Abstract
Cue-induced cocaine craving is a major cause of relapse in abstinent addicts. In rats, cue-induced craving progressively intensifies (incubates) during withdrawal from extended-access cocaine self-administration. After ~1 month of withdrawal, incubated craving is mediated by Ca2+-permeable AMPARs (CP-AMPARs) that accumulate in the nucleus accumbens (NAc). We found that decreased mGluR1 surface expression in the NAc precedes and enables CP-AMPAR accumulation. Thus, restoring mGluR1 tone by administering repeated injections of an mGluR1 positive allosteric modulator (PAM) prevented CP-AMPAR accumulation and incubation, whereas blocking mGluR1 transmission at even earlier withdrawal times accelerated CP-AMPAR accumulation. In studies conducted after prolonged withdrawal, when CP-AMPAR levels and cue-induced craving are high, we found that systemic administration of an mGluR1 PAM attenuated the expression of incubated craving by reducing CP-AMPAR transmission in the NAc to control levels. These results demonstrate a strategy whereby recovering addicts could use a systemically active compound to protect against cue-induced relapse.
Relapse, often triggered by environmental cues previously associated with cocaine use, is a major problem in treating cocaine addiction. Addicts remain vulnerable to relapse long after the acute withdrawal phase. In a rat model of this phenomenon, cue-induced cocaine craving progressively intensifies (“incubates”) during the first months of withdrawal from extended-access cocaine self-administration1. Incubation models a human scenario in which heavy drug use is interrupted by hospitalization or incarceration2, and involves neuroadaptations in the circuitry underlying motivation and addiction1. The nucleus accumbens (NAc) is a brain region that plays a central role in this circuitry, and is comprised mainly of medium spiny neurons (MSN). These MSN mediate motivated behaviors by serving as an interface between cortical and limbic regions and the motor circuitry3.
AMPA receptor (AMPAR) transmission onto NAc MSN is critical for drug-seeking in animal models of cocaine addiction4. In drug-naïve rats and rats with limited cocaine exposure, AMPAR transmission in the NAc is mediated by GluA2-containing Ca2+-impermeable AMPARs (CI-AMPARs)5. However, high conductance Ca2+-permeable AMPARs (CP-AMPARs) accumulate in NAc synapses during withdrawal from extended-access cocaine self-administration6,7. After elevation of CP-AMPAR transmission has occurred, intra-NAc core injection of the selective CP-AMPAR antagonist naspm markedly reduces cue-induced cocaine-seeking6. These results establish that CP-AMPAR transmission in the NAc mediates the expression of incubation after prolonged withdrawal. Subsequently, we showed that the synaptic incorporation of CP-AMPARs enhances the baseline responsiveness of NAc MSN to glutamate transmission8. Together, these findings suggest that when glutamate is released in the NAc in response to presentation of cocaine-associated cues, MSN are able to respond more robustly due to the presence of CP-AMPARs. This in turn leads to enhanced cocaine-seeking.
The presence of CP-AMPARs in MSN of the NAc can be detected as an elevated rectification index (RI) for evoked AMPAR-mediated EPSCs6–11. Using this measure, we found that CP-AMPAR-mediated transmission becomes elevated after ~1 month of withdrawal and endures through at least withdrawal day (WD) 80 and perhaps much longer5. Thus, once abstinence is achieved, the risk of relapse might be decreased if CP-AMPARs were removed from NAc synapses. In the present study, we approached this goal through mGluR1 positive allosteric modulation. This was inspired by work in other brain regions showing that mGluR1 produces a postsynaptically expressed form of long-term depression (LTD) that relies on removal of CP-AMPARs from synapses12–16. This form of mGluR-LTD had not been demonstrated in MSN of drug-naïve animals – instead, synaptic depression produced by the group I mGluR agonist DHPG depends on mGluR5 and is expressed presynaptically via CB1R-mediated inhibition of glutamate release17,18. However, in NAc slices prepared after incubation and CP-AMPAR accumulation, we found that DHPG-induced synaptic depression in MSN is associated with normalization of the elevated RI through an mGluR1 and postsynaptic PKC-dependent mechanism10. This indicates a dramatic shift in group I mGluR regulation of NAc synaptic transmission after incubation and points to the potential utility of mGluR1-based strategies for reducing incubated cocaine craving.
