SUMMARY
The homeostatic balance of hepatic glucose utilization, storage and production is exquisitely controlled by hormonal signals and hepatic carbon metabolism during fed and fasted states. How the liver senses extracellular glucose to cue glucose utilization versus production is not fully understood. Here, we show that the physiologic balance of hepatic glycolysis and gluconeogenesis is regulated by BAD, a dual function protein with roles in apoptosis and metabolism. BAD deficiency reprograms hepatic substrate and energy metabolism towards diminished glycolysis, excess fatty acid oxidation and exaggerated glucose production that escapes suppression by insulin. Genetic and biochemical evidence suggest that BAD’s suppression of gluconeogenesis is actuated by phosphorylation of its BH3 domain and subsequent activation of glucokinase. The physiologic relevance of these findings is evident from the ability of a BAD phospho-mimic variant to counteract unrestrained gluconeogenesis and improve glycemia in leptin resistant and high-fat diet models of diabetes and insulin resistance.
INTRODUCTION
Hepatic glucose production (HGP) is central to metabolic adaptation during fasting and its abnormal elevation is a chief determinant of fasting hyperglycemia in diabetes (Lin and Accili, 2011; Rizza, 2010). During short term fasting, glucose is produced by both net glycogenolysis and gluconeogenesis (Rothman et al., 1991), whereas upon prolonged fasting, glucose is synthesized almost exclusively from gluconeogenesis. Gluconeogenesis and glycolysis are competing pathways that are reciprocally regulated by glucagon and insulin during fasted and fed states, respectively. In addition to transcriptional regulation, changes in hepatic carbon flux can exert allosteric effects on gluconeogenic enzymes (Magnuson et al., 2003). Hepatic substrate flux is further intertwined with fatty acid oxidation (FAO), TCA cycle and mitochondrial electron transport chain activity, which supply the gluconeogenic pathway with carbon substrates, reducing equivalents and ATP (Satapati et al., 2012).
We have previously shown that the BCL-2 family protein BAD has an alternative function in glucose metabolism separate from its role in apoptosis (Danial, 2008; Danial et al., 2003; Danial et al., 2008). BAD’s metabolic effect is dependent on its ability to activate glucokinase (GK) and modulate glucose oxidation (Danial, 2008; Danial et al., 2008). GK is the product of the maturity onset diabetes of the young type 2 (MODY2) gene that exerts tissue-restricted functions (Matschinsky, 2009). In liver, GK regulates substrate utilization and storage by stimulating glycogen and lipid synthesis while suppressing hepatic glucose production (Postic et al., 1999; Rossetti et al., 1997; Velho et al., 1996). In β-cells, GK is critical for glucoseregulation of insulin secretion (Matschinsky, 2009). We previously reported that in β-cells, GK activation and glucose sensing are attuned to BAD phosphorylation on S155 within an amphipathic α-helical region known as the BCL-2 Homology (BH)-3 domain. BAD S155 is the target of multiple kinases that either phosphorylate S155 directly or modify upstream priming phosphorylation sites, facilitating S155 phosphorylation [reviewed in (Danial, 2008)]. Phosphorylation of the BH3 domain not only triggers BAD’s metabolic function but also suppresses its apoptotic activity. Importantly, the phospho-BAD BH3 helix is both required and sufficient for BAD’s metabolic function (Danial et al., 2008). Recent biochemical and structural dissection of the BAD-GK complex has revealed that the phospho-BAD BH3 helix directly engages GK near its active site and increases the Vmax of the enzyme without drastic effects on its glucose affinity (Szlyk et al., 2013).
Beyond β-cell abnormalities, the contribution of altered hepatic metabolism to glucose homeostasis defects associated with loss of BAD has not been examined. This is especially relevant as the regulatory networks that modulate GK activity in liver are distinct from β-cells (Matschinsky, 2009). Here, we use a combination of informative genetic models and BAD mutants to investigate the significance of BAD and its partnership with GK in hepatic substrate utilization and glucose production. Moreover, we probe the physiologic relevance of hepatic BAD in restoring proper control of glucose production in the leptin resistant and high-fat diet models of diabetes and insulin resistance.
RESULTS
BAD-dependent modulation of hepatic glucose metabolism
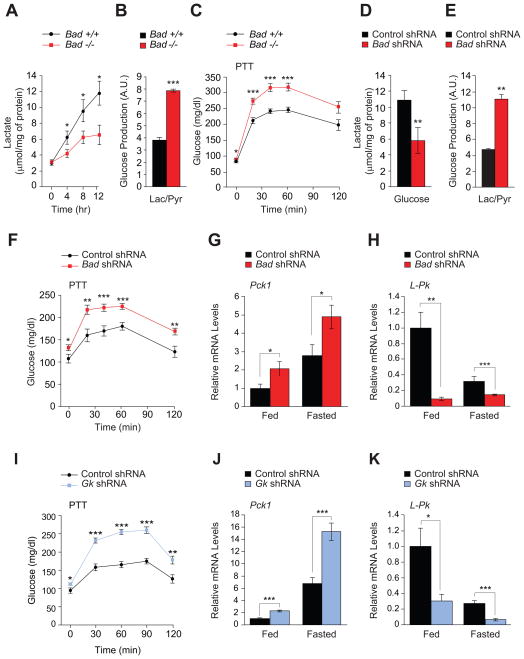
To define the contribution of altered hepatic metabolism to glucose homeostasis abnormalities in Bad −/− mice, we initially focused on glycolytic and gluconeogenic pathways. In primary Bad −/− hepatocytes, lactate production in response to glucose was diminished (Figure 1A), and gluconeogenesis was significantly exaggerated as apparent from glucose release in response to lactate/pyruvate (Figure 1B). These observations suggest that the net balance of glycolysis and gluconeogenesis is shifted towards gluconeogenesis in the absence of BAD. Pyruvate tolerance tests (PTT) confirmed that Bad −/− mice display significantly higher gluconeogenic conversion of pyruvate to glucose in vivo (Figure 1C). Importantly, knockdown of Bad in primary wild-type hepatocytes was sufficient to reduce glycolysis and enhance gluconeogenesis (Figures 1D and 1E), indicating an acute and cell autonomous role for BAD in these processes.
Figure 1. Hepatic glucose metabolism in the absence of BAD.
(A) Glucose-stimulated lactate production by primary Bad +/+ and −/− hepatocytes (n=5–8).
(B) Glucose release by Bad +/+ and −/− hepatocytes treated with lactate/pyruvate (n=6).
(C) PTT in Bad +/+ and −/− mice (n=14–20).
(D) Lactate production in Bad knockdown hepatocytes 8 hr after glucose stimulation (n=9).
(E) Glucose production in Bad knockdown hepatocytes treated with lactate/pyruvate (n=3–5).
(F) PTT in C57BL/6J mice after hepatic knockdown of Bad (n=12–17).
(G–H) Relative hepatic mRNA levels of Pck1 (G) and L-Pk (H) in fed and overnight fasted C57BL/6J mice after hepatic knockdown of Bad (n=10).
(I) PTT in C57BL/6J mice after hepatic knockdown of Gk (n=8–12).
(J–K) Relative hepatic mRNA levels of Pck1 (J) and L-Pk (K) in fed and overnight fasted C57BL/6J mice after hepatic knockdown of Gk (n=5).
Error bars, ± SEM. *p < 0.05; **p < 0.01, ***p < 0.001.
See also Figure S1.
