Abstract
Objective
In the current era, giant paraesophageal hernia repair by experienced minimally-invasive surgeons has excellent perioperative outcomes when performed electively. Nonelective repair, however, is associated with significantly greater morbidity and mortality, even when performed laparoscopically. We hypothesized that clinical prediction tools using pretreatment variables could be developed that would predict patient-specific risk of postoperative morbidity and mortality.
Methods
We assessed 980 patients who underwent giant paraesophageal hernia repair (1997-2010; 80% elective; 97% laparoscopic). The association between clinical predictor covariates, including demographics, comorbidity and urgency of operation, and risk for in-hospital or 30-day mortality and major morbidity was assessed. Using forward, stepwise logistic regression, clinical prediction models for mortality and major morbidity were developed.
Results
Urgency of operation was a significant predictor of mortality (elective 1.1% [9/778] versus nonelective 8% [16/199]; p<0.001) and major morbidity (elective 18% [143/781] versus nonelective 41% [81/199]; p<0.001). The most common adverse outcomes were pulmonary complications (n=199; 20%). A 4-covariate prediction model consisting of age 80 or greater, urgency of operation and two Charlson comorbidity index variables (congestive heart failure and pulmonary disease) provided discriminatory accuracy for postoperative mortality of 88% while a 5-covariate model (sex, age by decade, urgency of operation, congestive heart failure and pulmonary disease) for major postoperative morbidity was 68% predictive.
Conclusions
Predictive models using pretreatment patient characteristics can accurately predict mortality and major morbidity after giant paraesophageal hernia repair. After prospective validation, these models could provide patient-specific risk prediction, tailored for individual patient characteristics, and contribute to decision-making regarding surgical intervention.
Background
Giant paraesophageal hernias (GPEH) can be asymptomatic, cause chronic low-grade symptoms, or present acutely. Chronic GPEH-related symptoms, such as heartburn, chest discomfort and dyspnea, are clear indications for surgical repair, which can be approached laparoscopically with acceptably low rates of mortality and morbidity when performed electively.(1-4) Quality of life after laparoscopic repair is excellent, with relief of heartburn, dysphagia, regurgitation, and dyspnea symptoms in the majority of patients. Preoperative pulmonary function abnormalities and anemia are also improved after GPEH repair.(5-9) The improved perioperative mortality and morbidity associated with elective laparoscopic GPEH repair has prompted ongoing debate regarding the appropriate timing for repair and the safety of watchful waiting.(10-12) Proponents of watchful waiting cite evidence that the historical risks, including high mortality and morbidity associated with gastric volvulus and strangulation, are overestimated.(13) However, multiple retrospective reports have shown that mortality and major morbidity associated with nonelective GPEH repair was significantly higher than after elective GPEH repair.(14-15) Clearly, the ability to weigh potential risks and benefits of operative intervention would be extremely useful in guiding treatment decisions.
Clinical prediction rules allow clinicians to determine the probability of an outcome using existing clinical covariates, either for their own decision making or in counseling patients.(16)For example, the Pneumonia Severity Index, developed to determine allocation of care in the treatment of community-acquired pneumonia, uses clinical variables obtained at emergency room presentation to assign an index risk score.(17) The patient's score guides clinical decisions regarding hospital admission and oral versus intravenous antibiotics. Similar clinical prediction rules to assign risk categories for mortality and major morbidity after GPEH repair could inform provider decisions regarding surgical intervention.
We hypothesized that clinical, risk-stratification tools based on known preoperative covariates could provide patient-specific perioperative mortality and major morbidity estimates in the preoperative setting. The aim of this study was to develop easy-to-use clinical prediction rules, incorporating readily available preoperative covariates that have discriminatory accuracy for predicting patient risk for perioperative mortality and major morbidity after laparoscopic repair of GPEH.
Methods
Patient Selection
Adult patients (n=980) who underwent primary transabdominal (laparoscopic or open) repair of GPEH (January 1, 1997 to August 31, 2010) were retrospectively identified from a prospectively maintained database. GPEH was defined as more than 30% gastric herniation into the posterior mediastinum.(18) Patients undergoing elective (n=781; 80%) and nonelective surgery were included. Nonelective surgery was defined as urgent (n=173; patient requiring admission for GPEH symptom management and repaired during the same admission) or emergent (n=26; immediate operation in patient with acute GPEH-related complications). Patients with prior antireflux surgery or GPEH repair were excluded. This study was approved by our Institutional Review Board.
Operative Approach
A laparoscopic approach was planned in 951 patients (97%). Our approach to laparoscopic GPEH repair has been previously described.(1, 19) Hernia reduction, extensive esophageal mobilization, and crural reapproximation were performed in all patients. Definitive GPEH repair, with an antireflux procedure to minimize postoperative reflux, was performed in 888 patients (91%). Fundoplication was partial (Toupet or Dor) in 206 patients and circumferential (Nissen) in the remaining 682 patients. Of the 888 patients treated with fundoplication, a Collis gastroplasty for esophageal lengthening was added in 465 (52%) patients. Mesh repair was performed in 116 patients (12%). Patients who received GPEH repair without fundoplication were also included (n=92 [9%]; e.g. gastropexy, Roux-en-Y near-esophagojejunostomy and gastroesophageal resection). Type of fundoplication and the need for mesh cruroplasty or esophageal lengthening were determined intraoperatively. GPEH repairs were performed by 19 surgeons over the time period of study with 498 (51%) performed by the senior surgeon (JDL).
Data Collection and Outcome Measures
Identification of Predictor Covariates
Predictor variables were defined as patient demographics, symptom complaints, covariates defined by the Charlson comorbidity index (CCI),(20) urgency of operation, and laboratory and radiographic findings that were known when the decision for surgery was made. Covariates in the CCI that were present in less than 3% of patients were grouped together as one variable (rare CCI conditions). These included diabetes mellitus with organ damage, peripheral vascular disease, renal disease, liver disease, hemiplegia or paraplegia, lymphoma, leukemia, metastatic cancer, and acquired immunodeficiency syndrome.
Definition of Outcome Variables
Postoperative adverse outcomes, including hospital or 30-day mortality and major morbidity were assessed using the Society of Thoracic Surgeons (STS) definitions for postoperative complications.(21) A binary outcome for mortality was defined as death during the initial hospital stay or within 30-days after surgery, whichever was longer. Similarly, a binary outcome measure for major morbidity was defined as at least one major adverse event during the initial hospital stay or within 30-days after surgery, whichever was longer and included pneumonia, reintubation, tracheostomy, pulmonary embolism, myocardial infarction, congestive heart failure, acute renal failure, cerebral vascular accident, septic shock or bacteremia, postoperative gastric or esophageal leak, perioperative hernia recurrence, and readmission or reoperation within 30-days. Mortality data was missing for 3 patients who survived to discharge in less than 30-days but were subsequently lost to follow-up. Therefore, a total of 977 patients were assessed for predictors of mortality and 980 patients for predictors of major morbidity.
