Summary
Objectives
The complex overlap between asthma and obesity may be explained in part by activity avoidance in asthma. We compared responses to a walking intervention between matched groups of children with and without asthma. We expected youth with asthma to have lower baseline and post-intervention activity levels. Psychosocial, demographic, and physiologic correlates of activity were also examined.
Design/Participants
We compared baseline and post-intervention activity levels among 59 children aged 10–16 with well-controlled asthma and 59 healthy matched controls. Participants completed spirometry, physical examination, anthropometric measurement, and psychosocial questionnaires.
Intervention/Outcome Measure
Participants wore blinded calibrated pedometers for a baseline typical activity week, returning to complete the Wonders of Walking (WOW) intervention, followed by a week of post-intervention pedometer monitoring.
Results
Contrary to expectation, no differences between cases (median steps = 6,348/day) and controls (median steps = 6,825/day) in baseline activity were found. Response to the WOW intervention was comparable, with both groups demonstrating an increase of approximately 1,485 steps per day (equivalent to more than 5 additional miles walked during the post-intervention week). Health beliefs did not correlate to activity at baseline or intervention response. No significant associations between activity and asthma control, FEV1, or duration of diagnosis were found. Intervention response was comparable across racial/ethnic groups, children versus adolescents, and between normal weight and overweight youth.
Conclusions
Contrary to expectation, we found similar rates of objectively measured physical activity among youth with well-controlled asthma and controls. Importantly, we documented statistically significant increases in physical activity across both groups following a brief, pedometer-based intervention. The intervention was successful even among typically sedentary groups, and represents an effective, safe, and inexpensive walking program.
Keywords: asthma, adolescent, obesity, physical activity, intervention
INTRODUCTION
Asthma and obesity have simultaneously become leading public health problems over the past several decades, resulting in consideration of underlying factors linking these health priorities.1 While debate exists, excess body weight appears associated with the development of asthma across the lifespan2 and particularly among women.3,4 Moreover, asthma appears to be a risk factor for the development of obesity,5 and weight loss improves lung function, reduces active symptoms, and minimizes reliance on asthma medications.6 Research has examined a variety of potential mechanisms underlying the obesity and asthma connection, including genetic links,1 environmental factors impeding physical activity,7 and shared biological and atopic markers.8,9 Despite these efforts, clear pathways have yet to be identified. More definitively, comorbid presentation of obesity and asthma complicates the diagnosis, course, and treatment of both chronic conditions,8,10 emphasizing the importance of preventing obesity and encouraging regular physical activity, particularly among those with a preexisting diagnosis of asthma.
One potentially important, yet understudied mechanism linking asthma and obesity involves sedentary lifestyle and physical activity avoidance. Children and adolescents have become increasingly sedentary related to excessive television viewing, decreased physical education in schools, and diminished reliance on walking for transportation.11–13 Additionally, safety concerns are often cited as a reason for discouraging physical activity and outdoor play,14 and access to low-nutrient and high calorie foods and drinks are also implicated in the development of obesity.15 Vigorous physical activity has become particularly rare among youth, and has been shown to decline significantly during the transition to adolescence, especially among girls.16,17 Nearly half of all children and adolescents do not participate in any regular vigorous activity, with African-American females evidencing the lowest levels of activity participation.18Youth with asthma, by virtue of activity-triggering bronchospasm, may be at an even greater risk of adopting sedentary lifestyles and launching the trajectory toward obesity at early ages, however, few studies have utilized objective activity measurements and rigorous study designs to answer this question. One study documented decreased physical activity among pre-schoolers with a history of wheezing,19 and another study reported lower levels of activity among youth with asthma versus a control group.20,21 In contrast, another reported comparable levels of activity between those with and without asthma,22 underscoring the need for further study. Little is known about whether children with asthma require specialized exercise promotion interventions, or whether they would benefit equally from generic public health interventions to encourage more activity. The present study examined the effectiveness of a single-session activity promotion intervention among children with asthma 10–16 years of age compared to a control group. Additionally, psychosocial, demographic, and physiologic correlates of activity were examined to help understand the factors that foster and hinder exercise among youth with asthma.
MAIN RESEARCH QUESTIONS
The following questions were examined: (1) Do children with asthma have lower levels of objectively measured physical activity compared to otherwise healthy controls at baseline? (2) Will children with asthma demonstrate a smaller response to a brief, pedometer-based activity intervention compared to controls? (3) What are the psychosocial, demographic, and physiologic correlates of activity? We hypothesized that youth with asthma would have lower levels of baseline activity and a lower magnitude response to the intervention compared to controls. Additionally, psychosocial correlates of activity, demographic variables, markers of asthma control, and body mass index (BMI) were expected to correlate to intervention response.