Based on these findings, we examined the effect of intra-NAc and systemic administration of mGluR1 positive allosteric modulators (PAMs) on cue-induced craving after withdrawal from the extended-access cocaine self-administration regimen leading to incubation. We also conducted measures of CP-AMPAR transmission during withdrawal and after treatment with mGluR1 PAMs or antagonists, as well as biochemical studies of group I mGluRs and Homer signaling proteins. We focused on the NAc core because this subregion is critical in cocaine-seeking elicited by cues19 and, more specifically, blockade of CP-AMPARs in the NAc core is sufficient to prevent expression of incubation6. Our results show that mGluR1 surface expression is decreased during withdrawal, just prior to the onset of increased CP-AMPAR levels, suggesting that mGluR1 normally exerts inhibitory tone on CP-AMPAR levels, which is lost during incubation. Supporting this hypothesis, we found that enhancing mGluR1 transmission during a critical period of withdrawal, through repeated mGluR1 PAM injections, blocked the emergence of enhanced CP-AMPAR transmission and incubation. Conversely, decreasing mGluR1 tone in early withdrawal accelerated CP-AMPAR accumulation. Importantly, mGluR1 stimulation after incubation had occurred was sufficient to reduce CP-AMPAR transmission to control levels and attenuate the expression of incubation. The protective effects of a systemically administered mGluR1 PAM lasted ~1 day after a single injection and ~2–3 days after repeated treatment, suggesting strong translational potential.
RESULTS
mGluR1 activation in NAc core attenuates incubated seeking
All cocaine self-administration groups in this study underwent extended-access cocaine self-administration training (6 h/day for 10 days). Compared to limited-access cocaine self-administration, extended-access regimens produce different or enhanced behaviors that more closely model the compulsive drug-seeking and drug-taking characteristic of addiction20. Furthermore, incubation of cocaine craving is most robust after extended-access regimens, and withdrawal-associated CP-AMPAR accumulation occurs after extended-access, but not limited-access, cocaine self-administration8.
We first asked whether enhancing NAc mGluR1 function in vivo may promote a reduction in CP-AMPAR transmission sufficient to prevent the expression of incubated cocaine craving. Using rats that underwent prolonged withdrawal from the cocaine regimen described above (Fig. 1a,b), hereafter referred to as “incubated rats”, we injected DHPG or vehicle into the NAc core just before a test for cue-induced cocaine-seeking (cannulae placements shown in Supplementary Fig. 1). During the seeking test, nose-poking in the active hole (previously associated with cocaine and cue delivery) now delivers only the cue. Thus, responding under these conditions provides a measure of cue-induced craving. As predicted from DHPG’s ability to attenuate CP-AMPAR-mediated transmission in vitro10, “incubated rats” that received intra-NAc DHPG infusion exhibited significantly reduced cocaine-seeking compared to vehicle-infused rats (t25=2.35, p=0.03) (Fig. 1c). The same effect was produced by intra-NAc infusion of the selective mGluR1 PAM Ro67-7476 (t11=1.95, p=0.04) (Fig. 1d). Neither drug altered inactive hole responding during the seeking test (Fig. 1c,d) or self-administration of a natural reward (sucrose; Supplementary Fig. 2), indicating that their effects on cocaine-seeking were not attributable to nonspecific motor depression or a generalized decrease in motivation to seek reward.
Figure 1. Incubated cocaine-seeking is attenuated by intra-NAc infusion of DHPG or mGluR1 PAMs.