Statistical Analysis and Development of Clinical Prediction Rules
Descriptive statistics were summarized with frequencies and percentages for categorical variables, and median with interquartile range (IQR) for continuous variables. Fisher's exact test and Student's t-test were used to compare differences between groups. Predictive models for mortality and major morbidity were then derived using forward, stepwise logistic regression. The performance of each predictive model was then estimated using bootstrap leave-one-out cross-validation analysis. Statistical analysis was performed using IBM SPSS 19 (IBM® Corp., Armonk, NY) and STATA SE 11.0 (StataCorp LP®, College Station, TX) software.
Because the number of patients suffering postoperative mortality was small (n=25) and, thus, limited the number of potential predictors that could be assessed, an a priori ‘p-value for inclusion’ of 0.05 was chosen to indicate a statistically significant change in the model at each step in the regression. In addition, because urgency of operation is well-established as a clinically important predictor of adverse outcome after GPEH repair, we began regression modeling for mortality by forcing the urgency variable into the model as the first covariate and then began forward, step-wise regression from that point. At each step, one additional variable was assessed using logistic regression; if the addition of that variable produced a significant change (p=0.05) in the predictive accuracy of the model, that variable was included and the next step performed by re-running the logistic regression with the next variable. This process continued until no more variables could be added and, at that point, the model was considered final. Variable inclusion order depended on the variable that caused the most significant model change, i.e. the smallest p-value for that step.
An identical process was performed for the major morbidity prediction model; however, because the number of patients experiencing major morbidity was large (n=224, an a priori p-value of 0.15 was selected as the ‘p-value for inclusion’ cut-off. This number of events allowed us to assess up to 22 variables in the model, enabling a larger p-value for inclusion and reducing the chance that important predictors would be overlooked.
Out of 980 patients, 26 (2.7%) were excluded from the regression analyses for mortality and 23 (2.3%) from the regression analyses for morbidity due to one or more missing predictor variables in any of the potential predictors considered in the model. We also excluded peptic ulcer disease, a CCI variable, from the mortality model due to inconsistency in the documentation of peptic ulcer disease in the medical record.
To determine the discriminatory accuracy of the predictive model, the area under the receiver operating characteristic (ROC) curve (AUC) was determined for each outcome model. Risk scores for mortality and major morbidity were then calculated. Using the same method developed to assign risk score in the CCI,(20) a risk score for each covariate in the model was determined by assessing the odds ratio and assigning a whole number points value. As was done in the original description of the CCI, for odds ratios ≥1.2 and <1.5, 1 point was assigned; for odds ratios ≥1.5 and <2.5, 2 points were assigned; for odds ratios ≥2.5 and <3.5, 3 points were assigned and so on. Categories were then created based on the risk score with 3 groups for the mortality risk model and 4 groups for the major morbidity risk model. The rate of mortality and major morbidity for each group was determined.
Results
The majority of patients were female (n=733; 75%) with a median age of 71 years (IQR 62, 78). The median body mass index was 29 kg/m2 (IQR 26, 33). At least 50% of the stomach was herniated into the mediastinum in 82% of patients and 24% had completely intrathoracic stomach. (Table 1) At least one preoperative CCI comorbidity was present in 60% of patients (n=592). The most common comorbid condition was pulmonary disease. The most common preoperative symptoms were respiratory problems (dyspnea, recurrent pneumonia or aspiration), chest or abdominal pain, heartburn and regurgitation. (Table 1)
Table 1. Association of patient demographics, comorbid diseases and symptoms with major adverse outcome and mortality after GPEH repair.
| Major Adverse Outcome | 30-day Mortality | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Patient Demographics: (n, [%]) | Overall n=980 |
No n=756 |
Yes n=224 |
p-value | Alive n=952 |
Dead n=25 |
p-value | |
| Sex | Female | 733 (75) | 574 (78) | 159 (22) | 0.137 | 713 (78) | 18 (2) | 0.815 |
| Male | 247 (25) | 182 (74) | 65 (26) | 239 (97) | 7 (3) | |||
|
| ||||||||
| Age group at operation | <50 | 60 (6) | 53 (88) | 7 (12) | <0.001 | 59 (98.3) | 1 (1.7) | <0.001 |
| 50-59 | 148 (15) | 124 (84) | 24 (16) | 147 (100) | 0 (0) | |||
| 60-69 | 261 (27) | 212 (81) | 49 (19) | 260 (99.6) | 1 (0.4) | |||
| 70-70 | 319 (32) | 245 (77) | 74 (23) | 315 (98.7) | 4 (1.3) | |||
| 80+ | 192 (20) | 122 (64) | 70 (36) | 171 (90) | 19 (10) | |||
|
| ||||||||
| Body Mass Index by WHO classificationa | Underweight | 16 (2) | 10 (63) | 6 (37) | 0.042 | 14 (87.5) | 2 (12.5) | 0.001 |
| Ideal | 185 (19) | 131 (71) | 54 (29) | 175 (94.6) | 10 (5.4) | |||
| Overweight | 356 (37) | 281 (79) | 75 (21) | 345 (97.7) | 8 (2.3) | |||
| Obese | 258 (27) | 210 (81) | 48 (19) | 257 (99.6) | 1 (0.4) | |||