PARTICIPANTS AND METHODS
Design
A case–control intervention design compared objectively measured physical activity during a baseline and intervention follow-up period among youth with well-controlled asthma and an otherwise healthy matched cohort. The study received human subjects approval by the National Jewish Medical and Research Center Institutional Review Board and the Colorado Multiple Institutional Review Board.
Participants
Children and adolescents were recruited between December 2005 and July 2007 from urban primary care clinics serving disadvantaged populations in Denver, Colorado and from a database of previous research participants. Inclusion criteria included 10–16 years of age, no chronic health conditions (other than asthma), and no physical challenges that could interfere with movement. Inclusion for the asthma cohort included a physician diagnosis of asthma of at least a year’s duration and one or more prescriptions for an asthma medication. Exclusion criteria included FEV1 below 85% predicted, a chronic or acute health condition interfering with activity (other than asthma), or poorly controlled asthma, which was defined as a severe exacerbation marked by hospitalization, emergency room visit, and/or oral steroid burst in the past 2 months. Asthma was ruled out in the control group by physical examination, clinical history, and spirometry. Medications were not withheld prior to spirometry. Case–control pairs were matched based on racial/ethnic background (Caucasian, Hispanic or African-American), gender, and age ± 24 months. Attempts were made to schedule visits for matched pairs within a 2-week window to minimize differences in weather conditions.
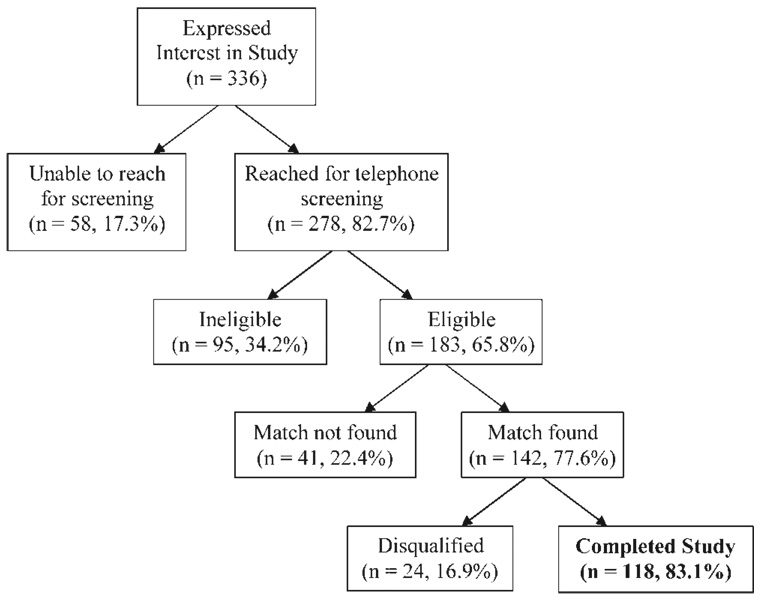
As shown in Figure 1, a total of 336 families expressed an interest in participating in the study, and 278 (82.7%) were reached for telephone screening. Of screened families, 183 (65.8%) were eligible. Reasons for ineligibility included a history of asthma not meeting criteria (n = 10), a sibling enrolled in the study (n = 32), other illness (n = 22), inappropriate age (n = 12), lack of English proficiency (n = 11), or lack of interest in the study (n = 8). Matches were identified for 142 (77.6%) families, and matches were not found for 41 eligible participants (22.4%). Of those matched families, 24 (16.9%) did not complete the study beyond the first visit due to ineligibility determination from low FEV1 results (n = 13) or physical examination findings (n = 2), pedometer device failure or loss (n = 6), or no-show at subsequent study visits (n = 3). No group differences in youth with and without asthma were found in the number of children disqualified after their first study visit for any reason. No significant differences were found between completers and non-completers for income, age, gender, race/ethnicity, or BMI. The final complete data set included 59 matched pairs (N = 118) of youth with asthma and otherwise healthy matched counterparts.
Fig. 1.
Enrollment flowchart.
Power and Sample Size
Based on a prior study,21 a sample size of approximately 60 subjects per group was required to obtain 90% power (at the α = 0.05 level) to detect a between-group difference in daily pedometer steps of 320 with an estimated standard deviation of 533.