Impaired PTT in Bad −/− mice is consistent with changes in hepatic glucose metabolism, however, secondary changes due to chronic whole body deletion of Bad cannot be excluded. To directly examine the relevance of hepatic BAD in vivo, Bad was knocked down in the liver of C57BL/6J mice following tail vein delivery of adenoviruses bearing Bad shRNA or control (scrambled) sequence (Figure S1A). Hepatic depletion of BAD manifested in excess glucose production during PTT and fasting hyperglycemia (Figure 1F). In addition, acute hepatic knockdown of Bad resulted in impaired glucose tolerance (Figure S1B) similar to Bad −/− mice (Danial et al., 2003). Notably, hepatic depletion of BAD did not alter insulin secretion in response to a glucose challenge in vivo (Figure S1C). In addition, fed/fasted insulin levels were comparable in mice treated with Bad shRNA vs. control shRNA (Figure S1D). Collectively, the above in vitro and in vivo observations indicate that hepatic BAD influences the fate of pyruvate and is relevant for the proper regulation of glucose utilization and production in the liver.
Hepatic pyruvate metabolism is regulated, at least in part, at the transcriptional level downstream of hormonal and nutrient signaling (Lin and Accili, 2011). Initial examination of gluconeogenic and glycolytic genes in fed and fasted Bad −/− and Bad knockdown liver indicated changes in several genes. In particular, mRNA levels of the gluconeogenic enzyme phosphoenolpyruvate carboxykinase (Pck) 1 were significantly elevated, while mRNA levels of the glycolytic enzyme liver-type pyruvate kinase (L-Pk) were significantly diminished (Figures 1G, 1H, and S1E). Other gluconeogenic genes [fructose 1,6-bisphosphatase (Fbp) 1 and glucose 6-phosphatase (G6Pase)] were also increased in Bad −/− liver (Figure S1E). This is further consistent with BAD-dependent reciprocal changes in hepatic glycolysis and gluconeogenesis.
In addition to transcriptional control during fed/fasted states, L-Pk and Pck1 expression can be regulated by glucose. Specifically, the promoters of both genes are glucose-sensitive in that increased glucose metabolism through GK stimulates L-Pk transcription and represses that of Pck1 (Cournarie et al., 1999; Scott et al., 1998; Yamada and Noguchi, 1999). Based on these reports, we predicted that glucose-modulation of L-Pk and Pck1 transcription may be altered upon hepatic depletion of BAD and the attendant diminution of glucose metabolism. This was indeed the case. In wild-type hepatocytes, L-Pk mRNA levels were elevated at high compared to low glucose concentrations (25 mM vs. 5 mM), whereas Pck1 mRNA levels showed the opposite profile (Figures S1F and S1G). Bad knockdown, however, resulted in higher Pck1 mRNA levels that were especially exaggerated at low glucose (Figure S1F). In comparison, glucose-stimulation of L-Pk transcripts was significantly reduced (Figure S1G).
The above observations in both cultured hepatocytes and whole animals using a combination of biochemical and transcriptional readouts suggest that BAD is required for proper interpretation of glucose signals. Notably, the metabolic outcome of BAD depletion in the liver was phenocopied in mice treated with Gk shRNA (Figure S1H). This is evident from exaggerated glucose levels during PTT, significant elevation of hepatic Pck1 mRNA levels and diminution of L-Pk transcripts, as well as impaired glucose tolerance (Figures 1I, 1J, 1K, and S1I). Given the biochemical interaction between BAD and GK (Danial et al., 2003; Danial et al., 2008; Szlyk et al., 2013), these shared metabolic outcomes suggest their functional partnership may be relevant for the proper control of glycolysis and gluconeogenesis in the liver (see below).
We have previously reported that BAD’s capacity to activate GK in β-cells is triggered by S155 phosphorylation (aa enumeration based on the mouse sequence of BAD) (Danial et al., 2008). Importantly, S155 phosphorylation is sensitive to nutrient status in both β-cells (Danial et al., 2008) and in liver (Figure 2A). Specifically, BAD S155 phosphorylation is induced upon re-feeding. In addition, the phosphorylation of two priming serine residues upstream of the BAD BH3 domain, S112 and S136, is similarly increased upon re-feeding (Figure 2A). These observations suggest that BAD phosphorylation and metabolic activity may be integrated with nutritional states. Consistent with this idea, interference with BAD phosphorylation through a Bad S155A non-phosphorylatable knock-in allele was associated with fasting hyperglycemia and excess glucose release during PTT (Figure 2B).
Figure 2. The effect of BAD phosphorylation on gluconeogenesis.
(A) Immunoblot analysis and quantification of relative BAD phosphorylation on S155, S136 and S112 in liver samples derived from C57BL/6J mice fasted overnight and re-fed for 6 hr after overnight fasting.
(B) PTT in Bad +/+ and S155A knock-in mice (n=10–11).
(C) PTT in Bad +/+ and −/− mice following hepatic reconstitution with the indicated adenoviruses (n=16–24). Asterisks in (C) compare Bad −/−: BAD S155D vs. Bad −/−: GFP and the symbols # compare Bad −/−: BAD WT vs. Bad −/−: GFP.
(D) Glucose production in primary Bad −/− hepatocytes reconstituted with the indicated adenoviruses and treated with lactate/pyruvate (n=7–12).
Error bars, ± SEM. *p < 0.05; **p < 0.01, ***p < 0.001; n.s., non-significant.
See also Figure S2.
To interrogate the direct significance of BAD phosphorylation specifically in the liver, we carried out whole animal liver reconstitution assays in Bad −/− mice using adenoviruses expressing wild-type or defined phosphorylation mutants within the BAD BH3 domain. Hepatic reconstitution with wild-type BAD lowered the glucose values during PTT, indicating that exaggerated gluconeogenesis in Bad −/− is directly related to the loss of BAD function in the liver (Figure 2C). Given BAD’s dual capacity to engage GK and influence glucose metabolism on one hand, and to bind select BCL-2 protein (BCL-2, BCL-XL and BCL-w) and influence the sensitivity of cells to apoptosis on the other, a definitive role for BAD’s metabolic function in regulation of gluconeogenesis could not be inferred solely based on the phenotype of wild-type BAD in these assays. Therefore, we examined three separate BAD phosphorylation mutants that are competent or deficient in activating GK (Figure S2A). The phospho-mimic S155D mutation triggers BAD’s capacity to activate GK while blocking its interaction with BCL-2 protein partners (Danial et al., 2008). Hepatic reconstitution with this mutant was sufficient to restore GK activity and correct the PTT profile of Bad −/− mice (Figures 2C and S2B). The corresponding phospho-deficient variant, BAD S155A, which is compromised in its capacity to restore hepatic GK activity in Bad −/− mice (Figure S2B), did not correct the glucose values during PTT (Figure 2C). Although diminished in its ability to activate GK, BAD S155A retains its capacity to interact with BCL-2 family partners (Danial, 2008), and is therefore not a direct control for S155D GK-activating variant, which does not bind to BCL-2 family proteins (Figure S2A). For these reasons, we used a second phospho-deficient BAD variant that has diminished GK-activating capacity but cannot bind BCL-2 family partners (BAD L151A, S155A, and D156A triple mutant referred to as BAD AAA) (Figures S2A and S2B) (Danial et al., 2008). Parallel comparison of this mutant with BAD S155D allowed assessment of BAD’s capacity to engage and activate GK separate from its ability to bind BCL-2 family partners. Unlike BAD S155D, BAD AAA did not restore the PTT profile in Bad −/− mice (Figure 2C). Furthermore, in vitro genetic reconstitution studies using these variants were consistent with the above observations in that BAD S155D, but not BAD S155A or BAD AAA corrected gluconeogenesis in Bad −/− hepatocytes to levels comparable to GFP-expressing Bad +/+ controls (Figure 2D). Thus in vivo and in vitro interference with BAD S155 phosphorylation is associated with unrestrained gluconeogenesis and phenocopies the changes in hepatic metabolism observed in Bad −/− and Bad knockdown liver.
