the day).
Predictors can be added at the person level as well as the measurement level, and the regression coefficients can also be allowed to be random (e.g., specific circumstances may have an effect on analyte levels, but this effect may depend on the individual). Influences on salivary measures on the lower level may include momentary states and feelings (e.g., mood states) and activities reported at the time of sampling. Documenting everyday events and emotions that help explain changes in analyte levels or activity across a time period of interest may strengthen causal inference when paired with samples across multiple days. For example, in studies focusing on cortisol, samples are collected approximately 20 min after a diary entry. Computerized handheld devices have made self-assessments quite feasible (e.g., Stone et al., 2003). Furthermore, multilevel techniques may also be applied for the construction of growth curves in order to capture changes in salivary analyte levels over time and to test individual differences in reactivity to a stressful event or in diurnal rhythm.
Analytes in Saliva of Interest to Nursing
To date, most health research has focused on a small number of salivary analytes: cortisol, testosterone, DHEA, and sAA. In fact, however, the salivary proteome has recently been characterized and includes more than 1,000 analytes (Hu et al., 2007). These analytes provide information about (1) systemic body processes, (2) local oral biology, (3) surrogate markers of physiological activity, (4) antibodies, (5) medications and environmental exposures, and (5) genetic factors. This broader range of analytes may expand the utility of saliva for health research. We discuss each category of analytes briefly below and summarize them in Table 2.
Table 2.
Salivary Analytes of Potential Interest to Nursing Research.
| Endocrine | |
| Aldosterone | Estradiol, estriol, esterone |
| Androstenedione | Melatonin |
| Cortisol | Progesterone; 17-OH progesterone |
| Dihydroepiandrosterone, and -sulfate | Testosterone |
| Immune/Inflammation | |
| Beta-2-microgolublin (B2M) | Neopterin |
| C-reactive protein (CRP) | Secretory immunoglobulin A (SIgA) |
| Cytokines | Soluble cytokine receptors |
| Autonomic Nervous System | |
| Alpha-amylase (sAA) | Vasoactive intestinal peptide (VIP) |
| Neuropeptide Y (NPY) | |
| Nucleic Acids | |
| Bacterial | Mitochondrial |
| Human genomic | mRNA |
| Microbial | Viral |
| Antibodies Specific for Antigens | |
| Epstein Barr | Malaria |
| Hepatitis A | Measles |
| Hepatitis B | Mumps |
| Herpes simplex | Rubella |
| Human immunodeficiency virus (HIV) | |
| Pharmaceuticals | |
| Alcohol | Lithium |
| Barbituates | Marijuana (THC) |
| Bisphenol-A (BPA) | Meth-, Amphetamine |
| Caffeine | Methadone |
| Cocaine | Opioids |
| Cotinine | Phenytoin |
The first group of analytes is present in saliva because oral fluid represents an ultra-filtrate of analytes found in the bloodstream (i.e., serum constituents). Because of high serum/saliva correlations, measuring these analytes in saliva enables investigators to make inferences about systemic physiological states. Adrenal and gonadol hormones are exemplars of this category of salivary markers (e.g., see Table 2).
Most analytes in saliva are produced locally in the oral cavity and are secreted from salivary glands (Nieuw Amerongen, Ligtenberg, & Veerman, 2007; Veerman et al., 1996). While individual differences in these salivary analytes may reflect systemic processes, a major contributor is local oral biology (e.g., local inflammatory processes, oral health and disease). Many salivary immune and inflammatory markers such as neopterin, beta-2-microglobulin and cytokines (see Table 2) fall into this category (e.g., Brandtzaeg, 2007). Markers in this group may be less interesting to investigators outside the fields of oral biology and oral health.
A third group of salivary analytes is produced locally by salivary glands, but the levels vary predictably with systemic physiological activation. For example, sympathetic nervous system activation affects the release of catecholamines from nerve endings, and these compounds' action on adrenergic receptors influences the activity of the salivary glands. For instance, salivary alpha-amylase is considered a surrogate marker of autonomic nervous system (ANS) activation, as are salivary measures of neuropeptide Y and vasoactive intestinal peptide (e.g., Santavirta et al., 1997).