| Severely obese | 149 (15) | 119 (80) | 30 (20) | 148 (99.3) | 1 (0.7) | |||
|
| ||||||||
| Preoperative Hernia Size (%)b | 30-49% | 174 (18) | 146 (84) | 28 (16) | 0.009 | 169 (97.1) | 5 (2.9) | 0.171 |
| 50-74% | 354 (36) | 276 (78) | 78 (22) | 345 (97.7) | 8 (2.3) | |||
| 75-99% | 212 (22) | 166 (78) | 46 (22) | 210 (99.1) | 2 (0.9) | |||
| Intrathoracic Stomach | 240 (24) | 168 (70) | 72 (30) | 228 (95.8) | 10 (4.2) | |||
|
| ||||||||
| History of Pretreatment Charlson-defined patient comorbidity | ||||||||
|
| ||||||||
| Myocardial infarction or revascularization | No | 833 (85) | 653 (78) | 180 (22) | 0.033 | 812 (97.6) | 20 (2.4) | 0.402 |
| Yes | 147 (15) | 103 (70) | 44 (30) | 140 (96.5) | 5 (3.5) | |||
|
| ||||||||
| Congestive heart failure | No | 941 (96) | 741 (79) | 200 (21) | <0.001 | 918 (97.9) | 20 (2.1) | 0.002 |
| Yes | 39 (4) | 15 (38 | 24 (62) | 34 (87.2) | 5 (12.8) | |||
|
| ||||||||
| Cerebral vascular accident or transient attack | No | 904 (92) | 708 (78) | 196 (22) | 0.004 | 882 (97.9) | 19 (2.1) | 0.010 |
| Yes | 76 (8) | 48 (63) | 28 (37) | 70 (92.2) | 6 (7.9) | |||
|
| ||||||||
| Dementia | No | 937 (96) | 732 (78) | 205 (22) | 0.001 | 917 (98.2) | 17 (1.8) | <0.001 |
| Yes | 43 (4) | 24 (56) | 19 (44) | 35 (81.4) | 8 (18.6) | |||
|
| ||||||||
| Pulmonary disease | No | 690 (70) | 548 (79) | 142 (21) | 0.010 | 675 (98.3) | 12 (1.8) | 0.024 |
| Yes | 290 (30) | 208 (72) | 82 (28) | 277 (95.5) | 13 (4.5) | |||
|
| ||||||||
| Connective Tissue Disorder | No | 942 (96) | 728 (77) | 214 (23) | 0.560 | 916 (97.6) | 23 (2.5) | 0.253 |
| Yes | 38 (4) | 28 (74) | 10 (26) | 36 (94.7) | 2 (2.3) | |||
|
| ||||||||
| Peptic Ulcer Disease | No | 794 (81) | 617 (78) | 177 (22) | 0.384 | 777 (98.2) | 14 (1.8) | 0.003 |
| Yes | 186 (19) | 139 (18) | 47 (21) | 175 (94.1) | 11 (5.9) | |||
|
| ||||||||
| Diabetes requiring medical therapy | No | 909 (93) | 705 (78) | 204 (22) | 0.304 | 884 (97.6) | 22 (2.4) | 0.419 |
| Yes | 71 (7) | 51 (72) | 20 (28) | 68 (95.8) | 3 (4.2) | |||
|
| ||||||||
| Malignancy in the past 5 years | No | 924 (94) | 716 (77) | 208 (23) | 0.325 | 900 (97.7) | 21 (2.3) | 0.050 |
| Yes | 56 (6) | 40 (71) | 16 (29) | 52 (92.9) | 4 (7.1) | |||
|
| ||||||||
| Rare CCI variablesc | No | 939 (96) | 730 (78) | 209 (22) | 0.038 | 914 (97.7) | 22 (2.4) | 0.083 |
| Yes | 41 (4) | 26 (63)) | 15 (37) | 38 (92.7) | 3 (7.3) | |||
|
| ||||||||
| Preoperative Symptoms | ||||||||
|
| ||||||||
| Respiratory Symptoms | No | 232 (34) | 183 (79) | 49 (21) | 0.443 | 223 (96.1) | 9 (3.9) | 0.341 |
| Yes | 450 (66) | 342 (76) | 108 (24) | 436 (97.5) | 11 (2.5) | |||
|
| ||||||||
| Chest and/or Abdominal pain | No | 273 (32) | 221 (81) | 52 (19) | 0.081 | 266 (97.8) | 6 (2.2) | 1.000 |
| Yes | 589 (68) | 444 (75) | 145 (25) | 574 (97.8) | 13 (2.2) | |||
|
| ||||||||
| Heartburn | No | 335 (35) | 233 (70) | 102 (30) | <0.001 | 323 (97) | 10 (3) | 0.365 |
| Yes | 626 (65) | 513 (82) | 113 (18) | 613 (98.1) | 12 (1.9) | |||
|
| ||||||||
| Dysphagia | No | 533 (55) | 406 (76)) | 127 (24) | 0.353 | 519 (97.6) | 13 (2.4) | 1.000 |
| Yes | 431 (45) | 340 (79) | 91 (21) | 418 (97.4) | 11 (2.6) | |||
|
| ||||||||
| Regurgitation | No | 377 (39) | 286 (76) | 91 (24) | 0.345 | 370 (98.7) | 5 (1.3) | 0.061 |
| Yes | 590 (61) | 463 (78) | 127 (22) | 569 (96.6) | 20 (3.4) | |||
|
| ||||||||
| Bloating | No | 619 (66) | 476 (77) | 143 (23) | 0.411 | 602 (97.6) | 15 (2.4) | 0.649 |
| Yes | 325 (34) | 258 (79) | 67 (21) | 318 (98.2) | 6 (1.9) | |||
Underweight BMI <18.5; Ideal BMI 18.5 up to 25; Overweight BMI 25 up to 30; Obese BME 30 up to 35; Severely Obese BMI 35 or greater.
BMI was available for 964 patients with 30-day mortality data for 961.
Percent of stomach within the mediastinum by barium esophagram, preoperative endoscopy or by intraoperative description
Rare CCI variables (<3% of cohort) include peripheral vascular disease, renal disease, liver disease, diabetes mellitus with organ damage, hemiplegia or BMI - body mass index (kg/m2); WHO - World Health Organization; CCI – Charlson Comorbidity Index
Predictors of Mortality and Major Morbidity
In-hospital or 30-day mortality occurred in 2.6% of patients (n=25). Mortality was 1.1% after elective surgery and 8.0% after nonelective surgery (p<0.01). Additional factors associated with mortality included older age at operation, lower body mass index, history of congestive heart failure, cerebral vascular accident, dementia, pulmonary disease, peptic ulcer disease, and malignancy within the past 5 years. There was a trend toward increased mortality in patients with regurgitation as a presenting symptom. (Table 1)
At least one major morbidity was identified in 22.9% of patients (n=224). Older age at operation, lower body mass index, and larger preoperative hernia were significantly associated with an increased rate of major postoperative morbidity. (Table 1) CCI variables associated with major postoperative morbidity include history of myocardial infarction or coronary revascularization, congestive heart failure, cerebral vascular accident or transient ischemic attack, dementia and pulmonary disease. (Table 1) The only preoperative symptom that was significantly associated with adverse outcome was heartburn, which was less likely to be present in patients who had at least one adverse event (Table 1) Patients with preoperative heartburn were also younger (p<0.001), more likely to have smaller hernia (p<0.001), and less likely to have urgent operation (p<0.001).