BAD’s effect on mitochondrial energy metabolism in hepatocytes
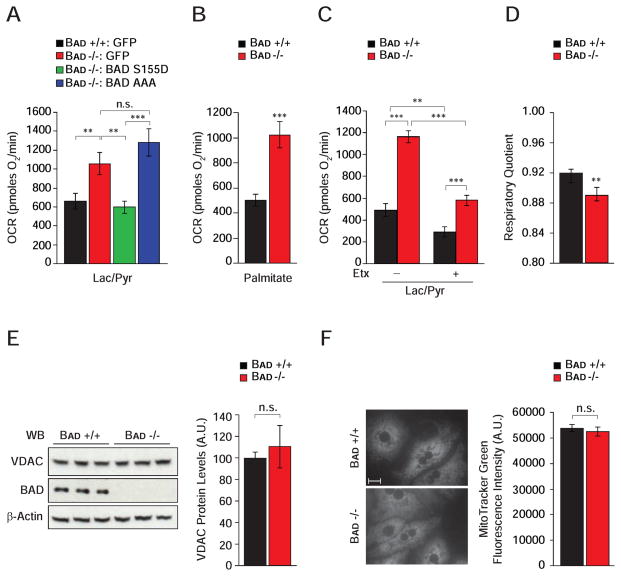
Mitochondria play a pivotal role in gluconeogenesis through provision of carbon substrates, reducing equivalents, and ATP (Burgess et al., 2007; Satapati et al., 2012). Metabolic flux analysis has also uncovered a tight connection between PCK1, mitochondrial bioenergetics and TCA cycle (Burgess et al., 2007; Hakimi et al., 2005; Satapati et al., 2012). To assess how Bad −/− hepatocytes derive the ATP required to support excess gluconeogenesis, we focused on mitochondrial energy metabolism. We reasoned that gluconeogenesis from exogenous lactate/pyruvate would create a state of energy demand driving ATP production by mitochondrial oxidative phosphorylation, which can be deduced from the fraction of mitochondrial oxygen consumption rate (OCR) that is sensitive to the mitochondrial ATP-synthase inhibitor oligomycin. In the presence of lactate/pyruvate, Bad −/− hepatocytes showed a significantly higher induction of mitochondrial ATP production (Figure 3A and Table S1), which was restored to wild-type values after genetic introduction of the BAD S155D variant but not the BAD AAA mutant (Figure 3A and Table S1).
Figure 3. BAD-dependent modulation of hepatic energy metabolism.
(A) Mitochondrial OCR in primary Bad +/+ and −/− hepatocytes transduced with the indicated adenoviruses and treated with lactate/pyruvate (n=4).
(B) Mitochondrial OCR in primary Bad +/+ and −/− hepatocytes treated with palmitate (n=10).
(C) Etomoxir (Etx) inhibition of OCR in primary hepatocytes treated with lactate/pyruvate (n=5).
(D) Respiratory quotient in Bad +/+ and −/− mice (n=8).
(E–F) Comparison of mitochondrial mass as examined by the protein levels of Voltage Dependent Anion Channel (VDAC) using western blot analysis (E) and by the fluorescence signal intensity of MitoTracker Green in Bad +/+ and −/− primary hepatocytes (F) (n=3). Scale bar in F, 10 μm.
Error bars, ± SEM. **p < 0.01, ***p < 0.001; n.s., non-significant.
Gluconeogenesis and mitochondrial FAO are interdependent. For example, fatty acids augment flux through pyruvate carboxylase leading to significant stimulation of OCR and gluconeogenesis in the presence of lactate/pyruvate (Gustafson et al., 2001; Pryor et al., 1987). Importantly, inhibition of carnitine palmitoyl transferase (CPT1), which is required for mitochondrial import of long-chain fatty acids, or interference with mitochondrial electron transport chain activity reverse these effects (Pryor et al., 1987), indicating the importance of mitochondria in energetic and substrate coupling between FAO and gluconeogenesis. This prompted examination whether increased gluconeogenesis in the absence of BAD is linked to elevated FAO. Indeed, mitochondrial OCR in response to palmitate was significantly higher in primary Bad −/− hepatocytes compared with controls (Figure 3B and Table S1). Similar results were also obtained in Bad knockdown hepatocytes (data not shown). As predicted, the rise in OCR normally seen upon treatment with lactate/pyruvate was inhibited by the mitochondrial FAO inhibitor etomoxir (Etx) in both Bad +/+ and −/− hepatocytes (Figure 3C). However, the etomoxir inhibitable portion of this respiratory rate was significantly larger in Bad −/− than control hepatocytes (Figure 3C and Table S1), which is in agreement with higher FAO in Bad −/− hepatocytes (Figure 3B). This is also consistent with lower respiratory quotient (RQ) in Bad −/− mice (Figure 3D), which reflects preferential utilization of fatty acids as energy source. Overall, these results suggest that loss of BAD is associated with an altered program of mitochondrial substrate and energy metabolism that matches excess gluconeogenesis. Notably, these alterations do not appear to be linked to changes in mitochondrial mass in the absence of BAD (Figures 3E and 3F).
The functional increase in FAO in the absence of BAD warranted examination of hepatic genes involved in fatty acid metabolism. Comparison of microarray expression profiles using gene set enrichment analysis (GSEA) identified the mitochondrial β-oxidation pathway as the top gene set enriched in fasted Bad −/− liver compared to Bad +/+ controls (Figure S3A). Quantitative real-time PCR provided independent evidence for augmented mRNA abundance of the FAO genes in Bad −/− liver (Figure S3B). Conversely, transcription of the lipogenic gene acetyl-CoA carboxylase (Acc) was markedly down-regulated in Bad −/− liver (Figure S3B). Examination of key transcriptional regulators of FAO also indicated significant induction of Pparα mRNA in Bad −/− liver (Figure S3B). Importantly, these transcriptional changes were also observed following acute hepatic knockdown of Bad (Figure S3C), and were further phenocopied by acute hepatic knockdown of Gk (Figure S3D) or liver-specific deletion of Gk, (Gklox/lox vs. Gklox/lox; Alb-cre, Figure S3E). These observations suggest a potential link between BAD- and GK-dependent metabolic signaling and hepatic gene expression.
Hepatic function of BAD is mediated by GK
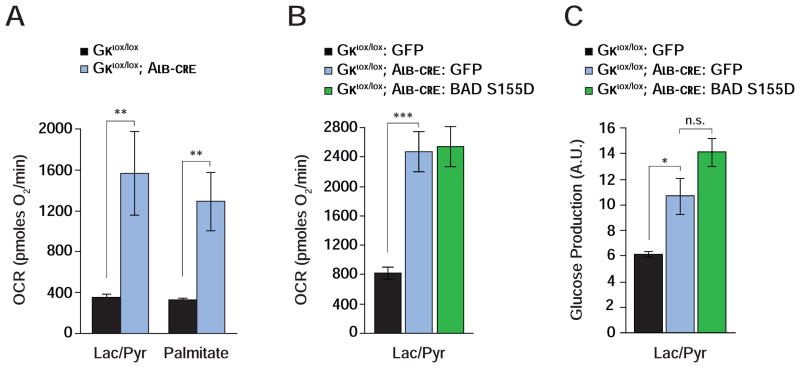
The differential effect of BAD S155D and AAA variants in genetic reconstitution assays suggests that BAD’s influence on glycolysis and gluconeogenesis is coordinated with its ability to activate GK. In addition, the common phenotype of exaggerated gluconeogenesis and FAO when either BAD or GK are acutely depleted in the liver suggests that the functional interaction of these two proteins is relevant for hepatic substrate metabolism. This predicts that loss of GK would phenocopy loss of BAD in mitochondrial energy metabolism and handling of gluconeogenic substrates; and that BAD’s effect on hepatic substrate utilization would be abrogated in the genetic background of Gk deficiency. We next tested each of these predictions. Primary Gk −/− hepatocytes display marked stimulation of mitochondrial OCR when cultured with lactate/pyruvate or palmitate (Gklox/lox vs. Gklox/lox; Alb-cre, Figures 4A and 4B; Table S1) analogous to Bad −/− hepatocytes. Gluconeogenesis was also significantly higher in Gk-null hepatocytes (Figure 4C). However, expression of BAD S155D in Gk −/− hepatocytes was ineffective in curtailing excess mitochondrial OCR and glucose production in the presence of lactate/pyruvate (Figures 4B and 4C). This contrasts with the capacity of BAD S155D to correct these parameters in Bad −/− hepatocytes (Figures 2D and 3A). These data provide genetic and mechanistic evidence consistent with GK as a downstream mediator of BAD’s effect on hepatic substrate and energy metabolism.