Antibodies to specific antigens (e.g., human immunodeficiency virus [HIV] antibodies) comprise another group of salivary analytes. Table 2 offers several additional examples. Antibodies in oral fluids reflect an individual's immunological history and pathogen/microbe exposure. Further, depending on the specific antibody measured, they may reflect local and/or systemic immune activity. To date, relatively few biobehavioral studies have taken advantage of the information provided by salivary antibodies.
A variety of pharmaceuticals, abused substances, and environmental contaminants can be quantitatively monitored in oral fluids (see Table 2). One example is bisphenol-A (BPA), a constituent of polycarbonate plastic and epoxy resins used in water bottles, baby bottles and food containers which may leach into food and drink. Daily BPA exposures below the U. S. human exposure limit (50 ug/kg/day) have been linked to permanent changes in genitalia, early puberty, and reversal of sex differences in brain structure (Maffini, Rubin, Sonnenschein, & Soto, 2006; Melnick et al., 2002).
A final group of analytes has been made possible by recent technical advances allowing high quantity and quality DNA to be extracted from whole saliva (Zimmerman, Park, & Wong, 2007). We recently documented that saliva samples collected using the same techniques described above to assess individual differences in salivary analytes and biomarkers can produce sufficient quality and quantity of DNA to yield reliable and valid information about genetic polymorphisms (Nemoda, Horvat-Gordon, Fortunato, Beltzer, Scholl, & Granger, in press). More specifically, our analysis revealed that the typical volumes (.10–1.0 mL) of saliva collected using procedures described above were more than sufficient to enable isolation of DNA for genetic analyses (see Table 3). Differences in collection device materials had only a negligible influence on the isolation of sufficient quantity and quality of DNA from saliva. The effects of room temperature (RT) storage of saliva samples on the quality and quantity of DNA recovered were minimal. Archived saliva samples that had been exposed to multiple freeze–thaw cycles yielded sufficient quantity and quality of DNA for genetic analyses. Finally, there were no differences in DNA quantity and quality according to where in the mouth the samples were collected.
Table 3.
Means (standard deviations) for DNA concentration and quality obtained from different sample volumes of whole saliva.
| Sample Volume | ||||
|---|---|---|---|---|
|
|
||||
| Measure | .10 ml | .25 ml | .50 ml | 1.0 ml |
| DNA concentration (ng/μl) | 28.25 (15.36) | 68.00 (39.66) | 116.90 (55.19) | 239.78 (144.27) |
| DNA quality (260/280 nm) | 1.78 (.10) | 1.75 (.10) | 1.74 (.14) | 1.79 (.11) |
| DNA total amount (μg) | 1.43 (.77) | 3.40 (1.98) | 6.24 (3.38) | 11.99 (5.71) |
Note. DNA = deoxyribonucleic acid.
Source: Nemoda et al., 2011
Conclusion and Future Directions
The growing body of nursing research using salivary biomarkers attests to the new opportunities oral fluids offer for health researchers. As the number of substances that can be reliably measured grows, collecting saliva may become an increasingly attractive alternative to collecting blood. For example, biomarkers in saliva provide opportunities to implement research protocols that would be unacceptably burdensome for many research participants if blood collection were required. Further, collecting saliva may be particularly useful when a less invasive alternative to venipuncture is required: such as research with healthy children, in which ethical considerations dictate no more than minimal risk.
As a gateway to the body, the mouth senses and responds to the external world and reflects what is happening inside the body (U. S. Department of Health and Human Services, 2000). Collecting saliva may also allow researchers new windows on real-world indicators of, disease risk, resilience, and environmental exposures. For example, salivary biomarkers are ideal for examining biological parameters that shift through the day. Having subjects collect their own specimens frees the investigator to examine physiological responses to everyday situations in context and removes the bias introduced by laboratory-based sampling conditions. With ongoing attention to appropriate saliva collection procedures, collection materials, sample storage, as well as the oral biology behind their analyte of choice, researchers can ensure maximal reliability and validity of these data.