Measures
Physical activity measurements
Pedometers were selected based on their accuracy, non-intrusive design, and cost-effectiveness.23,24 Pedometers have been used in research studies with adults25 and children26 for their ability to provide immediate activity feedback, and for their convergent27 and construct validity.28 The Omron Pedometer Model HJ-112® was selected due its capacity to store and download seven to 30 days of activity data, to measure movements other than steady forward walking, such as play-based behavior, and the ability to detect and record compliance with pedometer monitoring. Pedometer data were downloaded at visit 2 and visit 3 to generate baseline and post-intervention aggregate activity levels.
Psychosocial correlates of activity measures
Participants completed a Psychosocial Correlates of Physical Activity interview29,30 with strong psychometric properties and extensive previous use in literature.30–32 One parent from each family and the identified child were asked to respond to a set of items using Likert-type scales, with interviews lasting approximately 45 min. A sample item included asking the youth to respond to the following question along a five-point Likert-type scale of how much they agree with the statement: “I can be physically active during my free time on most days.” A parent and child psychosocial correlates composite score was generated to compile responses across domains of health beliefs, activity attitudes, self-efficacy, and social support for physical activity. Cronbach’s alphas for all measures ranged between 0.70 and 0.87 in the present study.
Demographic and Health Assessments
Parents completed a brief interview for basic demographic and health history information. Physical examination was conducted on all participants to confirm eligibility. Asthma control and severity were measured by spirometry (FEV1), and responses to the Asthma Therapy Assessment Questionnaire (ATAQ).33 Asthma morbidity was measured by the Rosier questionnaire.34 Height and weight were measured on calibrated equipment and BMI calculations were made using the Center for Disease Control and Prevention (CDC) National Center for Health Statistics year 2000 data (http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm). As recommended, BMI age- and sex-specific percentiles were calculated using the above referenced program to take into consideration the variability of growth parameters during child and adolescent development,35 and classified using American Academy of Pediatrics categories, with values ≥95th percentile considered “obese,” 85th–95th considered “overweight,”15 and <85th considered “normal weight.”
Procedures
Recruitment efforts were made in clinic waiting rooms and through mailings. After telephone screenings, families attended a first study visit for informed consenting, physical examination, spirometry, correlates of activity interview, and pedometer calibration. After the first visit and confirmation of study eligibility, youth were given blinded pedometers and instructed to participate in regular activities for a week. Week-long baseline and post-intervention phases were selected based on research that 7 days is an optimal time span for accomplishing reliability and capturing activity fluctuations.36
Intervention
After the baseline week, participants returned for the Wonders of Walking (WOW) activity promotion intervention. The WOW program is a family-based, single-session activity promotion intervention developed for the present study based on empirically supported principles of behavior change (e.g., goal setting, social support, problem-solving techniques). The WOW intervention was designed as a single session, manualized program that could be replicated in other settings, such as within a primary care practice. The WOW intervention was administered by research assistants without any advanced professional training under the supervision of a clinical psychologist. Each session lasted 45–60 min and featured education on the benefits of exercise, activity goal-setting, social support, and problem-solving to reduce sedentary time and maximize walking. The use of an “open-loop feedback system” encouraged youth to set specific walking goals and to frequently review pedometers to monitor goal attainment, an approach shown to increase activity in obese and normal weight children.37,38 Participants completed a post-intervention week of activity monitoring, and then returned to download pedometers. Families received compensation and small incentive prizes at each study visit.
Analysis
All statistical analyses were conducted with the SAS statistical analysis package (version 9.1; SAS Institute, Inc., Cary, NC). The main outcome of daily activity level was computed based on the total number of pedometer steps recorded between visits 1 and 2 (baseline) and visits 2 and 3 (follow-up) divided by the number of days the pedometer was worn. Parameters were set to maximize data integrity, such as a minimum of 4 hr of monitoring required in order to determine that a day’s data were valid. Log-transformed step data were used as primary outcomes for hypothesis testing, but the median and inter-quartile range (IQR) of pedometer measurements in their original scale are reported for ease of interpretation.
Group differences in activity (post-intervention vs. baseline) were analyzed using paired Student’s t-tests. Chi-square tests were used to examine differences across racial/ethnic, gender, and obesity groupings, and Pearson’s correlation coefficients were calculated between physical activity and physiological and psychological measures. Analysis of variance (ANOVA) model was used to assess the association between physical activity and BMI percentile, gender and racial group. Intra-class correlation coefficients were calculated for the step data using a one-way random effects ANOVA model. Distributions of obese (BMI ≥ 95th) and overweight (BMI ≥ 85th) groups at baseline were compared with the National Health and Nutrition Examination Survey (NHANES) 1999–2000 data39 using a one-sample test for a binomial proportion. Two-tailed tests and a significance level of less than 0.05 were used for all analyses.