Figure 4. Hepatic function of BAD is GK-dependent.
(A) Mitochondrial OCR in GK-deficient hepatocytes treated with lactate/pyruvate or palmitate (n=8–10).
(B–C) Mitochondrial OCR (B) and glucose production (C) in GK-deficient hepatocytes transduced with the indicated adenoviruses (n=9).
Error bars, ± SEM. *p < 0.05; **p < 0.01, ***p < 0.001; n.s., non-significant.
See also Table S1.
Hepatic insulin resistance in Bad −/− mice
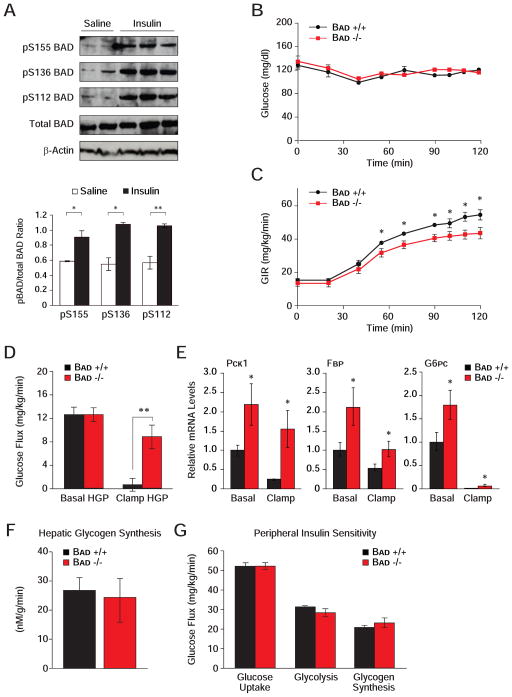
Insulin is a critical regulator of hepatic glucose metabolism through transcriptional and post-translational mechanisms, as well as modulation of substrate supply (Lin and Accili, 2011; Saltiel and Kahn, 2001). It stimulates transcription of several glycolytic genes, including Gk, and represses that of gluconeogenic genes Pck1, Fbp1 and G6p. In addition, insulin stimulates BAD phosphorylation in the liver (Figure 5A). Given the effect of BAD phosphorylation on GK activity and hepatic substrate utilization, these observations predict that BAD’s hepatic function may be subject to insulin regulation. To test the physiologic relevance of BAD in the context of insulin action, we performed euglycemic-hyperinsulinemic clamp analysis. While glucose was maintained at basal concentration (~6.7 mM) throughout the analysis (Figure 5B), Bad −/− mice displayed a small but statistically significant reduction in glucose infusion rates (GIR) (Figure 5C, 51.5 ± 2.5 vs. 43.4 ± 2.6 mg/kg/min, p < 0.05, Bad +/+ vs. Bad −/−) consistent with whole body insulin resistance. As reduced GIR can derive from impaired suppression of HGP by insulin and/or its diminished capacity to enhance glucose uptake in peripheral tissues, hepatic and peripheral insulin sensitivities were measured separately. Suppression of HGP by insulin in Bad −/− mice was markedly impaired consistent with hepatic insulin resistance (Figure 5D, 94.7 ± 14.7% vs. 32.1 ± 14.0%, p < 0.008, Bad +/+ vs. Bad −/−). This was further consistent with diminished capacity of insulin to fully inhibit the transcription of Pck1, Fbp1 and the catalytic subunit of G6p (G6pc) in Bad −/− liver (Figure 5E).
Figure 5. Hepatic insulin resistance in the absence of BAD.
A) Modulation of hepatic BAD phosphorylation by insulin in vivo. Immunoblot analysis and quantification of relative BAD phosphorylation on S155, S136 and S112 in liver samples derived from C57BL/6J mice fasted overnight and injected with saline or insulin.
(B–G) Euglycemic-hyperinsulinemic clamp analysis in Bad −/− and Bad +/+ mice (n=7–9), showing plasma glucose (B), glucose infusion rate (GIR) (C), hepatic glucose production (HGP) (D), relative mRNA abundance of hepatic gluconeogenic genes (E), hepatic glycogen synthesis rates (F), and peripheral insulin sensitivity (G).
Error bars, ± SEM. *p < 0.05; **p < 0.01, Bad−/− vs. Bad+/+ mice.
See also Figure S4.
To determine if hepatic insulin resistance in BAD-deficient mice extends to abnormalities in glucose storage, the net hepatic glycogen synthesis rates were also measured and found to be comparable in Bad +/+ and −/− mice (Figure 5F). These rates reflect the net balance of direct and indirect pathways of glycogen synthesis (Agius, 2008). While diminished hepatic glucose utilization in Bad −/− mice (Figure 1A) would predict lower glycogen synthesis via the direct pathway, increased gluconeogenesis in these animals may lead to a larger contribution from the indirect pathway. It is possible that a relative increase in the indirect pathway of glycogen synthesis may have offset any differences in the direct pathway, rendering total glycogen synthesis in Bad −/− mice comparable to controls. Notably, a prominent contribution of the gluconeogenic pathway to hepatic glycogen synthesis in glucokinase-deficient (MODY2) subjects has been reported (Velho et al., 1996).
Insulin resistance in Bad −/− mice is restricted to liver as insulin-mediated glucose uptake in skeletal muscle and white adipose tissues was not altered (Figures S4A and S4B). Whole body glucose uptake, glycolysis and glycogen synthesis did not reveal any differences in the two genotypes (Figure 5G). Other metabolic parameters such as fat and lean mass, energy expenditure, activity, as well as fasted serum levels of glucagon, FFA and triglycerides were comparable in both genotypes (Figures S4C–S4H). Overall, these results indicate that peripheral insulin sensitivity is preserved in Bad −/− mice and that diminished GIR in this genetic model can be chiefly attributed to impaired suppression of HGP.