Development of the Clinical Prediction Rule
Forward stepwise logistic regression was performed to identify combinations of variables most predictive of postoperative mortality and major morbidity. For postoperative mortality, a 4-variable model consisting of two CCI variables (history of congestive heart failure and history of pulmonary disease), age at operation (80 or greater versus <80), and urgency of operation (Table 2a; risk score range 0-20) predicted mortality with discriminatory accuracy of 88.4% (95% CI 82.0%-94.8%). Patients were then categorized into groups based on the mortality risk score. Low (mortality risk score 0-2), intermediate (mortality risk score 3) and high (mortality risk score 4+) risk categories were associated with increasing risk for postoperative mortality. (Figure 1)
Table 2. Clinical prediction rules for in-hospital or 30-day mortality (2a. mortality model) and major morbidity (2b. morbidity model): Variables included in predictive models after forward stepwise logistic regression analysis and points assigned for each risk factor present.
| Variables in each model | Points | Comparison | p-value | Adjusted Odds ratioc | 95% CI |
|---|---|---|---|---|---|
| 2a. Mortality | |||||
|
| |||||
| Congestive heart failurea | 5 | present vs. absent | 0.009 | 4.740 | 1.481, 15.172 |
| Pulmonary diseasea | 3 | present vs. absent | 0.009 | 3.342 | 1.345, 8.306 |
| Surgery typeb | 3 | nonelective vs. elective | 0.021 | 3.165 | 1.193, 8.397 |
| Age 80+ (years) | 9 | 80+ vs. <80 | <0.001 | 8.577 | 3.043, 24.174 |
|
| |||||
| 2b. Morbidity | |||||
|
| |||||
| Sex | 1 | male vs. female | 0.122 | 1.328 | 0.927, 1.901 |
| Congestive heart failurea | 4 | present vs. absent | <0.001 | 4.267 | 2.083, 8.737 |
| Pulmonary diseasea | 2 | present vs. absent | 0.015 | 1.515 | 1.083, 2.121 |
| Surgery typeb | 2 | nonelective vs. elective | <0.001 | 2.142 | 1.466, 3.128 |
| Age Group (years) | |||||
| 1 | 50-59 vs. <50 | 0.395 | 1.487 | 0.596, 3.712 | |
| 2 | 60-69 vs. <50 | 0.221 | 1.718 | 0.722, 4.086 | |
| 2 | 70-79 vs. <50 | 0.128 | 1.940 | 0.826, 4.558 | |
| 3 | ≥80 vs. <50 | <0.001 | 2.689 | 1.123, 6.441 | |
History of each comorbid disease as defined in the Charlson Comorbidity Index
Non-elective surgery includes urgent and emergent surgery as defined by the Society of Thoracic Surgeons
Adjusted for all other factors in the model
CI – confidence interval; CCI – Charlson Comorbidity Index
Figure 1. In-hospital or 30-day Mortality.
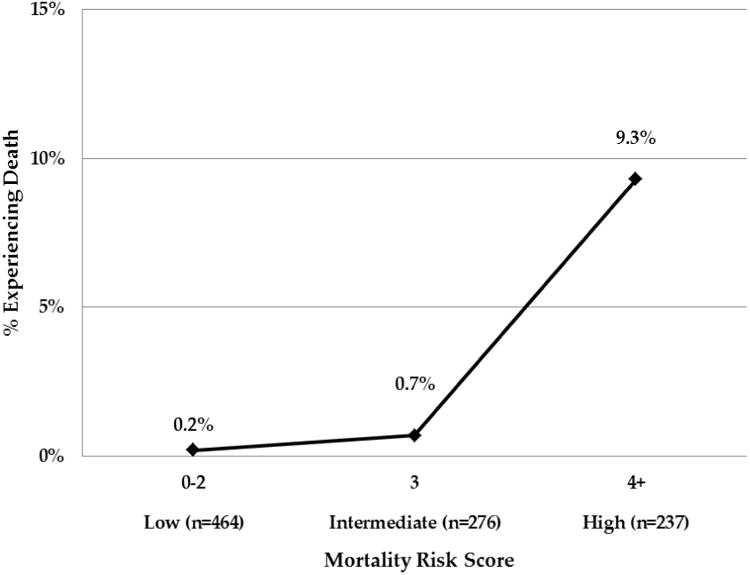
Patients were assigned points according to the presence of risk factors for mortality. Increasing risk score correlates with the incidence of mortality. Incidence of mortality (in bold) and numbers of patients within each group are shown.
For postoperative morbidity, a 5-variable model consisting of sex, age at operation (by decade), history of congestive heart failure, history of pulmonary disease, and urgency of operation (Table 2b; risk score range 0-12) predicted major morbidity with discriminatory accuracy of 67.8% (AUC 0.678; 95% CI 0.637, 0. 718). When risk score was categorized into minimal, low, intermediate and high risk, increasing risk category was associated with increasing incidence of at least one major adverse outcome. For patients in the minimal risk category (morbidity risk score 0-2), at least one major adverse outcome was identified in 13.5% compared to a rate of 40.6% in the patients in the high risk category (morbidity risk score 5+). Increasing risk category was also associated with progressively increasing rates of most of the major adverse outcomes assessed in the study. (Table 3)
Table 3. Distribution of postoperative morbidity within categories of predicted risk.
| Major Morbidity Risk Score | ||||||
|---|---|---|---|---|---|---|
| Minimal | Low | Intermediate | High | |||
|
|
||||||
| Post operative Outcome (n, [%]) | Total n=980 |
0-2 n=407 |
3 n=173 |
4 n=188 |
5+ n=212 |
p-value |
| At least one major adverse outcome | 224 (23) | 55 (13.5) | 34 (19.7) | 49 (26.1) | 86 (40.6) | <0.001 |
|
| ||||||
| Any pulmonary complicationa | 199 (20.3) | 51 (12.5) | 28 (16) | 38 (20) | 82 (39) | <0.001 |
| Pneumonia | 65 (6.7) | 15 (3.7) | 7 (4.1) | 14 (7.5) | 29 (13.8) | <0.001 |
| Reintubation | 42 (4.3) | 7 (1.7) | 3 (1.7) | 11 (5.9) | 21 (9.9) | <0.001 |
| Postoperative tracheostomy | 8 (0.8) | 1 (0.25) | 0 (0) | 2 (1.1) | 5 (2.4) | 0.027 |
| Pulmonary embolism | 34 (3.5) | 13 (3.2) | 6 (3.5) | 8 (4.3) | 7 (3.3) | 0.909 |
| Myocardial infarction | 11 (1.1) | 3 (0.74) | 0 (0) | 1 (0.5) | 7 (3.3) | 0.012 |
| Congestive heart failure | 36 (3.7) | 2 (0.5) | 4 (2.3) | 5 (2.7) | 25 (11.9) | <0.001 |
| Acute renal failure | 25 (2.6) | 0 (0) | 3 (1.7) | 3 (1.6) | 19 (9) | <0.001 |
| Cerebral vascular accident | 6 (0.6) | 1 (0.25) | 0 (0) | 1 (0.53) | 4 (1.9) | 0.068 |
| Septic shock or bacteremia | 21 (2.1) | 2 (0.5) | 3 (1.7) | 7 (3.7) | 9 (4.3) | 0.003 |
| Postoperative gastric or esophageal leak | 21 (2.2) | 6 (1.5) | 2 (1.2) | 7 (3.7) | 6 (2.8) | 0.229 |
| Perioperative hernia recurrence | 12 (1.2) | 4 (1) | 3 (1.7) | 1 (0.5) | 4 (1.9) | 0.528 |
| Readmission within 30-days of operation | 96 (9.8) | 21 (5.2) | 16 (9.3) | 22 (11.7) | 37 (17.5) | <0.001 |
| Reoperation within 30-days of operation | 60 (6.1) | 13 (3.2) | 8 (4.7) | 17 (9.0) | 22 (10.4) | 0.001 |
Any pulmonary complication includes: pneumonia; tracheostomy; unplanned bronchoscopy; pneumothorax or pleural effusion requiring drainage; reintubation; prolonged initial ventilation>48 hours
To assess how the models developed for major adverse outcome and post-operative mortality might generalize to an independent data set, we performed leave-one-out bootstrap cross-validation. The leave-one-out cross validation error rate was 21.503% for any major adverse outcome and 2.304% for postoperative mortality. These are nearly identical to the 21.399% and 2.304% error rates from the fitted models, indicating that application of the models to new patient data sets would yield similar results.