Acknowledgments
DAG is founder and Chief Strategy and Scientific Advisor at Salimetrics LLC (State College, PA). DAG's relationship with Salimetrics LLC is managed by the Conflict of Interest Committee at the Johns Hopkins University School of Medicine. The other authors do not have a commercial or financial involvement that might present an appearance of a conflict of interest related to the contribution. We appreciate the contributions of Doug Maple and Chris Schwartz in the preparation of the figures. DO was supported by a Rubicon award (446-10-026) from the Netherlands Organization for Scientific Research.
References
- Anhøj J, Møldrup C. Feasibility of collecting diary data from asthma patients through mobile phones and SMS (short message service): Response rate analysis and focus group evaluation from a pilot study. Journal of Medical Internet Research. 2004;6:e42. doi: 10.2196/jmir.6.4.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendorf TM, Bredekamp B, Cloete CA, Sauer G. Oral manifestations of HIV infection in 600 South African patients. Journal of Oral Pathology and Medicine. 1998;27:176–179. doi: 10.1111/j.1600-0714.1998.tb01936.x. [DOI] [PubMed] [Google Scholar]
- Beltzer EK, Fortunato CK, Guaderrama MM, Peckins MK, Garramone BM, Granger DA. Salivary flow on alpha–amylase: Collection technique, duration, and oral fluid type. Physiology and Behavior. 2010;101:289–296. doi: 10.1016/j.physbeh.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Falk EB, Lieberman MD. In the trenches of real-world self-control: Neural correlates of breaking the link between craving and smoking. Psychological Science. 2011;22:498–506. doi: 10.1177/0956797611400918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A, Johnson DR, Granger DA, Crouter AC, McHale S. Testosterone and child and adolescent adjustment: The moderating role of parent–child relationships. Developmental Psychology. 2003;39:85–98. doi: 10.1037//0012-1649.39.1.85. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Annals of the New York Academy of Science. 2007;1098:288–311. doi: 10.1196/annals.1384.012. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models. Newbury Park, CA: Sage; 1992. [Google Scholar]
- Butz AM, Halterman JS, Bellin M, Tsoukleris M, Donithan M, Kub J, Bollinger ME. Factors associated with second-hand smoke exposure in young inner-city children with asthma. Journal of Asthma. 2011;48:449–457. doi: 10.3109/02770903.2011.576742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Pournajafi–Nazarloo H, Kramer KM, Ziegler TE, White–Traut R, Bello D, Schwertz D. Oxytocin: Behavioral associations and potential as a salivary biomarker. Annals of the New York Academy of Science. 2007;1098:312–322. doi: 10.1196/annals.1384.006. [DOI] [PubMed] [Google Scholar]
- Chard T. An introduction to radioimmunoassay and related techniques. 4th. Amsterdam: Elsevier; 1990. [Google Scholar]
- Clingerman EM, Brown A. Stress in migrant farmworkers during premigration. Biological Research for Nursing. 2012;14:27–37. doi: 10.1177/1099800410396703. [DOI] [PubMed] [Google Scholar]
- Cone EJ, Huestis MA. Interpretation of oral fluid tests for drugs of abuse. Annals of the New York Academy of Science. 2007;1098:51–103. doi: 10.1196/annals.1384.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs JM., Jr Salivary testosterone measurements: Collecting, storing, and mailing saliva samples. Physiology and Behavior. 1991;49:815–817. doi: 10.1016/0031-9384(91)90323-g. [DOI] [PubMed] [Google Scholar]
- Dawidson I, Blom M, Lundeberg T, Theodorsson E, Angmar–Mansson B. Neuropeptides in the saliva of healthy subjects. Life Sciences. 1997;60:269–278. doi: 10.1016/s0024-3205(96)00627-3. [DOI] [PubMed] [Google Scholar]