RESULTS
Demographic Characteristics
Groups were accurately matched according to age (±24 months), gender, and race/ethnicity. The average age difference between matched pairs was 2.2 months (SD = 10.6 months). Every attempt was made to have matched pairs complete the study within a 2-week window of one another to minimize potential effects of weather differences on activity level. We reached this goal for 55.9% of pairs, with an average of 9.54 weeks duration between study completion dates for matched pairs (SD = 15.19 weeks).
The final sample included a diverse racial representation, with 52 Caucasian (44.1%), 34 African-American (28.8%), and 32 Hispanic (27.1%) youth. The sample included 60 (50.8%) females and 58 males (49.2%), and most families (66.1%) reported a total income of less than $50,000 (see Table 1). In order to examine developmental differences, 12 years of age was used as a cut off for child (<12) versus adolescent (≥12).
TABLE 1.
Demographics
| Group | |||
|---|---|---|---|
| Variable | Asthma (n = 59) |
Control (n = 59) |
P-value |
| Age in years (mean, SD) | 13.3 (1.8) | 13.2 (1.8) | ns |
| Sex (%) | |||
| Male | 50.8 | 50.8 | ns |
| Female | 49.2 | 49.2 | ns |
| Race (%) | |||
| Caucasian | 44.1 | 44.1 | ns |
| Hispanic | 27.1 | 27.1 | |
| African-American | 28.8 | 28.8 | |
| Family income | |||
| <$10,000 | 5.2 | 8.8 | ns |
| $10,000–15,000 | 10.5 | 6.9 | |
| $15,000–20,000 | 5.3 | 13.8 | |
| $20,000–25,000 | 12.3 | 8.6 | |
| $25,000–50,000 | 12.3 | 15.5 | |
| $50,000–75,000 | 14.0 | 5.2 | |
| >$75,000 | 22.8 | 25.9 | |
Comparisons of Activity Levels
To check the reproducibility of the pedometer measures between baseline and follow-up measures for each individual within each group, we calculated the intraclass correlation coefficient, which were in the “fair to good” range (0.61 and 0.75 for asthma and control, respectively). Contrary to expectation, comparable levels of baseline physical activity were found between youth with asthma and their matched counterparts (Table 2). Due to the lack of group differences, we combined the groups to examine the overall impact of the WOW program. Both groups demonstrated comparably significant increases in steps following the intervention, with a median increase of 1,485 steps per day (IQR = 2,605; P < 0.0001) across groups. This corresponds to an additional 5 miles during the follow-up week, reflecting an additional caloric expenditure of roughly 150 calories per day. No safety concerns, injuries or asthma exacerbations were reported.
TABLE 2.
Pedometer Results at Baseline and Follow-Up
| Median steps (inter-quartile range) |
1P-value testing if two groups were equal |
|||
|---|---|---|---|---|
| Both groups | Asthma group | Control group | ||
| Pre-intervention | 6662 (3600) | 6348 (3751) | 6825 (3887) | ns |
| Post-intervention | 8424 (4041) | 7644 (3814) | 9364 (4110) | ns |
| 2Change (post–pre) | 1485 (2605) | 1443 (2371) | 1683 (2617) | ns |
| 1P-value testing if change = 0 | <0.0001 | <0.0001 | <0.0001 | |
Log-transformed data were used for hypothesis testing using Student’s t-test.
Change for each individual was calculated as the difference between post- and pre-intervention steps. Showing above are the median (inter-quartile range) change for the group.
Psychosocial Correlates of Activity Level
No group differences were found in physical activity attitudes, self-efficacy, or social support for physical activity between cases and controls. Youth with asthma had lower health belief scores (mean = 72.9, SD = 9.5), reflecting less positive health attitudes, compared to controls (mean = 76.9, SD = 9.1) (P < 0.05). Despite this difference, health belief score did not relate to activity levels at baseline, follow-up or the change score (data not shown). Additionally, both groups demonstrated comparable psychosocial correlate composite scores, and these scores were not related to change in activity level for the entire group (r = −0.003, P = 0.97), youth with asthma (r = −0.16, P = 0.24), or controls (r = 0.16, P = 0.15).
Demographic Correlates of Activity Level
Girls walked fewer steps at baseline than boys across both groups (5,895 vs. 7,857 median steps, P < 0.0001), driven mainly by boys in the control group. At baseline, healthy boys walked significantly more than healthy girls (8,451 vs. 5,561 median steps, P < 0.0001) and significantly more than asthmatic boys (8,451 vs. 7,440 median steps, P < 0.05). Interestingly, however, both boys and girls demonstrated an equivalent response to the WOW intervention (P = 0.34), and the program was equally successful at increasing activity for girls and boys with and without asthma (P = 0.36 and 0.72, respectively). Ethnic/racial differences in activity level at baseline or post-intervention were not found. Additionally, differences in activity level at baseline or post-intervention were not observed between children and adolescents (data not shown).