Hepatic insulin signaling in the absence of BAD
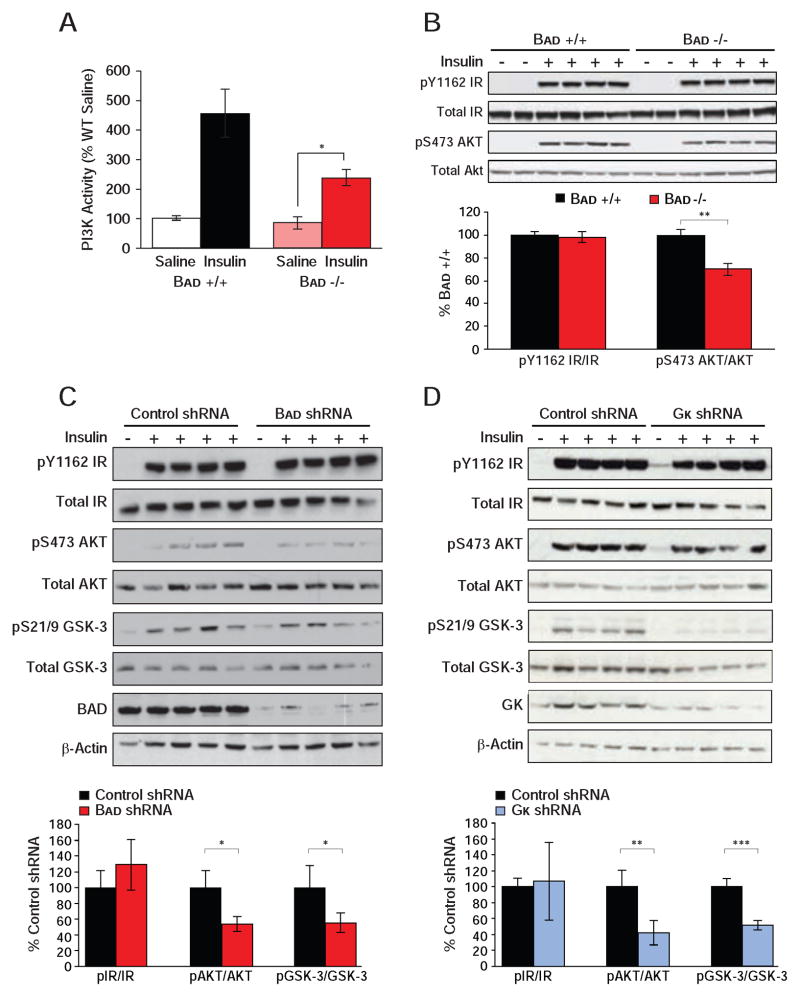
Impaired hepatic insulin action in Bad −/− mice warranted examination of insulin signaling. Insulin stimulation of PI3K activity was significantly diminished in Bad −/− liver but not in Bad −/− gastrocnemius muscle (Figures 6A and S5A). Lower hepatic PI3K activity in Bad −/− was consistent with reduced stimulation of AKT phosphorylation by insulin but could not be explained by changes in insulin receptor (IR) phosphorylation (Figure 6B). Importantly, insulin stimulation of AKT phosphorylation was not altered in muscle (Figure S5B), which is in agreement with selective loss of insulin action in liver but not in muscle (Figures 5D and S4A). Reduced AKT phosphorylation in response to insulin was further evident in primary Bad −/− hepatocytes treated with insulin (Figure S5C), indicating a cell autonomous effect of BAD deficiency on insulin signaling. Moreover, attenuated insulin signaling in Bad −/− mice was recapitulated in mice treated with Bad shRNA (Figure 6C). Reduced PI3K-AKT signaling without alterations in insulin receptor phosphorylation predicts inhibition at the level of IRS-1 through negative feedback loops (Copps and White, 2012). Examination of several IRS-1 inhibitory kinases revealed comparable hepatic JNK and ERK phosphorylation but diminished GSK-3 phosphorylation in Bad shRNA liver (Figure 6C and data not shown). Because phosphorylation inactivates GSK-3, these results indicate increased hepatic GSK-3 activity in BAD-depleted liver. However, GSK-3 phosphorylation is itself regulated by insulin similar to several other IRS-1 inhibitory kinases (Copps and White, 2012), and whether GSK-3 activity is the cause or consequence of reduced insulin signaling in these mice remains to be determined. Importantly, an analogous decline in hepatic insulin signaling was also observed in mice treated with Gk shRNA (Figure 6D). This is consistent with published reports that loss of GK or inhibition of its activity is associated with blunted suppression of HGP and hepatic insulin resistance (Barzilai et al., 1996; Clement et al., 1996; Rossetti et al., 1997). In aggregate, our findings indicate that, similar to hepatic depletion or inhibition of GK, loss of BAD and the attendant diminution of GK activity is associated with reduced hepatic insulin sensitivity and insulin signaling.
Figure 6. Altered insulin signaling upon hepatic manipulation of BAD and GK.
(A) IRS-1-associated PI3K activity in liver of Bad +/+ and −/− mice injected with saline or insulin after an overnight fast (n=10 per genotype).
(B) Phosphorylation of the insulin receptor (IR) and AKT in liver of Bad +/+ and −/− mice treated as in (A). Bar graphs quantitate relative insulin induction of IR and AKT phosphorylation (n=4).
(C–D) Insulin signaling in liver samples isolated from C57BL/6J mice following hepatic knockdown of Bad (C) and Gk (D). Bar graphs quantitate relative insulin induction of IR, AKT, and GSK-3 phosphorylation (n=4).
Error bars, ± SEM. *p < 0.05; **p < 0.01, ***p < 0.001.
See also Figure S5.
Hepatic GK and BAD are both regulated by insulin. Gk is a transcriptional target of SREBP1c downstream of the PI3K-AKT axis, and BAD phosphorylation, which stimulates GK activity (Figure S2B) (Szlyk et al., 2013), is induced by insulin. It is, therefore, possible that BAD may serve as an additional downstream mediator of insulin’s effect on GK, whereby insulin stimulates GK activity beyond induction of Gk transcription. Given insulin regulation of BAD and GK and reduced insulin signaling in Bad −/− mice, two potential scenarios may explain the diminished insulin suppression of HGP in these mice; diminished insulin stimulation of Gk expression or diminished capacity of insulin to enhance GK activity through BAD phosphorylation. To determine changes in Gk expression in this setting, we examined hepatic Srebp1c and Gk mRNA levels in Bad +/+ and −/− mice subjected to euglycemic-hyperinsulinemic clamp. Relative Srebp1c mRNA abundance was lower in Bad −/− liver (Figure S5D), which is consistent with reduced insulin stimulation of PI3K-AKT signaling in these mice. Surprisingly, however, Gk mRNA levels were not diminished in these samples (Figure S5E), suggesting potential SREBP1c-independent compensatory mechanisms that maintain Gk mRNA levels despite reduced insulin signaling in Bad −/− liver. Of note, several other transcription factors can directly activate the Gk promoter in the absence of SREBP1c (Bechmann et al., 2012; Kim et al., 2009; Oosterveer et al., 2012). Moreover, Gk mRNA levels can be regulated by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK2) independent of insulin and SREBP1c (Payne et al., 2005; Wu et al., 2004). Therefore, it is possible that one or more of these mechanisms may stabilize Gk expression in Bad −/− liver under these conditions. In addition, the potential contribution of residual insulin signaling in these mice cannot be ruled out. Regardless of the mechanisms that may stabilize Gk mRNA levels in Bad −/− mice, our results indicate that diminished insulin suppression of HGP in these animals cannot be explained by diminished Gk transcription. Considering insulin-induction of BAD phosphorylation on S155 and the direct stimulatory effect of the phospho-BAD BH3 domain on GK activity (Szlyk et al., 2013), our data are most consistent with the proposal that defective GK activity in Bad −/− liver, despite stabilized Gk mRNA levels, may curtail the full effect of insulin on proper modulation of HGP.
Metabolic benefits of BAD in models of diabetes and insulin resistance
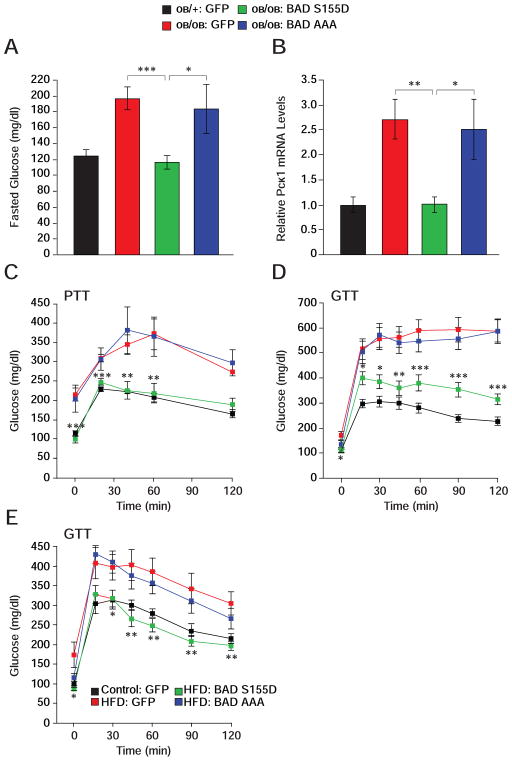
The capacity of BAD S155D to counterbalance exaggerated gluconeogenesis in Bad −/− liver warranted examination of its effect in models of diabetes and insulin resistance such as ob/ob mice. Hepatic BAD protein levels were comparable in ob/ob and control ob/+ mice despite elevated Bad mRNA levels in ob/ob animals, indicating that increased transcription of the Bad gene does not result in higher protein levels in this context (Figures S6A and S6B). Remarkably, however, BAD phosphorylation on S155 and S136 was significantly diminished in ob/ob liver (Figure S6C). These observations further motivated examination whether expression of a BAD S155D can alter hepatic metabolism in these animals. Hepatic delivery of BAD S155D, but not BAD AAA, significantly ameliorated fasting hyperglycemia in ob/ob mice (Figure 7A), which was accompanied by diminished fasting Pck1 mRNA abundance (Figure 7B). Importantly, glucose levels during PTT were markedly down-regulated in ob/ob mice reconstituted with BAD S155D adenoviruses so that they were comparable to ob/+ controls treated with GFP viruses (Figure 7C). BAD S155D expression further resulted in systemic metabolic benefits marked by improved glucose tolerance in ob/ob mice (Figure 7D).