- De Geus EJC, Kupper N, Boomsma DI, Snieder H. Bivariate genetic modeling of cardiovascular stress reactivity: Does stress uncover genetic variance? Psychosomatic Medicine. 2007;69:356–364. doi: 10.1097/PSY.0b013e318049cc2d. [DOI] [PubMed] [Google Scholar]
- Gill JM, Szanton S, Taylor TJ, Page GG, Campbell JC. Medical conditions and symptoms associated with posttraumatric stress disorders in low-income urban women. Journal of Women's Health. 2009;18:261–267. doi: 10.1089/jwh.2008.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Hormones and Behavior. 2008;53:96–1003. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Cicchetti D, Rogosch FA, Hibel LC, Teisl M, Flores E. Blood contamination in children's saliva: Prevalence, stability, and impact on the measurement of salivary cortisol, testosterone, and dehydroepiandrosterone. Psychoneuroendocrinology. 2007;32(6):724–733. doi: 10.1016/j.psyneuen.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR. Salivary alpha-amylase in biobehavioral research: Recent developments and applications. Annals of the New York Academy of Science. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, Whembolua GL. Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiology and Behavior. 2007;92:583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Granger DA, Schwartz EB, Booth A, Curran M, Zakaria D. Assessing dehydroepiandrosterone in saliva: A simple radioimmunoassay for use in studies of children, adolescents and adults. Psychoneuroendocrinology. 1999;24(5):567–579. doi: 10.1016/s0306-4530(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwartz EB. The “trouble” with salivary testosterone. Psychoneuroendocrinology. 2004;29(10):1229–1240. doi: 10.1016/j.psyneuen.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Groschl M, Rauh M. Influence of commercial collection devices for saliva on the reliability of salivary steroids analysis. Steroids. 2006;71:1097–1100. doi: 10.1016/j.steroids.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Mangelsdorf S, Larson M, Hertsgaard L. Attachment, temperament, and adrenocortical activity in infancy: A study of psychoendocrine regulation. Developmental Psychology. 1989;3:355–363. [Google Scholar]
- Gunnar MR, Vasquez D. Low cortisol and a flattening of the expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Haeckel R, Bucklitsch I. Procedures for saliva sampling. Journal of Clinical Chemistry and Biochemistry. 1987;25:199–204. doi: 10.1515/cclm.1987.25.4.199. [DOI] [PubMed] [Google Scholar]
- Harmon AG, Hibel LC, Rumyansteva O, Granger DA. Measuring salivary cortisol in studies of child development: Watch out––What goes in may not come out of saliva collection devices. Developmental Psychobiology. 2007;49:495–500. doi: 10.1002/dev.20231. [DOI] [PubMed] [Google Scholar]
- Harmon AG, Towe NR, Fortunato CK, Granger DA. Differences in saliva collection location and disparities in baseline and diurnal rhythms of alpha–amylase: A preliminary note of caution. Hormones and Behavior. 2008;54:592–596. doi: 10.1016/j.yhbeh.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Fries E, Schweusthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Hodgson N, Freedman VA, Granger DA, Erno A. Biobehavioral correlates of relocation in the frail elderly: Salivary cortisol, affect, and cognitive function. Journal of the American Geriatric Society. 2004;52:1856–1862. doi: 10.1111/j.1532-5415.2004.52505.x. [DOI] [PubMed] [Google Scholar]
- Hox J. Multilevel analysis: Techniques and applications. London: Lawrence Erlbaum Associates; 2002. [Google Scholar]
- Hu S, Loo JA, Wong DT. Human saliva proteome analysis. Annals of the New York Academy of Science. 2007;1098:323–329. doi: 10.1196/annals.1384.015. [DOI] [PubMed] [Google Scholar]