Physiologic Correlates of Activity Level
Asthma Factors
Activity level at baseline and change between baseline and follow-up were not associated with duration of asthma diagnosis, asthma severity or control (FEV1 values and ATAQ scores), or asthma morbidity (Rosier values) (data not shown).
Obesity Factors
Rates of overweight and obesity were significantly greater than general population estimates, due to the prevalence of obesity among the asthma cohort, where 52.5% were ≥85th percentile and 30.5% were ≥95th percentile (see Table 3). Ethnic/racial minorities across groups were at the greatest risk of obesity, with 60.6% ≥85th percentile (vs. 17.3% for whites, P < 0.0001), and 37.9% ≥95th percentile (vs. 3.9% of whites, P < 0.0001). While we expected overweight and obese youth, particularly those with asthma, to show lower activity levels at baseline and extent of change at follow-up, no associations were found between BMI category and activity level. The WOW program was equally successful regardless of weight status for both those with and without asthma (data not shown).
TABLE 3.
Rates of Overweight and Obesity versus Population Estimates1
| Current study | Prevalence from general population |
P-value for comparison (Chi-square test) |
|||
|---|---|---|---|---|---|
| BMI group | N | % | |||
| All participants | At or above 85th | 49 | 41.5 | 30.35 | <0.01 |
| (N = 118) | At or above 95th | 27 | 22.9 | 15.43 | <0.01 |
| Asthma cohort | At or above 85th | 31 | 52.5 | 30.35 | <0.0001 |
| (N = 59) | At or above 95th | 18 | 30.5 | 15.43 | <0.001 |
| Healthy cohort | At or above 85th | 18 | 30.5 | 30.35 | ns |
| (N = 59) | At or above 95th | 9 | 15.3 | 15.43 | ns |
The general population’s obesity data were from the NHANES 1999–2000 study.39
DISCUSSION
Maximizing physical activity and minimizing sedentary behaviors are priorities for all children and adolescents, 18 but are especially important for youth with asthma due to the increased risk of comorbid asthma and obesity. While debate continues as to the exact causal pathway underlying the asthma and obesity overlap, overweight patients with asthma clearly experience more complicated diagnosis, treatment, and outcomes.10 The etiology of obesity is essentially an energy imbalance whereby caloric intake outpaces caloric output.40 Hence, encouraging regular physical activity is key to obesity prevention and treatment. Youth with asthma face barriers to regular physical activity. Upwards of 90% of individuals with asthma experience respiratory symptoms secondary to exercise, and activity limitation is a consequence of poorly controlled disease.41 Additionally, negative beliefs about physical endurance and enthusiasm have been reported among those with asthma.42 Consequently, youth with asthma appear to be at a greater risk of adopting sedentary lifestyles promoting the energy imbalance that leads to obesity, requiring the development and dissemination of activity promotion programs that are suited to all youth, regardless of asthma status.
Several key findings emerged from this study. First, statistically significant differences in baseline physical activity levels were not found between youth with asthma and matched healthy controls. While some researchers have reported diminished physical activity levels among youth with asthma, consistent with the present findings, others have not found differences.22 Some have speculated that differences in activity level may be subtle during childhood, and become more pronounced during the transition from adolescence to adulthood.43 While our findings of comparable baseline activity levels between those with and without asthma are encouraging, it is important to emphasize that pedometer measurement does not specify activity intensity. Accordingly, youth with asthma may still have lower overall energy expenditure, despite our finding of similar exertion duration.