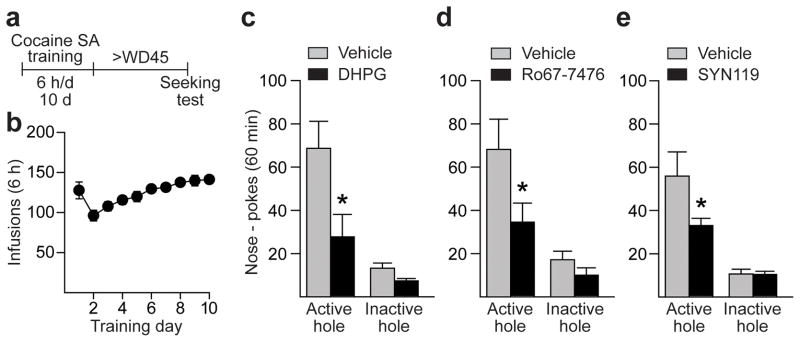
(a) Timeline. SA, self-administration. (b) Training: Nose-pokes in the active hole result in cocaine infusion (0.5 mg/kg) paired with a light cue. (c–e) Seeking tests: Shown are nose-pokes (mean ± s.e.m) in the previously active hole (a measure of cocaine-seeking) and inactive hole during a 60-min test performed under extinction conditions (nose-pokes deliver cue but not cocaine). Infusion of DHPG (0.25nmol/0.5μl/side or 500μM; control, n=16 rats; DHPG, n=11 rats) (c), Ro67-7476 (0.005nmol/0.5μl/side or 10μM; control, n=7 rats; Ro67-7476, n=6 rats) (d), or SYN (0.005nmol/0.5μl/side or 10μM; control, n=7 rats; SYN, n=9 rats) (e) into the NAc core 10 min prior to the test decreased seeking compared to vehicle-infused controls (*p<0.05; see Results for specific p values). Seeking was also reduced significantly by a lower dose of DHPG (0.125nmol/0.5μl/side or 250μM; 41% reduction, t13=2.23, p=0.04 versus vehicle; control, n=8 rats; DHPG, n=7 rats; data not shown).
Systemic SYN119 attenuates seeking and CP-AMPAR function
If mGluR1 PAMs are to be useful therapeutics, they must exert anti-craving effects after systemic administration. We therefore continued our studies using SYN119, a selective mGluR1 PAM that is behaviorally active after systemic injection21. First, we confirmed that SYN119 reduced cue-induced cocaine-seeking after infusion into the NAc of “incubated rats” (t14=2.17, p=0.048) (Fig. 1e). We then conducted MSN recordings in NAc brain slices obtained from “incubated rats” and showed that bath application of SYN119 normalized the elevated RI of AMPAR transmission (exposure x treatment interaction: F1,8=18.04, p=0.003) (Fig. 2a,b) and reduced EPSC-70mV amplitude by ~25% (t5=4.67, p=0.01 vs. baseline, n=6 cells/5 rats; data not shown). This reduction was due to elimination of CP-AMPAR-mediated transmission since bath application of the CP-AMPAR antagonist naspm produced no further synaptic depression (Fig. 2c). In the absence of SYN119, naspm reduced EPSC-70mV amplitude by ~30% in MSNs from “incubated rats” (Fig. 2c). Furthermore, naspm-induced attenuation of EPSC-70mV amplitude was not altered by the presence of a kainate receptor antagonist (Supplementary Fig. 3).
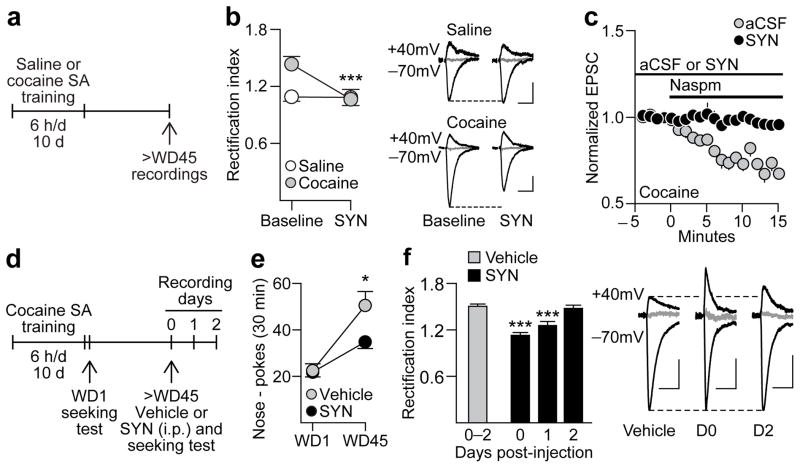
Figure 2. A single systemic injection of the mGluR1 PAM SYN119 reduces cocaine-seeking and CP-AMPAR function.