Factors Associated with Nonelective Operation
Because nonelective operation was a significant, independent predictor of mortality and major morbidity in our models, we assessed the clinical covariates associated with urgent or emergent GPEH repair. (Table 4) Patients undergoing nonelective operation were more likely to be male, in the 70+ age groups, underweight or ideal body weight, and have larger hernia. Charlson comorbid diseases were more common in patients who underwent nonelective repair. Symptoms that were more commonly present in patients who underwent nonelective repair included respiratory symptoms, chest and/or abdominal pain, heartburn, regurgitation and bloating. Age 80 or greater at operation, preoperative hernia size of 75% or greater, and a history of dementia or peptic ulcer disease were independent predictors of nonelective repair in multivariate analysis. Patients who had a nonelective operation were also significantly more likely to have a planned open repair (23/199; 12%) than patients who underwent elective repair (6/781; 0.8% [p<0.001]).
Table 4. Association of patient demographics, comorbid diseases and symptoms with urgent or emergent paraesophageal hernia repair.
| Nonelective operation | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Patient Demographics: (n, [%]) | Overall n=980 |
Elective n=781 |
Nonelective n=199 |
p-value | Adjusted ORd | 95% CI | |
| Sex | Female | 733 (75) | 595 (81) | 138 (19) | 0.055 | ||
| Male | 247 (25) | 186 (75) | 61 (25) | ||||
|
| |||||||
| Age group at operation | <50 | 60 (6) | 51 (85) | 9 (15) | <0.001 | ref | ref |
| 50-59 | 148 (15) | 130 (88) | 18 (12) | 0.80 | (0.33, 1.96) | ||
| 60-69 | 261 (27) | 234 (90) | 27 (10) | 0.61 | (0.26, 1.42) | ||
| 70-70 | 319 (33) | 265 (83) | 55 (17) | 0.93 | (0.42, 2.09) | ||
| 80+ | 192 (20) | 102 (53) | 90 (47) | 2.66 | (1.16, 6.10) | ||
|
| |||||||
| Body MassIndex by WHO classificationa | Underweight | 16 (2) | 9 (56) | 7 (44) | <0.001 | ref | ref |
| Ideal | 185 (19) | 126 (68) | 59 (32) | 0.50 | (0.13, 1.73) | ||
| Overweight | 356 (37) | 295 (83) | 61 (17) | 0.35 | (0.10, 1.12) | ||
| Obese | 258 (27) | 220 (85) | 38 (15) | 0.36 | (0.10, 1.27) | ||
| Severely obese | 149 (15) | 127 (85) | 22 (15) | 0.42 | (0.12, 1.54) | ||
|
| |||||||
| Preoperative Hernia Size (%)b | 30-49% | 174 (18) | 157 (90) | 17 (10) | <0.001 | ref | ref |
| 50-74% | 354 (36) | 300 (85) | 54 (15) | 1.40 | (0.74, 2.60) | ||
| 75-99% | 212 (22) | 159 (75) | 53 (25) | 2.60 | (1.25, 4.88) | ||
| Intrathoracic Stomach | 240 (24) | 165 (69) | 75 (31) | 3.00 | (1.59, 5.58) | ||
|
| |||||||
| History of Pretreatment Charlson-defined patient comorbidity | |||||||
|
| |||||||
| Myocardial infarction or revascularization | No | 833 (85) | 677 (81) | 156 (19) | 0.005 | 1.27 | (0.80, 2.02) |
| Yes | 147 (15) | 104 (71) | 43 (29) | ||||
|
| |||||||
| Congestive heart failure | No | 941 (96) | 761 (81) | 180 (19) | <0.001 | 1.84 | (0.83, 4.05) |
| Yes | 39 (4) | 20 (51) | 19 (48) | ||||
|
| |||||||
| Cerebral vascular accident or transient attack | No | 904 (92) | 737 (82) | 167 (18) | <0.001 | 1.60 | (0.87, 2.83) |
| Yes | 76 (8) | 44 (58) | 32 (42) | ||||
|
| |||||||
| Dementia | No | 937 (96) | 763 (81) | 174 (19) | <0.001 | 2.43 | (1.13, 5.22) |
| Yes | 43 (4) | 18 (42) | 25 (58) | ||||
|
| |||||||
| Pulmonary disease | No | 690 (70) | 550 (80) | 140 (20) | 1.000 | ||
| Yes | 290 (30) | 231 (80) | 59 (20) | ||||
|
| |||||||
| Connective Tissue Disorder | No | 942 (96) | 750 (80) | 192 (20) | 1.000 | ||
| Yes | 38 (4) | 31 (82) | 7 (18) | ||||
|
| |||||||
| Peptic Ulcer Disease | No | 794 (81) | 655 (82) | 139 (18) | <0.001 | 2.20 | (1.50, 3.30) |
| Yes | 186 (19) | 126 (68) | 60 (32) | ||||
|
| |||||||
| Diabetes requiring medical therapy | No | 909 (93) | 728 (80) | 181 (20) | 0.284 | ||
| Yes | 71 (7) | 53 (75) | 18 (25) | ||||
|
| |||||||
| Malignancy in the past 5 years | No | 924 (94) | 740 (80) | 184 (20) | 0.231 | ||
| Yes | 56 (6) | 41 (73) | 15 (27) | ||||
|
| |||||||
| Rare CCI variablesc | No | 939 (96) | 753 (80) | 186 (20) | 0.074 | ||
| Yes | 41 (4) | 28 (68) | 13 (32) | ||||
|
| |||||||
| Preoperative Symptoms | |||||||
|
| |||||||
| Respiratory Symptoms | No | 232 (34) | 164 (71) | 68 (29) | <0.001 | ||
| Yes | 450 (66) | 373 (83) | 77 (17) | ||||
|
| |||||||
| Chest and/or Abdominal pain | No | 273 (32) | 236 (86) | 37 (14) | 0.001 | ||
| Yes | 589 (68) | 449 (76) | 140 (24) | ||||
|
| |||||||
| Heartburn | No | 335 (35) | 226 (67) | 109 (33) | <0.001 | ||
| Yes | 626 (65) | 545 (87) | 81 (13) | ||||
|
| |||||||
| Dysphagia | No | 533 (55) | 418 (78) | 115 (22) | 0.122 | ||
| Yes | 431 (45) | 356 (83) | 75 (17) | ||||
|
| |||||||
| Regurgitation | No | 377 (39) | 316 (84) | 61 (16) | 0.014 | ||
| Yes | 590 (61) | 456 (77) | 134 (23) | ||||
|
| |||||||
| Bloating | No | 619 (66) | 483 (78) | 136 (22) | 0.016 | ||
| Yes | 325 (34) | 275 (85) | 50 (15) | ||||
Underweight BMI <18.5; Ideal BMI 18.5 up to 25; Overweight BMI 25 up to 30; Obese BMI 30 up to 35; Severely Obese BMI 35 or greater
Percent of stomach within the mediastinum by barium esophagram, preoperative endoscopy or by intraoperative description
Rare CCI variables (<3% of cohort) include peripheral vascular disease, renal disease, liver disease, diabetes mellitus with organ damage, hemiplegia or paraplegia, lymphoma, leukemia, metastatic cancer and acquired immunodeficiency syndrome
Each adjusted for the other variables in the model (age group, BMI, size of hernia, myocardial infarction, congestive heart failure, cerebral vascular accident or transient attack, dementia, peptic ulcer disease)
BMI - body mass index (kg/m2); WHO - World Health Organization; CCI - Charlson Comorbidity Index
Discussion