Figure 7. Metabolic effects of BAD in ob/ob and HFD-treated mice.
(A–D) Fasting glucose levels (A), liver Pck1 mRNA abundance (B), pyruvate (C) and glucose
(D) tolerance tests following hepatic reconstitution of ob/ob mice with the indicated adenoviruses (n=10–14). Asterisks in (C–D) compare ob/ob mice treated with BAD S155D vs. GFP adenoviruses.
(E) GTT in C57BL/6J mice subjected to high-fat diet for 10 weeks prior to hepatic reconstitution with the indicated adenoviruses (n=9). Asterisks in (E) compare HFD mice treated with BAD S155D vs. GFP adenoviruses.
Error bars, ± SEM. *p < 0.05; **p < 0.01, ***p < 0.001.
See also Figure S6.
We next set out to determine whether the differential effects of BAD S155D and BAD AAA in ob/ob liver parallel their GK-activating capacity. Previous studies have reported an age-dependent progressive decline in hepatic Gk mRNA and GK activity in leptin resistant rodent models (Torres et al., 2009). Importantly, increased GK activity through chemical or genetic approaches improved glucose homeostasis in these models (Grimsby et al., 2003; Torres et al., 2009). Hepatic expression of BAD S155D or BAD AAA variants did not alter Gk mRNA levels in ob/ob mice (Figure S6D), but had distinct effect on GK activity. Specifically, BAD S155D increased hepatic GK activity whereas the BAD AAA variant was ineffective (Figure S6E). Thus, the differential effect of BAD phospho-mutants in hepatic reconstitution of ob/ob mice co-segregates with their effect on GK activity.
The ob/ob genetic model is a severe model of diabetes with a complex myriad of chronic pathophysiologic perturbations. We also tested the metabolic consequence of BAD in the high-fat diet (HFD) model. Acute hepatic delivery of BAD S155D to wild-type mice after 10 weeks of high-fat feeding effectively improved fasting hyperglycemia and glucose tolerance, while the BAD AAA variant was inert in this setting (Figure 7E). The GTT profiles of HFD mice treated with BAD S155D were comparable to normal chow (control)-fed mice treated with GFP viruses.
DISCUSSION
Our combined use of multiple genetic tools, including the Bad-null and phosphorylation knock-in mice, liver-specific Bad knockdown, as well as informative BAD mutants enabled biochemical and systemic evaluation of BAD’s significance in hepatic substrate metabolism. These studies show that hepatic loss of BAD or interference with its phosphorylation is linked to reduced glycolysis, enhanced FAO, and unrestrained gluconeogenesis. Moreover, BAD deficiency is associated with impaired insulin-mediated suppression of HGP and attenuated insulin signaling. Our collective observations suggest that exaggerated gluconeogenesis in the absence of BAD can be mapped to a combination of altered substrate and energy metabolism, as well as loss of hepatic insulin sensitivity.
BAD’s capacity to modulate hepatic glucose utilization and production is regulated by its phosphorylation, which is in turn sensitive to the fed/fasted nutritional states and insulin. When phosphorylated, BAD activates GK, promoting glycolysis and inhibiting FAO. Upon BAD dephosphorylation, glycolysis is diminished, FAO is dis-inhibited, and pyruvate is preferentially partitioned to gluconeogenesis. These observations are consistent with the possibility that BAD phosphorylation may help mitochondria distinguish the glycolytic versus gluconeogenic source of pyruvate and its metabolic fate. The idea that GK is an important mechanistic component of BAD modulation of hepatic metabolism is supported by two lines of investigation. First, the selective capacity of BAD BH3 variants to modulate hepatic metabolism in both primary hepatocytes and whole animal liver reconstitution assays co-segregates with their differential GK-activating property. Second, BAD’s ability to coordinately regulate hepatic energy metabolism and gluconeogenesis is abolished in the absence of GK, providing evidence in favor of an “epistatic” relationship between these partner proteins where GK is a downstream mediator of BAD’s metabolic effects.
The notion that the BAD-GK axis is relevant for proper regulation of glycolysis and gluconeogenesis is further consistent with several metabolic alterations that are phenocopied when each protein is depleted in the liver, including exaggerated gluconeogenesis and impaired glucose tolerance. Hepatic GK is subject to several regulatory mechanisms, including distinct binding interactions and sub-cellular localization (Matschinsky, 2009). BAD deficiency does not lead to reduced hepatic GK expression at mRNA or protein levels, but is associated with diminished GK activity. Given the multiple mechanisms that converge on hepatic GK regulation, loss of BAD is not expected to recapitulate the full phenotypic spectrum of GK loss-of-function in the liver. Within this context, the finding that several metabolic alterations are shared between mice with hepatic knockdown of Bad and animals with hepatic knockdown of Gk is remarkable.
Changes in hepatic metabolism following depletion of BAD or GK also include altered expression of several glycolytic and gluconeogenic genes, and a robust transcriptional activation of β-oxidation. This gives rise to the question as to how BAD- or GK- dependent changes in hepatic metabolism modify gene expression. Because these changes can be acutely triggered by the knockdown of either BAD or GK, it is likely that select alterations in glucose- or fatty acid-derived metabolites may modulate the activity of glucose- and fatty acid-sensing transcription factors such as ChREBP and nuclear receptors (Agius, 2013; Jump et al., 2013). Another possible scenario is alterations in chromatin- and histone-modifying enzymes that can be modulated by defined metabolites or cellular metabolic state (Kaelin and McKnight, 2013).
Our findings indicate that deregulated gluconeogenesis in the absence of BAD also extends to diminished insulin suppression of HGP. BAD phosphorylation, which can down-regulate gluconeogenesis through GK activation (Figures 2C, 2D, S2B, 4C), is normally induced by insulin. This observation together with the finding that insulin suppression of HGP is diminished in Bad −/− mice suggest that BAD may serve as a downstream effector of insulin in modulation of hepatic glucose production. Moreover, hepatic insulin resistance in the absence of BAD is accompanied by changes in insulin signaling. Hepatic nutrient and energy sensing pathways and insulin signaling are tightly integrated (Newgard, 2012; Samuel and Shulman, 2012), and the shared phenotype of reduced insulin signaling upon acute hepatic depletion of BAD or GK predicts a potential interplay between reduced glucose utilization and/or increased FAO and the components of the insulin signaling axis. Notably, precedent for a link between glucose metabolism, ROS, and NADH, and the regulation of PI3K-AKT-GSK-3 signaling exists in other tissues (Pelicano et al., 2006; Yeshao et al., 2005). The precise molecular link between BAD- and GK-dependent changes in substrate metabolism and alterations in insulin signaling awaits additional studies.