(a) Timeline for assessing the effects of SYN119 in NAc brain slices obtained following self-administration (SA) of saline (controls) or cocaine (“incubated rats”). (b) Following prolonged withdrawal from cocaine self-administration, MSN exhibit an elevated RI that was normalized by bath-applied SYN119 (1μM, 15 min) ***p<0.001 versus baseline; control, n=4 cells/4 rats; SYN119, n=6 cells/5 rats (left). Example traces (right) illustrate the effects of SYN119 in MSN from controls and “incubated rats” (calibration bar=50 pA, 40 ms). The reversal potentials for both examples are +10 mV. Overall, the reversal potentials did not differ significantly between Saline and Cocaine groups (p=0.10). (c) Naspm (100μM) significantly reduced the RI in the absence of SYN119 (t7=9.15, p<0.001; baseline vs. last 5 min of naspm application), but this was not observed in the presence of SYN119, indicating that SYN119’s ability to normalize the RI is due to elimination of CP-AMPAR transmission (control, n=8 cells/5 rats; SYN119, n=5 cells/4 rats). (d) Timeline for assessing the effects of systemically administered SYN119 (panels e-f). (e) Nose-pokes (mean ± s.e.m) in the previously active hole during seeking tests on WD1 and >WD45. Injection of SYN119 (10 mg/kg i.p.) 20 min before the second test significantly reduced seeking compared to vehicle-injected controls (*p=0.048; control, n=13 rats; SYN119, n=21 rats), although this reduction was partial since WD1 and WD45 differed significantly within the SYN group (*p=0.02). (f) Left: Recordings from a subset of these animals demonstrated that SYN119 normalized the RI for ~24 h. Right: Representative traces of EPSC-70mV, EPSC+40mV and the reversal potential (in gray, 0 mV) for cells from Vehicle, SYN Day 0 (D0), and SYN Day 2 (D2) groups (calibration bar=50 pA, 40 ms). Overall, the reversal potentials did not differ significantly between Vehicle- and SYN-treated groups (p=0.16). ***p<0.001 versus vehicle group; Vehicle, n=11 cells/3 rats; SYN D0, n=12 cells/3 rats; SYN D1, n=12 cells/3 rats; SYN D2, n=4 cells, 2 rats.
Next, we assessed the effect of systemic SYN119 on cocaine craving by administering a single injection of SYN119 or vehicle (i.p.) to “incubated rats” just before a test for cue-induced cocaine-seeking (Fig. 2d). Relative to the vehicle-injected group, cocaine-seeking was significantly blunted by systemic injection of SYN119 (withdrawal day x treatment interaction: F1,32=6.21, p=0.02) (Fig. 2e). As with DHPG and Ro67-7476, this was not a nonspecific effect attributable to depression of motor activity because there was no group difference in inactive hole responding during the seeking test (Supplementary Fig. 4). Furthermore, SYN119’s effect was specific to cocaine-seeking, as rats tested after 10 days of sucrose self-administration and 30 days of withdrawal showed “incubated” cue-induced sucrose seeking that was not reduced by SYN119 injection (Supplementary Fig. 5).
To determine if the reduction in cocaine-seeking after i.p. SYN119 injection was attributable to reduced CP-AMPAR-mediated transmission, recordings were performed in slices prepared from a subset of SYN119- and vehicle-treated rats from the cocaine experiment (Fig. 2d,f). As expected, “incubated rats” injected with vehicle prior to the seeking test exhibited an elevated RI regardless of whether recordings were performed immediately after the test (day 0), one day later (day 1) or two days later (day 2). In contrast, SYN119-injected rats recorded the day of the seeking test (day 0) exhibited a normalized RI (Fig. 2f) resembling that observed in saline animals (see Fig. 2b for comparison). The partial nature of SYN119’s effect on seeking on day 0 (Fig. 2e), in contrast to complete reversal of the elevated RI, could indicate that NAc synapses are strengthened not only by postsynaptic CP-AMPAR accumulation but also by presynaptic adaptations22 that would not be affected by SYN119. The RI measured in SYN119-injected rats remained significantly attenuated on day 1, but returned to the elevated level characteristic of incubation by day 2 (group effect: F3,35=15.44, p<0.001) (Fig. 2f). Thus, a single systemic injection of SYN119 inhibited CP-AMPAR-mediated transmission in NAc synapses for ~24 h.