Most importantly, this study documented the feasibility and efficacy of the WOW intervention, a brief, safe, low-cost, and generalizable walking promotion program. WOW was successful regardless of illness status, gender, age, race/ethnicity, or BMI. The program was successful in encouraging even the most typically sedentary youth to increase walking. Of note, our asthma cohort demonstrated significantly higher rates of overweight and obesity than the general population, with more than half of participants with asthma at or above the 85th percentile for BMI, and nearly one-third at or above the 95th percentile. These findings are consistent with the other reports of high rates of obesity among youth with asthma, underscoring the urgency of identifying the etiology and optimal treatment for comorbid asthma and obesity.8 The role of physical inactivity among youth with asthma has been implicated as a potential mechanism underlying the asthma and obesity overlap, and physical activity promotion programs for youth with asthma have been considered as an important tool to address this comorbidity. Interestingly, most empirically supported activity promotion programs for youth with asthma have involved highly detailed and structured protocols, using exercise physiologists to encourage cardiopulmonary conditioning,44 precluding their widespread use. In contrast, the WOW program was delivered by research assistants in less than an hour. Several features of WOW were most likely to have facilitated the program’s success. First, an open-loop feedback system using objective pedometer measures, allowed families to review real-time feedback on activity levels.38 Additionally, established cognitive-behavioral strategies were featured within WOW, including goal setting, behavioral rewards and contingencies, positive self-statements, and relapse prevention. Additionally, a family-based approach was used, whereby parents, siblings, and identified youth were provided a joint intervention. Finally, WOW emphasized on walking, rather than more physically demanding and sophisticated activities.
Limitations in the study design and implementation are important to highlight. We only included youth with well-controlled asthma. As a result, we were not able to compare baseline activity levels between healthy youth and those with more severe and/or active asthma, and it will be important to test the efficacy of WOW among those with more problematic disease before concluding that the program is applicable to all youth with asthma. Additionally, we do not know whether intervention effects were sustained beyond the study and play any role in shaping longer-term behavior change or health outcomes. Future applications of the WOW program should include follow-up modules to reinforce behavior change and maximize the potential for prolonged lifestyle improvements, such as weight loss and increased cardiopulmonary conditioning. Additionally, the WOW program could be adapted for a group-based delivery, which may hold a specific appeal for girls, as females tend to seek social interaction as part of physical activity.45
CONCLUSION
The WOW intervention is a successful walking promotion program for those with and without asthma, and is applicable for groups at particularly high risk of sedentary lifestyles, such as ethnic minorities and obese youth. Future directions should consider replication of this study among youth with more active and severe asthma, incorporation of a longer baseline and follow-up phase, and measurement of activity intensity, rather than just quantity of accrued steps.
ACKNOWLEDGMENTS
We thank the participating CaReNet clinicians (The Children’s Hospital-Child Health Clinic, Denver Health Eastside Pediatric Clinic, Denver Health Kids Care Clinic, Park Hill Family Health Center, Denver Health Lowry Family Health Center, and Denver Health Mariposa Family Health Center) for their dedicated recruitment efforts.
Grant sponsor: American Lung Association Social Behavioral Research Grant; Grant numbers: SB-8393-N, M01-RR00051.
REFERENCES
- 1.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174:112–119. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilliland FD, Berhane K, Islam T, McConnell R, Gauderman WJ, Gilliland SS, Avol E, Peters JM. Obesity and the risk of newly diagnosed asthma in school-age children. Am J Epidemiol. 2003;158:406–415. doi: 10.1093/aje/kwg175. [DOI] [PubMed] [Google Scholar]
- 3.Kim S, Camargo CA., Jr Sex-race differences in the relationship between obesity and asthma: the behavioral risk factor surveillance system, 2000. Ann Epidemiol. 2003;13:666–673. doi: 10.1016/s1047-2797(03)00054-1. [DOI] [PubMed] [Google Scholar]