- Kivlighan KT, Granger DA, Schwartz EB, Nelson V, Curran M, Shirtcliff EA. Quantifying blood leakage into the oral mucosa and its effects on the measurement of cortisol, dehydroepiandrosterone, and testosterone in saliva. Hormones and Behavior. 2004;46:39–46. doi: 10.1016/j.yhbeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Kreft I, De Leeuw J. Introducing multilevel modeling. London: Sage Publications; 1998. [Google Scholar]
- Krishna S, Boren SA, Balas EA. Healthcare via cell phones: A systematic review. Telemedicine and e-Health. 2009;15:231–240. doi: 10.1089/tmj.2008.0099. [DOI] [PubMed] [Google Scholar]
- Kudelka, Schommer, Hellhammer, Kirschbaum Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29(8):983–992. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Kugler J, Hess M, Haake D. Secretion of salivary immunoglobulin A in relation to age, saliva flow, mood states, secretion of albumin, cortisol, and catecholamines in saliva. Journal of Clinical Immunology. 1992;12:45–49. doi: 10.1007/BF00918272. [DOI] [PubMed] [Google Scholar]
- Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: The case of bisphenol-A. Molecular and Cellular Endocrinology. 2006;254:179–186. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Magnano CL, Diamond EJ, Gardner JM. Use of salivary cortisol measurements in young infants: A note of caution. Child Development. 1989;60(5):1099–1101. [PubMed] [Google Scholar]
- Malamud D, Niedbala RS. Introduction. Annals of the New York Academy of Sciences. 2007;1098:xiv–xv. doi: 10.1196/annals.1384.046. [DOI] [PubMed] [Google Scholar]
- Malamud D, Tabak L. Saliva as a diagnostic fluid. Annals of the New York Academy of Sciences. 1993;694:216–233. doi: 10.1111/j.1749-6632.1993.tb18379.x. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Bell RQ. An introduction to latent growth models for developmental data analysis. In: Little TD, Schnabel KU, Baumert J, editors. Modeling longitudinal and multiple-group data: Practical issues, applied approaches, and specific examples. Hillsdale, NJ: Lawrence Erlbaum Associates; 2000. pp. 69–107. [Google Scholar]
- McArdle JJ, Nesselroade J. Using multivariate data to structure developmental change. In: Cohen SH, Reese HW, editors. Life-span developmental psychology. Hillsdale, NJ: Lawrence Erlbaum Associates; 1994. pp. 223–267. [Google Scholar]
- Melnick R, Lucier G, Wolfe M, Hall R, Stancel G, Prins G, Kohn M. Summary of the National Toxicology Program's report of the endocrine disruptors low-dose peer review. Environmental Health Perspectives. 2002;110:427–431. doi: 10.1289/ehp.02110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. Determinants of the diurnal course of salivary alpha–amylase. Psychoneuroendocrinology. 2007;32:392–401. doi: 10.1016/j.psyneuen.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. An introduction to behavioral endocrinology. Sunderland, MA: Sinauer Associates; 2005. [Google Scholar]
- Nemoda Z, Horvat-Gordon M, Fortunato CK, Beltzer EK, Scholl JL, Granger DA. Assessing genetic polymorphisms using DNA extracted from cells present in saliva samples. BMC Medical Research Methodology. doi: 10.1186/1471-2288-11-170. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu M, Laudenslager ML, Robinson J. Coregulation in salivary cortisol during maternal holding of premature infants. Biological Research for Nursing. 2009;10(3):226–240. doi: 10.1177/1099800408327789. [DOI] [PubMed] [Google Scholar]
- Nieuw Amerongen AV, Ligtenberg AJM, Veerman ECI. Implications for diagnostics in the biochemistry and physiology of saliva. Annals of the New York Academy of Science. 2007;1098:1–6. doi: 10.1196/annals.1384.033. [DOI] [PubMed] [Google Scholar]
- O'Donnell K, Kammerer M, O'Reilly R, Taylor A, Glover V. Salivary alpha-amylase stability, diurnal profile and lack of response to the cold hand test in young women. Stress. 2009;12(6):549–554. doi: 10.3109/10253890902822664. [DOI] [PubMed] [Google Scholar]