Decreased surface mGluR1 precedes CP-AMPAR accumulation
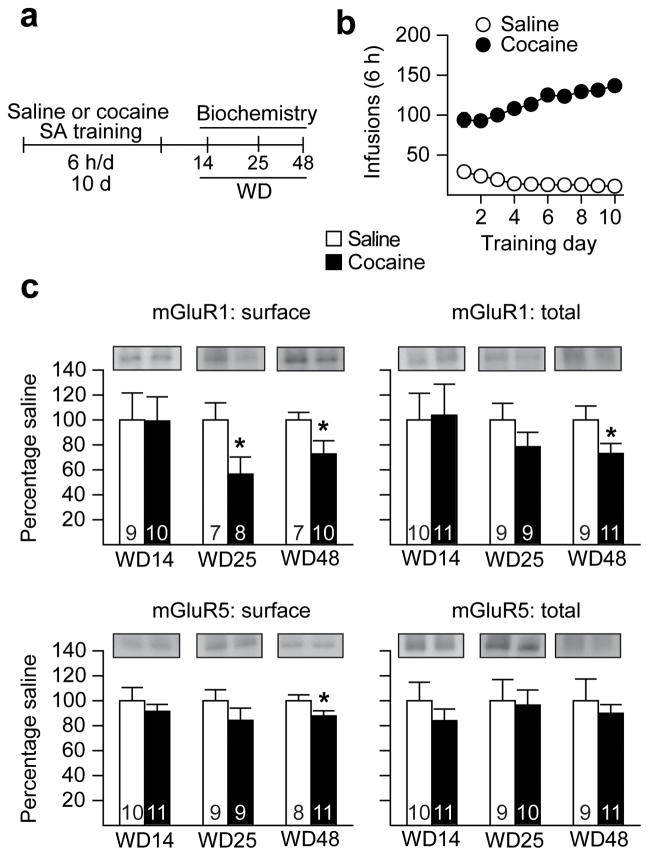
Having shown that mGluR1 activation inhibits CP-AMPAR function and thus reduces craving (Figs. 1 and 2), we hypothesized that mGluR1 normally exerts a “braking” effect on CP-AMPAR accumulation in the NAc. This would explain why CP-AMPARs account for only 5–10% of excitatory transmission in NAc MSN from adult, drug-naïve rats6,23. A similar negative regulatory role for basal mGluR1 transmission has been described in other regions7,15,24. We further hypothesized that, during withdrawal from extended-access cocaine self-administration, a decrease in NAc mGluR1 transmission may occur that removes this “brake”, permitting CP-AMPARs to accumulate. One possible way this could occur is through a decrease in mGluR1 surface expression. To test this possibility, we used biotinylation to measure mGluR1 surface and total protein levels during withdrawal from extended-access cocaine or saline self-administration. Based on the time-course of CP-AMPAR accumulation during withdrawal5, we selected three time-points for study: WD14 (before any change in AMPAR subunit composition), WD25 (when the RI is just beginning to increase) and WD48 (when CP-AMPARs are stably expressed). While saline and cocaine groups did not differ on WD14, the cocaine group exhibited decreased mGluR1 surface expression on WD25 (t13=2.24, p=0.04) and WD48 (t15=1.99, p=0.03) (Fig. 3a–c and Supplementary Fig. 6), suggesting that persistently decreased mGluR1 surface expression during cocaine withdrawal may contribute to CP-AMPAR accumulation. We speculate that the remaining mGluR1 receptors, while insufficient to maintain normal inhibitory tone on CP-AMPAR accumulation, can be stimulated pharmacologically when mGluR1-activating drugs (i.e. SYN119) are applied to a brain slice (Fig. 2) or injected directly into the NAc of an “incubated rat” (Fig. 1).
Figure 3. A withdrawal-dependent decrease in NAc mGluR1 surface expression just precedes CP-AMPAR accumulation.
(a) Timeline. SA, self-administration. (b) Training data for Saline and Cocaine groups (see legend to Fig. 1b for description of training). (c) Cell surface and total protein levels of mGluR1 and mGluR5 were measured on three withdrawal days (WD): before (WD14), at the onset (WD25) and after (WD48) elevation of CP-AMPAR levels. Data are expressed as percent of Saline group (± s.e.m.) at each time-point (n values are provided within each bar). *p=0.04 (surface mGluR1, WD25), *p=0.03 (surface mGluR1, WD48), *p=0.03 (total mGluR1, WD48; t18=2.0), and *p=0.03 (surface mGluR5, WD48) versus respective saline groups. Differences in optical density for Saline groups on different withdrawal days reflect differences in exposure time for different blots rather than time-dependent changes in protein levels after saline self-administration. Full-length blots are presented in Supplementary Figure 9 .