Using known pretreatment covariates, we have developed clinical prediction rules for mortality and major morbidity after GPEH repair that provide good discriminatory accuracy and could be easily implemented in the clinical setting. We found that the combination of nonelective operation, age 80 or greater, history of pulmonary disease and history of congestive heart failure clearly identified a group of patients at increased risk for perioperative mortality. Importantly, using these variables, our models also identified a group of patients at very low risk for perioperative mortality. Similarly, our 5-covariate model for major morbidity, including sex, age at operation, urgency of operation, history of congestive heart failure, and history of pulmonary disease facilitated assignment of patients into minimal, low, intermediate and high risk categories based on their pre-treatment scores. Importantly, the risk for perioperative mortality more than tripled and the risk for major morbidity more than doubled when nonelective repair was required. While a limited number of previous reports have examined the association of preoperative variables to adverse outcomes after GPEH repair,(2, 22) our study is the first to develop prediction models for adverse outcome based on pretreatment variables.
Impact of Nonelective Repair on Patient Risk
There is little debate that the risks of operative intervention outweigh the benefits in completely asymptomatic patients with GPEH. In symptomatic patients, however, the literature is somewhat conflicting. Historically, it has been recommended that all patients with paraesophageal hernia undergo repair at the time of diagnosis to prevent the catastrophic complications that can occur with acute mechanical symptoms, including gastric strangulation, massive hemorrhage or perforation.(13) More recently, however, it was suggested that minimally symptomatic patients can be managed expectantly.(10) Using Markov modeling, Stylopoulos and colleagues created theoretical cohorts with minimal symptoms and determined the outcomes for two approaches - immediate repair or watchful waiting. In their decision analysis, the mortality difference between elective and emergent surgery was the only factor influencing the value of elective surgery. They concluded that, assuming a 17% mortality rate of emergency surgery, elective laparoscopic GPEH repair becomes the optimal management if mortality with elective laparoscopic repair is less than 1%. If the mortality rate of emergency surgery is 6%, a value close to the mortality rate of 8% seen in our series, elective repair is recommended only if it can be achieved with mortality of 0.5% or less.(10)
When considering whether to adopt the recommendations for watchful waiting into clinical practice, it is important to note that the model developed by Stylopoulos and colleagues was designed to establish mortality cutoffs for minimally symptomatic patients. In this model, minimally symptomatic was defined as patients with symptoms (belching and heartburn) that do not affect the quality of life of the patient.(10) However, we and others have reported the significant impact of GPEH on quality of life, even in patients whose primary complaint is heartburn.(1, 3, 5, 9, 23-28) The vast majority of patients, particularly those with larger hernias, have mechanical symptoms such as pain, postprandial bloating and bleeding (acute and occult). Careful assessment for symptoms frequently reveals symptoms of chest and abdominal pain, postprandial bloating, dysphagia, chronic anemia, weight loss, change in eating habits or food avoidance.(6-7, 9) In patients with any of these symptoms, particularly when 75% or more of the stomach is herniated into the mediastinum, careful consideration for elective repair should be entertained.
In the current study, we found a strong independent association of nonelective repair with increased risk of mortality and major morbidity after GPEH repair. This is consistent with many other reports in the literature; recently published analyses of administrative datasets show mortality for emergent repair ranges from 5.1% to 16.4%.(10, 12, 15) It has also been noted that the risk of nonelective repair increases dramatically in the very elderly as does the risk for associated mortality. Poulose and colleagues assessed the impact of nonelective repair on postoperative mortality in octogenarians using the 2005 National Inpatient Sample;(14) they found that, similar to the results presented in our series, 43% of the octogenarians in the nationwide sample underwent nonelective repair (47% in our series). Mortality after nonelective repair in the nationwide sample was markedly higher than for nonelective repair (16% for non-elective versus 2.4% for elective repair). We also found a marked increase in mortality (14%) for nonelective repair in the octogenarians compared with 6% for elective repair, although it did not reach statistical significance level because of the smaller numbers of patients. Nonelective repair has also been shown to be associated with longer hospital stay and higher cost.(12)
Impact of Increasing Age, Comorbidity and Hernia Size at Time of Repair on Patient Risk
Despite the higher risk, age alone should not be a contraindication to elective laparoscopic repair in symptomatic patients, as the majority of elderly patients do not suffer postoperative mortality or major morbidity.(2, 11, 15, 22, 27, 29-30) In addition to age, a history of congestive heart failure requiring medical therapy and a history of pulmonary disease are predictors of adverse outcome after GPEH repair. While none of the electively repaired patients in this current study had decompensated congestive heart failure, awareness of the increased risk for adverse outcome associated with these comorbid conditions would allow for medical optimization prior to elective surgery for symptomatic GPEH. Recognition of the increased risk may also inform the decision to forego surgical repair altogether if the hernia is small, the patient is very elderly with other comorbid conditions and the symptoms are predominantly regurgitation related rather than obstructive.