- 4.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 5.Gennuso J, Epstein LH, Paluch RA, Cerny F. The relationship between asthma and obesity in urban minority children and adolescents. Arch Pediatr Adolesc Med. 1998;152:1197–1200. doi: 10.1001/archpedi.152.12.1197. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115:897–909. doi: 10.1016/j.jaci.2004.11.050. quiz 910. [DOI] [PubMed] [Google Scholar]
- 7.Brisbon N, Plumb J, Brawer R, Paxman D. The asthma and obesity epidemics: the role played by the built environment—a public health perspective. J Allergy Clin Immunol. 2005;115:1024–1028. doi: 10.1016/j.jaci.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Story RE. Asthma and obesity in children. Curr Opin Pediatr. 2007;19:680–684. doi: 10.1097/MOP.0b013e3282f1ddfa. [DOI] [PubMed] [Google Scholar]
- 9.Matricardi PM, Gruber C, Wahn U, Lau S. The asthma-obesity link in childhood: open questions, complex evidence, a few answers only. Clin Exp Allergy. 2007;37:476–484. doi: 10.1111/j.1365-2222.2007.02664.x. [DOI] [PubMed] [Google Scholar]
- 10.Vortmann M, Eisner MD. BMI and health status among adults with asthma. Obesity (Silver Spring) 2008;16:146–152. doi: 10.1038/oby.2007.7. [DOI] [PubMed] [Google Scholar]
- 11.Amisola RV, Jacobson MS. Physical activity, exercise, and sedentary activity: relationship to the causes and treatment of obesity. Adolesc Med. 2003;14:23–35. [PubMed] [Google Scholar]
- 12.Proctor MH, Moore LL, Gao D, Cupples LA, Bradlee ML, Hood MY, Ellison RC. Television viewing and change in body fat from preschool to early adolescence: The Framingham Children’s Study. Int J Obes Relat Metab Disord. 2003;27:827–833. doi: 10.1038/sj.ijo.0802294. [DOI] [PubMed] [Google Scholar]
- 13.Steinbeck KS. The importance of physical activity in the prevention of overweight and obesity in childhood: a review and an opinion. Obes Rev. 2001;2:117–130. doi: 10.1046/j.1467-789x.2001.00033.x. [DOI] [PubMed] [Google Scholar]
- 14.Weir LA, Etelson D, Brand DA. Parents’ perceptions of neighborhood safety and children’s physical activity. Prev Med. 2006;43:212–217. doi: 10.1016/j.ypmed.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120:S193–S228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- 16.Crespo CJ, Smit E, Troiano RP, Bartlett SJ, Macera CA, Andersen RE. Television watching, energy intake, and obesity in US children: results from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2001;155:360–365. doi: 10.1001/archpedi.155.3.360. [DOI] [PubMed] [Google Scholar]
- 17.Pate RR, Freedson PS, Sallis JF, Taylor WC, Sirard J, Trost SG, Dowda M. Compliance with physical activity guidelines: prevalence in a population of children and youth. Ann Epidemiol. 2002;12:303–308. doi: 10.1016/s1047-2797(01)00263-0. [DOI] [PubMed] [Google Scholar]
- 18.DHHS. Atlanta, GA: CDC, National Center for Chronic Disease Prevention and Health Promotion; 1996. Physical activity and health: Report of the Surgeon General. [Google Scholar]
- 19.Firrincieli V, Keller A, Ehrensberger R, Platts-Mills J, Shufflebarger C, Geldmaker B, Platts-Mills T. Decreased physical activity among Head Start children with a history of wheezing: use of an accelerometer to measure activity. Pediatr Pulmonol. 2005;40:57–63. doi: 10.1002/ppul.20214. [DOI] [PubMed] [Google Scholar]
- 20.Glazebrook C, McPherson AC, Macdonald IA, Swift JA, Ramsay C, Newbould R, Smyth A. Asthma as a barrier to children’s physical activity: implications for body mass index and mental health. Pediatrics. 2006;118:2443–2449. doi: 10.1542/peds.2006-1846. [DOI] [PubMed] [Google Scholar]
- 21.Lang DM, Butz AM, Duggan AK, Serwint JR. Physical activity in urban school-aged children with asthma. Pediatrics. 2004;113:e341–e346. doi: 10.1542/peds.113.4.e341. [DOI] [PubMed] [Google Scholar]
- 22.van Gent R, van der Ent CK, van Essen-Zandvliet LE, Rovers MM, Kimpen JL, de Meer G, Klijn PH. No differences in physical activity in (un)diagnosed asthma and healthy controls. Pediatr Pulmonol. 2007;42:1018–1023. doi: 10.1002/ppul.20672. [DOI] [PubMed] [Google Scholar]
- 23.Trost SG. Objective measurement of physical activity in youth: current issues, future directions. Exerc Sport Sci Rev. 2001;29:32–36. doi: 10.1097/00003677-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Rowlands AV, Eston RG, Ingledew DK. Measurement of physical activity in children with particular reference to the use of heart rate and pedometry. Sports Med. 1997;24:258–272. doi: 10.2165/00007256-199724040-00004. [DOI] [PubMed] [Google Scholar]