- Out D, Bakermans–Kranenburg MJ, Granger DA, Cobbaert CM, Van IJzendoorn MH. State and trait variance in salivary α–amylase: A behavioral genetic study. Biological Psychology. 2011;88:147–154. doi: 10.1016/j.biopsycho.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Dewhirst FE. Bacterial diversity in human subgingival plaque. Journal of Bacteriology. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner J, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raff H, Homar PJ, Skoner DP. New enzyme immunoassay for salivary cortisol. Clinical Chemistry. 2003;49:203–204. doi: 10.1373/49.1.203. [DOI] [PubMed] [Google Scholar]
- Rees TD. Oral effects of drug abuse. Critical Reviews in Oral Biology and Medicine. 1992;3:163–184. doi: 10.1177/10454411920030030101. [DOI] [PubMed] [Google Scholar]
- Reibel J. Tobacco and oral diseases. Update on the evidence, with recommendations. Medical Principles and Practice. 2003;(Suppl. 1):22–32. doi: 10.1159/000069845. [DOI] [PubMed] [Google Scholar]
- Santavirta N, Konttinen YT, Tornwall J, Segerberg M, Santavirta S, Matucci–Cerinic M, Bjorvell H. Neuropeptides of the autonomic nervous system in Sjogren's syndrome. Annals of Rheumatoid Disease. 1997;56:737–740. doi: 10.1136/ard.56.12.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannapieco FA, Papandonatos GD, Dunford RG. Associations between oral conditions and respiratory disease in a national sample survey population. Annals of Periodontology. 1998;3:251–256. doi: 10.1902/annals.1998.3.1.251. [DOI] [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Schwartz EB, Curran MJ. Use of salivary biomarkers in biobehavioral research: Cotton based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001;26:165–173. doi: 10.1016/s0306-4530(00)00042-1. [DOI] [PubMed] [Google Scholar]
- Smyth JM, Ockenfels MC, Gorin AA, Cately D, Porter LS, Kirschbaum C, Stone AA. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22:89–105. doi: 10.1016/s0306-4530(96)00039-x. [DOI] [PubMed] [Google Scholar]
- Sreebny LM, Schwartz SS. A reference guide to drugs and dry mouth – 2nd edition. Gerodontology. 1997;14:33–47. doi: 10.1111/j.1741-2358.1997.00033.x. [DOI] [PubMed] [Google Scholar]
- Stone AA, Broderick JE, Schwartz JE, Shiffman S, Litcher–Kelly L, Calvanese P. Intensive momentary reporting of pain with an electronic diary: Reactivity, compliance, and patient satisfaction. Pain. 2003;104:343–351. doi: 10.1016/s0304-3959(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Szanton SL, Gill JM. Facilitating resilience using a society-to-cells framework: A theory of nursing essentials applied to research and practice. Advances in Nursing Science. 2010;33:329–343. doi: 10.1097/ANS.0b013e3181fb2ea2. [DOI] [PubMed] [Google Scholar]
- Tabak LA. Point-of-care diagnostics enter the mouth. Annals of the New York Academy of Science. 2007;1098:7–14. doi: 10.1196/annals.1384.043. [DOI] [PubMed] [Google Scholar]
- Twisk JWR. Applied multilevel analysis: A practical guide. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- U.S. Department of Health and Human Services. Oral Health in America: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health; 2000. Available at http://www.surgeongeneral.gov/library/oralhealth/ [Google Scholar]
- Veerman ECI, Van Den Keijbus PAM, Vissink A, Nieuw Amerongen AV. Human glandular saliva: Their separate collection and analysis. European Journal of Oral Science. 1996;104:346–352. doi: 10.1111/j.1600-0722.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Woods DL, Mentes JC. Spit: Saliva in nursing research, uses and methodological considerations in older adults. Biological Research for Nursing. 2011;13(3):320–327. doi: 10.1177/1099800411404211. [DOI] [PubMed] [Google Scholar]
- Zimmerman BG, Park NJ, Wong DT. Genomic targets in saliva. Annals of the New York Academy of Science. 2007;1098:184–191. doi: 10.1196/annals.1384.002. [DOI] [PMC free article] [PubMed] [Google Scholar]