To address these risk factors, our current approach to patients with GPEH is routine cardiac testing on the majority of patients prior to elective and urgent repair if the patient's clinical status will allow.This is due to the fact that the vast majority of patients are elderly, have a history of smoking and/or obesity or other cardiac risk factors such as family history, personal history of cardiac disease, hypertension, hyperlipidemia, or diabetes mellitus. In addition, chest pain is often one of the primary complaints. While the chest pain is often due to the GPEH itself, there are occasional patients in whom concomitant coronary artery disease is identified. For those patients in whom repair is elective and coronary disease is identified, optimization with medical or invasive techniques is performed prior to GPEH repair. It is important to note that all patients who are under the management of a cardiologist are sent to their provider for preoperative optimization and clearance. Pulmonary function testing is obtained on any patients with pulmonary complaints and smoking cessation is strongly encouraged for current smokers. This proactive approach likely reduces postoperative mortality and major morbidity and improves outcomes for these patients.
The clinical prediction rules presented here may provide a more accurate understanding of patient-specific risk for adverse outcomes with elective repair at an earlier age compared with the risk of nonelective repair when older if watchful waiting is undertaken. For example, a 65 year old female with pulmonary disease and GPEH would have a risk score for mortality of 3 (mortality rate 0.7%) if the operation was performed electively and a risk score of 6 (mortality rate 9.3%) if performed nonelectively. With regard to major postoperative morbidity, the patient's current risk category for elective repair is intermediate (risk score 4 for age and pulmonary disease). Waiting for acute presentation and nonelective repair increases the risk category for major morbidity to high risk (40%). Given an estimated life-time risk of developing acute symptoms of 18% and an estimated annual probability of symptom progression of ∼14%,(10) elective repair would be warranted, preferably before the patient reaches 80 years of age, when perioperative mortality rises steeply.
While paraesophageal hernia size did not remain in the final model due to high collinearity with age and nonelective operation, it is important to note that the risk of nonelective operation did increase with increased hernia size. Combined with the other high risk criteria in the clinical prediction models, waiting for an acute presentation in a patient with the majority of stomach herniated into the mediastinum could prove fatal. On the other hand, a patient with other high risk criteria and a small GPEH may be advised to pursue medical therapy, especially if the symptoms are predominantly heartburn.
Study Strengths and Limitations
Our study has several strengths and limitations. With the large numbers of patients in our extensive, prospectively maintained database for GPEH, and definition-driven abstraction of covariates, we are uniquely positioned to develop these clinical predication rules. However, our study findings may be limited by the fact that the overall mortality in our series was low and restricted our ability to identify additional predictors of mortality, such as stratifying risk by expanded age groups. Hence, our model may have overlooked other important predictors. Our findings may also be hard to generalize because our center has extensive expertise in the management of GPEH and the majority of both elective and nonelective repairs were performed laparoscopically. It is possible that open repair is a significant predictor of adverse outcome after GPEH repair; this could not be assessed in our series because of the significant collinearity between nonelective repair and a planned open approach to operation. As a result, the rate of adverse outcomes in our series may not represent the observed rates in other centers.
Ideally, our model would also have undergone external validation; however, we were able to perform internal cross-validation bootstrapping using the leave-one-out strategy, which showed error rates that were similar to the rates for the developed models for both outcomes. Further validation of the models in other settings is clearly required. Finally, the patients' goals for symptom relief and improved quality of life are also important considerations in guiding the decision to offer surgery.
In conclusion, we have developed clinical prediction rules for postoperative mortality and major morbidity after GPEH repair in a high volume center with extensive expertise in the management of GPEH that have good discriminatory accuracy and facilitate assignment of patients to risk categories for adverse outcome. Our models included patient age at operation, sex, urgency of surgery, congestive heart failure and pulmonary disease. After further refinement and prospective validation, these clinical prediction rules for mortality and major morbidity after GPEH repair could provide patient-specific risk prediction, tailored for individual patient characteristics, and contribute to decision-making regarding surgical intervention.
Acknowledgments
The authors wish to thank Sunee Hempel, Megan Lunz, Kathy Lovas and Shannon Wyszomierski for their expert data abstraction, database management and editorial assistance. Without their dedication, this work would not be possible.