- 25.Rooney B, Smalley K, Larson J, Havens S. Is knowing enough? Increasing physical activity by wearing a pedometer. Wis Med J. 2003;102:31–36. [PubMed] [Google Scholar]
- 26.Tudor-Locke C, Bell RC, Myers AM, Harris SB, Ecclestone NA, Lauzon N, Rodger NW. Controlled outcome evaluation of the First Step Program: a daily physical activity intervention for individuals with type II diabetes. Int J Obes Relat Metab Disord. 2004;28:113–119. doi: 10.1038/sj.ijo.0802485. [DOI] [PubMed] [Google Scholar]
- 27.Tudor-Locke C, Williams JE, Reis JP, Pluto D. Utility of pedometers for assessing physical activity: convergent validity. Sports Med. 2002;32:795–808. doi: 10.2165/00007256-200232120-00004. [DOI] [PubMed] [Google Scholar]
- 28.Tudor-Locke C, Williams JE, Reis JP, Pluto D. Utility of pedometers for assessing physical activity: construct validity. Sports Med. 2004;34:281–291. doi: 10.2165/00007256-200434050-00001. [DOI] [PubMed] [Google Scholar]
- 29.Saunders RP, Pate RR, Felton G, Dowda M, Weinrich MC, Ward DS, Parsons MA, Baranowski T. Development of questionnaires to measure psychosocial influences on children’s physical activity. Prev Med. 1997;26:241–247. doi: 10.1006/pmed.1996.0134. [DOI] [PubMed] [Google Scholar]
- 30.Motl RW, Dishman RK, Trost SG, Saunders RP, Dowda M, Felton G, Ward DS, Pate RR. Factorial validity and invariance of questionnaires measuring social-cognitive determinants of physical activity among adolescent girls. Prev Med. 2000;31:584–594. doi: 10.1006/pmed.2000.0735. [DOI] [PubMed] [Google Scholar]
- 31.Dishman RK, Motl RW, Saunders RP, Dowda M, Felton G, Ward DS, Pate RR. Factorial invariance and latent mean structure of questionnaires measuring social-cognitive determinants of physical activity among black and white adolescent girls. Prev Med. 2002;34:100–108. doi: 10.1006/pmed.2001.0959. [DOI] [PubMed] [Google Scholar]
- 32.Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity of children and adolescents. Med Sci Sports Exerc. 2000;32:963–975. doi: 10.1097/00005768-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Skinner EA, Diette GB, Algatt-Bergstrom PJ, Nguyen TT, Clark RD, Markson LE, Wu AW. The Asthma Therapy Assessment Questionnaire (ATAQ) for children and adolescents. Dis Manag. 2004;7:305–313. doi: 10.1089/dis.2004.7.305. [DOI] [PubMed] [Google Scholar]
- 34.Rosier MJ, Bishop J, Nolan T, Robertson CF, Carlin JB, Phelan PD. Measurement of functional severity of asthma in children. Am J Respir Crit Care Med. 1994;149:1434–1441. doi: 10.1164/ajrccm.149.6.8004295. [DOI] [PubMed] [Google Scholar]
- 35.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 36.Trost SG, Pate RR, Freedson PS, Sallis JF, Taylor WC. Using objective physical activity measures with youth: how many days of monitoring are needed? Med Sci Sports Exerc. 2000;32:426–431. doi: 10.1097/00005768-200002000-00025. [DOI] [PubMed] [Google Scholar]
- 37.Goldfield GS, Kalakanis LE, Ernst MM, Epstein LH. Open-loop feedback to increase physical activity in obese children. Int J Obes Relat Metab Disord. 2000;24:888–892. doi: 10.1038/sj.ijo.0801248. [DOI] [PubMed] [Google Scholar]
- 38.Roemmich JN, Gurgol CM, Epstein LH. Open-loop feedback increases physical activity of youth. Med Sci Sports Exerc. 2004;36:668–673. doi: 10.1249/01.mss.0000121947.59529.3b. [DOI] [PubMed] [Google Scholar]
- 39.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 40.Hill JO, Melanson EL. Overview of the determinants of overweight and obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S515–S521. doi: 10.1097/00005768-199911001-00005. [DOI] [PubMed] [Google Scholar]
- 41.Milgrom H, Taussig LM. Keeping children with exercise-induced asthma active. Pediatrics. 1999;104:e38. doi: 10.1542/peds.104.3.e38. [DOI] [PubMed] [Google Scholar]
- 42.Kitsantas A, Zimmerman BJ. Self-efficacy, activity participation, and physical fitness of asthmatic and nonasthmatic adolescent girls. J Asthma. 2000;37:163–174. doi: 10.3109/02770900009055439. [DOI] [PubMed] [Google Scholar]
- 43.Welsh L, Roberts RG, Kemp JG. Fitness and physical activity in children with asthma. Sports Med. 2004;34:861–870. doi: 10.2165/00007256-200434130-00001. [DOI] [PubMed] [Google Scholar]
- 44.Ram FS, Robinson SM, Black PN, Picot J. Physical training for asthma. Cochrane Database Syst Rev. 2005;4 doi: 10.1002/14651858.CD001116.pub2. CD001116. [DOI] [PubMed] [Google Scholar]
- 45.Ridgers ND, Stratton G, Fairclough SJ. Physical activity levels of children during school playtime. Sports Med. 2006;36:359–371. doi: 10.2165/00007256-200636040-00005. [DOI] [PubMed] [Google Scholar]