The observation that hepatic delivery of BAD S155D reinstates proper regulation of gluconeogenesis and allays fasting hyperglycemia in the ob/ob and HFD-treated mice provides a powerful endorsement for the physiologic relevance of BAD phospho-regulation. We have shown that the BAD S155D variant and hydrocarbon stapled BAD BH3 helices that bear the phospho-mimic S155D modification (BAD SAHB compounds) can directly trigger GK activity and stimulate insulin secretion in primary islets (Danial et al., 2008; Szlyk et al., 2013). The studies presented here suggest that, in addition to boosting β-cell function, BAD BH3 phospho-mimic strategies may improve hepatic metabolism. This is consistent with published reports that GK activation imparts benefits to both β-cells and hepatocytes. Hepatic GK activation using genetic approaches or pharmacologic tools such as small molecule allosteric GK activators (GKAs) leads to increased glucose utilization, decreased glucose production, amelioration of hyperglycemia, and resistance to HFD (Ferre et al., 1996; Grimsby et al., 2003; Torres et al., 2009). However, these benefits have to be carefully leveraged against potential risk of hypoglycemia and hyperlipidemia associated with chronic GK activation above physiologic levels (Meininger et al., 2011). With advances in the development of different classes of GKAs that may elicit distinct mechanisms of action and the possibility of combination therapy with other anti-diabetic agents, the benefits of GK activation may ultimately outweigh these drawbacks (Matschinsky, 2009). Within this context, it is important to note that the mechanism by which the phospho-BAD BH3 helix activates GK is distinct from allosteric GKAs (Szlyk et al., 2013), suggesting phospho-BAD BH3 mimetic compounds may define a separate class of GKAs. The findings reported here highlight the potential therapeutic utility of phospho-BAD BH3 mimetic strategies in diabetes and metabolic syndrome.
EXPERIMENTAL PROCEDURES
Mice
Bad −/− and S155A knock-in mice have been previously described (Danial et al., 2008). These mice have been bred into the C57BL/6J genetic background for at least 14 generations and validated by genome scanning to be 99.9% congenic with C57BL/6J. Conditional Gk mice (Gklox/lox) were a generous gift of Dr. Mark Magnuson (Vanderbilt University) (Postic et al., 1999). C57BL/6J alb-cre mice and ob/ob mice were purchased from Jackson Laboratory and Taconics, respectively. Mice received a standard chow or high-fat diet (55% fat energy, TD93075, Harlan Laboratories), and were housed in a barrier facility with 12 hr light and dark cycles. Unless otherwise indicated, male mice at 10–12 weeks of age were used. All animal procedures were approved by the Institutional Animal Care and Use Committee of Dana-Farber Cancer Institute and Yale University School of Medicine.
Metabolic studies
PTT, GTT, basal metabolic characterization, and euglycemic-hyperinsulinemic clamps were performed as previously described (Choi et al., 2007; Danial et al., 2008; Lin et al., 2004). Adenoviral transduction was carried out as described in Supplemental Experimental Procedures.
Mitochondrial Respirometry
OCR was measured in intact hepatocytes in real time using the XF24 extracellular flux analyzer and the XF24 v1.5.0.69 software (Seahorse Biosciences). Cells were seeded on collagen-coated XF24 V7 plates at 4x104 cells/well, allowed to attach for at least 2 hr, rinsed once and kept in 600 μl of sodium bicarbonate-free M199 medium supplemented with 1% BSA, 1 μM dexamethasone and 12 μM forskolin. After baseline measurements, the following order of additions were delivered through the instrument’s individual injection ports; substrates (lactate/pyruvate or palmitate), ADP, and oligomycin. L-lactate (Sigma) and sodium pyruvate (Cellgro, Mediatech) were injected at 10 and 1 mM, respectively. Sodium palmitate (Sigma) was injected at 200 μM together with 10 mM L-malate (Sigma). When palmitate was tested, 0.5 mM carnitine (Sigma) was included in the incubation/equilibration medium. Where indicated, etomoxir (Sigma) was included in incubation/equilibration medium at 50 μM final concentration. After addition of the substrates, 5 mM ADP (Sigma) was injected to drive maximum respiration. Lastly, 1 μM oligomycin (Calbiochem) was injected to derive the portion of OCR used for ATP synthesis. OCR values in Figures 3 and 4 represent the difference between the maximal rate in the presence of ADP and the rate after addition of oligomycin, thus indicating ATP production by mitochondrial oxidative phosphorylation. Additional experimental details and a summary table of OCR values in the presence and absence of ADP for all substrates and genotypes tested are provided in Table S1 and Supplemental Experimental Procedures.
Statistical Analysis
Data are presented as mean ± SEM of the indicated number of independent experiments or mice per genotype. Statistical significance among the groups was tested with unpaired or paired Student’s t-test and analysis of variance (ANOVA) when appropriate. Differences were considered significant at p < 0.05.
Supplemental Experimental Procedures include analysis of BAD phosphorylation, GK activity, mitochondrial mass, gene expression, and insulin signaling.
Supplementary Material
HIGHLIGHTS.
BAD modulates hepatic glucose output by coordinating glycolysis and gluconeogenesis
BAD is required for suppression of hepatic glucose production by insulin
The effect of BAD on glucose utilization and production is mediated by glucokinase
Hepatic expression of BAD improves glycemia in models of diabetes and obesity
Acknowledgments
We thank J. Quijada for technical assistance and animal husbandry, J. Lemasters for advice on OCR studies, B. Lowell, P. Puigserver, members of the Spiegelman laboratory, L. Agius and F. Matschinsky for helpful discussions. A.G.C. and L.G.H were supported by postdoctoral fellowships from the Ministerio de Educación y Ciencia (MEC, Spain). M.A.O. was supported by a postdoctoral fellowship from the Juvenile Diabetes Research Foundation. N.N.D. is a recipient of the Burroughs Wellcome Fund Career Award in Biomedical Sciences. This work was supported by the US National Institute of Health grants K01CA10659 (N.N.D.), R01DK078081 (N.N.D.), R01 DK-40936 (G.I.S.), U24 DK-059635 (G.I.S.), R01DK083567 (Y.B.K.), and the Korea Healthcare technology R&D Project A102060, Ministry for Health, Welfare & Family Affairs, Republic of Korea (C.S.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem J. 2008;414:1–18. doi: 10.1042/BJ20080595. [DOI] [PubMed] [Google Scholar]
- Agius L. High-carbohydrate diets induce hepatic insulin resistance to protect the liver from substrate overload. Biochemical pharmacology. 2013;85:306–312. doi: 10.1016/j.bcp.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Hawkins M, Angelov I, Hu M, Rossetti L. Glucosamine-induced inhibition of liver glucokinase impairs the ability of hyperglycemia to suppress endogenous glucose production. Diabetes. 1996;45:1329–1335. doi: 10.2337/diab.45.10.1329. [DOI] [PubMed] [Google Scholar]
- Bechmann LP, Gastaldelli A, Vetter D, Patman GL, Pascoe L, Hannivoort RA, Lee UE, Fiel I, Munoz U, Ciociaro D, Lee YM, Buzzigoli E, Miele L, Hui KY, Bugianesi E, Burt AD, Day CP, Mari A, Agius L, Walker M, Friedman SL, Reeves HL. Glucokinase links Kruppel-like factor 6 to the regulation of hepatic insulin sensitivity in nonalcoholic fatty liver disease. Hepatology. 2012;55:1083–1093. doi: 10.1002/hep.24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, Browning JD, Magnuson MA. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 2007;5:313–320. doi: 10.1016/j.cmet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, Zhang D, Cline GW, Wakil SJ, Shulman GI. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A. 2007;104:16480–16485. doi: 10.1073/pnas.0706794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement K, Pueyo ME, Vaxillaire M, Rakotoambinina B, Thuillier F, Passa P, Froguel P, Robert JJ, Velho G. Assessment of insulin sensitivity in glucokinase-deficient subjects. Diabetologia. 1996;39:82–90. doi: 10.1007/BF00400417. [DOI] [PubMed] [Google Scholar]
- Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournarie F, Azzout-Marniche D, Foretz M, Guichard C, Ferre P, Foufelle F. The inhibitory effect of glucose on phosphoenolpyruvate carboxykinase gene expression in cultured hepatocytes is transcriptional and requires glucose metabolism. FEBS Lett. 1999;460:527–532. doi: 10.1016/s0014-5793(99)01407-6. [DOI] [PubMed] [Google Scholar]
- Danial NN. BAD: undertaker by night, candyman by day. Oncogene. 2008;27(Suppl 1):S53–70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, Gygi SP, Korsmeyer SJ. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- Danial NN, Walensky LD, Zhang CY, Choi CS, Fisher JK, Molina AJ, Datta SR, Pitter KL, Bird GH, Wikstrom JD, Deeney JT, Robertson K, Morash J, Kulkarni A, Neschen S, Kim S, Greenberg ME, Corkey BE, Shirihai OS, Shulman GI, Lowell BB, Korsmeyer SJ. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med. 2008;14:144–153. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre T, Pujol A, Riu E, Bosch F, Valera A. Correction of diabetic alterations by glucokinase. Proc Natl Acad Sci U S A. 1996;93:7225–7230. doi: 10.1073/pnas.93.14.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsby J, Sarabu R, Corbett WL, Haynes NE, Bizzarro FT, Coffey JW, Guertin KR, Hilliard DW, Kester RF, Mahaney PE, Marcus L, Qi L, Spence CL, Tengi J, Magnuson MA, Chu CA, Dvorozniak MT, Matschinsky FM, Grippo JF. Allosteric activators of glucokinase: potential role in diabetes therapy. Science. 2003;301:370–373. doi: 10.1126/science.1084073. [DOI] [PubMed] [Google Scholar]
- Gustafson LA, Neeft M, Reijngoud DJ, Kuipers F, Sauerwein HP, Romijn JA, Herling AW, Burger HJ, Meijer AJ. Fatty acid and amino acid modulation of glucose cycling in isolated rat hepatocytes. Biochem J. 2001;358:665–671. doi: 10.1042/0264-6021:3580665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi P, Johnson MT, Yang J, Lepage DF, Conlon RA, Kalhan SC, Reshef L, Tilghman SM, Hanson RW. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutr Metab (Lond) 2005;2:33. doi: 10.1186/1743-7075-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump DB, Tripathy S, Depner CM. Fatty acid-regulated transcription factors in the liver. Annu Rev Nutr. 2013;33:249–269. doi: 10.1146/annurev-nutr-071812-161139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG, Jr, McKnight SL. Influence of metabolism on epigenetics and disease. Cell. 2013;153:56–69. doi: 10.1016/j.cell.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Kim H, Park JM, Im SS, Bae JS, Kim MY, Yoon HG, Cha JY, Kim KS, Ahn YH. Interrelationship between liver X receptor alpha, sterol regulatory element-binding protein-1c, peroxisome proliferator-activated receptor gamma, and small heterodimer partner in the transcriptional regulation of glucokinase gene expression in liver. J Biol Chem. 2009;284:15071–15083. doi: 10.1074/jbc.M109.006742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Magnuson MA, She P, Shiota M. Gene-altered mice and metabolic flux control. J Biol Chem. 2003;278:32485–32488. doi: 10.1074/jbc.R300020200. [DOI] [PubMed] [Google Scholar]
- Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov. 2009;8:399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- Meininger GE, Scott R, Alba M, Shentu Y, Luo E, Amin H, Davies MJ, Kaufman KD, Goldstein BJ. Effects of MK-0941, a novel glucokinase activator, on glycemic control in insulin-treated patients with type 2 diabetes. Diabetes Care. 2011;34:2560–2566. doi: 10.2337/dc11-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterveer MH, Mataki C, Yamamoto H, Harach T, Moullan N, van Dijk TH, Ayuso E, Bosch F, Postic C, Groen AK, Auwerx J, Schoonjans K. LRH-1-dependent glucose sensing determines intermediary metabolism in liver. J Clin Invest. 2012;122:2817–2826. doi: 10.1172/JCI62368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne VA, Arden C, Wu C, Lange AJ, Agius L. Dual role of phosphofructokinase-2/fructose bisphosphatase-2 in regulating the compartmentation and expression of glucokinase in hepatocytes. Diabetes. 2005;54:1949–1957. doi: 10.2337/diabetes.54.7.1949. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS, Hu Y, Ramdas L, Hu L, Keating MJ, Zhang W, Plunkett W, Huang P. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J Cell Biol. 2006;175:913–923. doi: 10.1083/jcb.200512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- Pryor HJ, Smyth JE, Quinlan PT, Halestrap AP. Evidence that the flux control coefficient of the respiratory chain is high during gluconeogenesis from lactate in hepatocytes from starved rats. Implications for the hormonal control of gluconeogenesis and action of hypoglycaemic agents. Biochem J. 1987;247:449–457. doi: 10.1042/bj2470449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza RA. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes. 2010;59:2697–2707. doi: 10.2337/db10-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L, Chen W, Hu M, Hawkins M, Barzilai N, Efrat S. Abnormal regulation of HGP by hyperglycemia in mice with a disrupted glucokinase allele. Am J Physiol. 1997;273:E743–750. doi: 10.1152/ajpendo.1997.273.4.E743. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science. 1991;254:573–576. doi: 10.1126/science.1948033. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satapati S, Sunny NE, Kucejova B, Fu X, He TT, Mendez-Lucas A, Shelton JM, Perales JC, Browning JD, Burgess SC. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res. 2012;53:1080–1092. doi: 10.1194/jlr.M023382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DK, O’Doherty RM, Stafford JM, Newgard CB, Granner DK. The repression of hormone-activated PEPCK gene expression by glucose is insulin-independent but requires glucose metabolism. J Biol Chem. 1998;273:24145–24151. doi: 10.1074/jbc.273.37.24145. [DOI] [PubMed] [Google Scholar]
- Szlyk B, Braun CR, Ljubicic S, Patton E, Bird GH, Osundiji MA, Matschinsky FM, Walensky LD, Danial NN. A phospho-BAD BH3 helix activates glucokinase by a mechanism distinct from that of allosteric activators. Nat Struct Mol Biol. 2013 doi: 10.1038/nsmb.2717. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres TP, Catlin RL, Chan R, Fujimoto Y, Sasaki N, Printz RL, Newgard CB, Shiota M. Restoration of hepatic glucokinase expression corrects hepatic glucose flux and normalizes plasma glucose in zucker diabetic fatty rats. Diabetes. 2009;58:78–86. doi: 10.2337/db08-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velho G, Petersen KF, Perseghin G, Hwang JH, Rothman DL, Pueyo ME, Cline GW, Froguel P, Shulman GI. Impaired hepatic glycogen synthesis in glucokinase-deficient (MODY-2) subjects. J Clin Invest. 1996;98:1755–1761. doi: 10.1172/JCI118974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Okar DA, Stoeckman AK, Peng LJ, Herrera AH, Herrera JE, Towle HC, Lange AJ. A potential role for fructose-2,6-bisphosphate in the stimulation of hepatic glucokinase gene expression. Endocrinology. 2004;145:650–658. doi: 10.1210/en.2003-1290. [DOI] [PubMed] [Google Scholar]
- Yamada K, Noguchi T. Nutrient and hormonal regulation of pyruvate kinase gene expression. Biochem J. 1999;337 ( Pt 1):1–11. [PMC free article] [PubMed] [Google Scholar]
- Yeshao W, Gu J, Peng X, Nairn AC, Nadler JL. Elevated glucose activates protein synthesis in cultured cardiac myocytes. Metabolism. 2005;54:1453–1460. doi: 10.1016/j.metabol.2005.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.