Grant Support: The project described was supported by Award Numbers K07CA151613 (KSN), UL1 RR024153, and UL1TR000005 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
This work was presented at the Plenary Session of the 38th Annual Meeting of the Western Thoracic Surgical Association on June 30, 2012, at the Grand Wailea, Maui, Hawaii
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luketich JD, Nason KS, Christie NA, et al. Outcomes after a decade of laparoscopic giant paraesophageal hernia repair. J Thorac Cardiovasc Surg. 2010 Feb;139(2):395–404. e1. doi: 10.1016/j.jtcvs.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larusson HJ, Zingg U, Hahnloser D, Delport K, Seifert B, Oertli D. Predictive factors for morbidity and mortality in patients undergoing laparoscopic paraesophageal hernia repair: age, ASA score and operation type influence morbidity. World J Surg. 2009 May;33(5):980–5. doi: 10.1007/s00268-009-9958-9. [DOI] [PubMed] [Google Scholar]
- 3.Nason KS, Luketich JD, Awais O, et al. Quality of life after collis gastroplasty for short esophagus in patients with paraesophageal hernia. The Annals of thoracic surgery. 2011 Nov;92(5):1854–60. doi: 10.1016/j.athoracsur.2011.06.030. discussion 60-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Targarona EM, Novell J, Vela S, et al. Mid term analysis of safety and quality of life after the laparoscopic repair of paraesophageal hiatal hernia. Surg Endosc. 2004 Jul;18(7):1045–50. doi: 10.1007/s00464-003-9227-1. [DOI] [PubMed] [Google Scholar]
- 5.Parameswaran R, Ali A, Velmurugan S, Adjepong SE, Sigurdsson A. Laparoscopic repair of large paraesophageal hiatus hernia: quality of life and durability. Surg Endosc. 2006 Aug;20(8):1221–4. doi: 10.1007/s00464-005-0691-7. [DOI] [PubMed] [Google Scholar]
- 6.Hayden JD, Jamieson GG. Effect on iron deficiency anemia of laparoscopic repair of large paraesophageal hernias. Dis Esophagus. 2005;18(5):329–31. doi: 10.1111/j.1442-2050.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 7.Low DE, Simchuk EJ. Effect of paraesophageal hernia repair on pulmonary function. Ann Thorac Surg. 2002 Aug;74(2):333–7. doi: 10.1016/s0003-4975(02)03718-9. discussion 7. [DOI] [PubMed] [Google Scholar]
- 8.Moskovitz M, Fadden R, Min T, Jansma D, Gavaler J. Large hiatal hernias, anemia, and linear gastric erosion: studies of etiology and medical therapy. Am J Gastroenterol. 1992 May;87(5):622–6. [PubMed] [Google Scholar]
- 9.Carrott PW, Hong J, Kuppusamy M, Kirtland S, Koehler RP, Low DE. Repair of giant paraesophageal hernias routinely produces improvement in respiratory function. The Journal of thoracic and cardiovascular surgery. 2012 Feb;143(2):398–404. doi: 10.1016/j.jtcvs.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 10.Stylopoulos N, Gazelle GS, Rattner DW. Paraesophageal hernias: operation or observation? Ann Surg. 2002 Oct;236(4):492–500. doi: 10.1097/00000658-200210000-00012. discussion -1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polomsky M, Jones CE, Sepesi B, et al. Should elective repair of intrathoracic stomach be encouraged? Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2010 Feb;14(2):203–10. doi: 10.1007/s11605-009-1106-1. [DOI] [PubMed] [Google Scholar]
- 12.Polomsky M, Hu R, Sepesi B, et al. A population-based analysis of emergent vs. elective hospital admissions for an intrathoracic stomach. Surg Endosc. 2010 Jun;24(6):1250–5. doi: 10.1007/s00464-009-0755-1. [DOI] [PubMed] [Google Scholar]
- 13.Hill LD. Incarcerated paraesophageal hernia. A surgical emergency. Am J Surg. 1973 Aug;126(2):286–91. doi: 10.1016/s0002-9610(73)80165-5. [DOI] [PubMed] [Google Scholar]
- 14.Poulose BK, Gosen C, Marks JM, et al. Inpatient mortality analysis of paraesophageal hernia repair in octogenarians. J Gastrointest Surg. 2008 Nov;12(11):1888–92. doi: 10.1007/s11605-008-0625-5. [DOI] [PubMed] [Google Scholar]
- 15.Sihvo EI, Salo JA, Rasanen JV, Rantanen TK. Fatal complications of adult paraesophageal hernia: a population-based study. J Thorac Cardiovasc Surg. 2009 Feb;137(2):419–24. doi: 10.1016/j.jtcvs.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 16.Reilly BM, Evans AT. Translating clinical research into clinical practice: impact of using prediction rules to make decisions. Annals of Internal Medicine. 2006 Feb 1;144(3):201–9. doi: 10.7326/0003-4819-144-3-200602070-00009. [DOI] [PubMed] [Google Scholar]
- 17.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. The New England journal of medicine. 1997 Jan 23;336(4):243–50. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 18.Pearson FG, Cooper JD, Ilves R, Todd TR, Jamieson WR. Massive hiatal hernia with incarceration: a report of 53 cases. The Annals of thoracic surgery. 1983 Jan;35(1):45–51. doi: 10.1016/s0003-4975(10)61430-0. [DOI] [PubMed] [Google Scholar]
- 19.Nason KS, Luketich JD, Witteman BP, Levy RM. The laparoscopic approach to paraesophageal hernia repair. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2012 Feb;16(2):417–26. doi: 10.1007/s11605-011-1690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.STS. STS General Thoracic Data Specifications, Version 2.081. Society of Thoracic Surgeons General Thoracic Surgery National Database. 2011 [Google Scholar]
- 22.Gupta A, Chang D, Steele KE, Schweitzer MA, Lyn-Sue J, Lidor AO. Looking beyond age and co-morbidities as predictors of outcomes in paraesophageal hernia repair. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2008 Dec;12(12):2119–24. doi: 10.1007/s11605-008-0685-6. [DOI] [PubMed] [Google Scholar]
- 23.Mark LA, Okrainec A, Ferri LE, Feldman LS, Mayrand S, Fried GM. Comparison of patient-centered outcomes after laparoscopic Nissen fundoplication for gastroesophageal reflux disease or paraesophageal hernia. Surg Endosc. 2008 Feb;22(2):343–7. doi: 10.1007/s00464-007-9628-7. [DOI] [PubMed] [Google Scholar]
- 24.Hazebroek EJ, Gananadha S, Koak Y, Berry H, Leibman S, Smith GS. Laparoscopic paraesophageal hernia repair: quality of life outcomes in the elderly. Dis Esophagus. 2008;21(8):737–41. doi: 10.1111/j.1442-2050.2008.00831.x. [DOI] [PubMed] [Google Scholar]
- 25.Velanovich V, Karmy-Jones R. Surgical management of paraesophageal hernias: outcome and quality of life analysis. Digestive surgery. 2001;18(6):432–7. doi: 10.1159/000050189. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 26.Louie BE, Blitz M, Farivar AS, Orlina J, Aye RW. Repair of symptomatic giant paraesophageal hernias in elderly (>70 years) patients results in improved quality of life. J Gastrointest Surg. 2011 Mar;15(3):389–96. doi: 10.1007/s11605-010-1324-6. [DOI] [PubMed] [Google Scholar]
- 27.Gangopadhyay N, Perrone JM, Soper NJ, et al. Outcomes of laparoscopic paraesophageal hernia repair in elderly and high-risk patients. Surgery. 2006 Oct;140(4):491–8. doi: 10.1016/j.surg.2006.07.001. discussion 8-9. [DOI] [PubMed] [Google Scholar]
- 28.Nason KS, Luketich JD, Qureshi I, et al. Laparoscopic repair of giant paraesophageal hernia results in long-term patient satisfaction and a durable repair. J Gastrointest Surg. 2008 Dec;12(12):2066–75. doi: 10.1007/s11605-008-0712-7. discussion 75-7. [DOI] [PubMed] [Google Scholar]
- 29.Hosking MP, Warner MA, Lobdell CM, Offord KP, Melton LJ., 3rd Outcomes of surgery in patients 90 years of age and older. JAMA. 1989 Apr 7;261(13):1909–15. [PubMed] [Google Scholar]
- 30.Kojima Y, Narita M. Postoperative outcome among elderly patients after general anesthesia. Acta Anaesthesiol Scand. 2006 Jan;50(1):19–25. doi: 10.1111/j.1399-6576.2005.00882.x. [DOI] [PubMed] [Google Scholar]