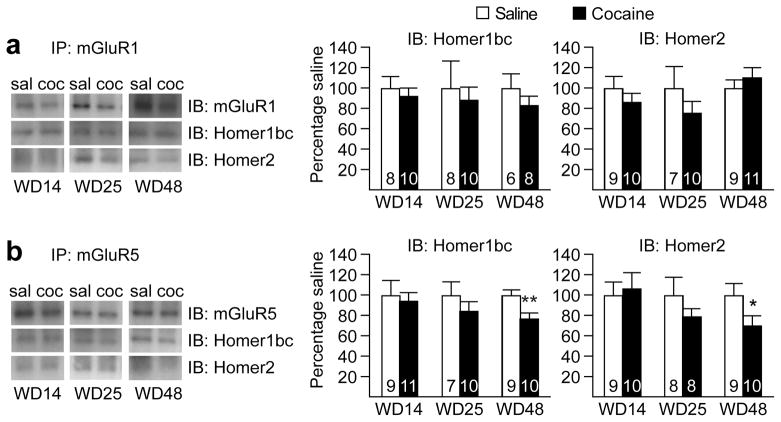
We next examined whether alterations in Homer protein expression or function might further contribute to depressed mGluR1 transmission during withdrawal. The Homer family of postsynaptic scaffolding proteins plays a critical role in group I mGluR signaling by regulating their association with signaling pathways25. Homer proteins are the product of three genes (Homer1-3) that encode several transcriptional variants. Most are long forms that are constitutively expressed (Homer1b–d, Homer2ab, Homer3) but two are truncated forms (Homer1a, ania-3) induced by synaptic activity. In general, the association of long Homers with group I mGluRs favors receptor coupling to IP3 receptor-dependent pathways, whereas short Homers disable this coupling and thus favor regulation of G protein-dependent ion channels26–29. In the NAc, mGluR1 appears to elicit CP-AMPAR removal through the canonical pathway linked to PLC activation, IP3 formation, Ca2+ release and PKC activation10. Thus, impaired signaling through the long Homers which promote this pathway could contribute – along with decreased mGluR1 surface expression – to reduced group I mGluR signaling during incubation. Indeed, there is evidence for decreased long Homer protein levels in the NAc after cocaine exposure25, including decreases in Homer1bc and Homer 2 after extended-access cocaine self-administration30. However, when we assessed Homer1bc and Homer 2 during cocaine withdrawal (WD14, WD25 and WD48), we found no significant changes in total protein levels of these Homers (Supplementary Fig. 7) or their physical association with mGluR1 assessed using co-immunoprecipitation (Fig. 4a and Supplementary Fig. 8). Together, these results suggest that CP-AMPAR accumulation is likely due to the observed decreases in mGluR1 surface expression rather than deficits in Homer signaling.
Figure 4. Prolonged withdrawal from extended-access cocaine self-administration does not alter mGluR1-Homer associations but decreases mGluR5-Homer associations in the NAc.
(a) Co-immunoprecipitation (co-IP) experiments assessing the physical associations between mGluR1 and Homer proteins on withdrawal day (WD) 14, 25 or 48 from extended-access cocaine (coc) or saline (sal) self-administration (see timeline in Fig. 3). No significant changes in association between mGluR1 and Homer proteins were observed at any of the 3 withdrawal time-points. (b) In the case of mGluR5, no change in association with Homer proteins was observed at the two earlier withdrawal time-points (WD14 and WD25). However, a significant decrease in association between mGluR5 and both Homer isoforms was found on WD48 in animals that previously self-administered cocaine. Data are expressed as percent of Saline group (± s.e.m.) at each time-point (n values are provided within each bar). **p=0.01 (Homer1bc) and *p=0.04 (Homer2) versus respective Saline groups. Full-length blots are presented in Supplementary Figure 9.
In light of our previous finding that mGluR5-mediated synaptic depression is disabled in the NAc of “incubated rats”10, it was also of interest to assess mGluR5 levels and Homer coupling. We observed a small but significant decrease in surface mGluR5 (t17=1.96, p=0.03) (Fig. 3c) and a significant decrease in association between mGluR5 and both Homer1bc (t17=2.75, p=0.01) and Homer2 (t17=1.91, p=0.04) on WD48 (Fig. 4b). We note that interpretation of the latter result is complex because uncoupling of Homers and group I mGluRs can both enhance and reduce signaling31.
The level of mGluR1 transmission regulates incubation
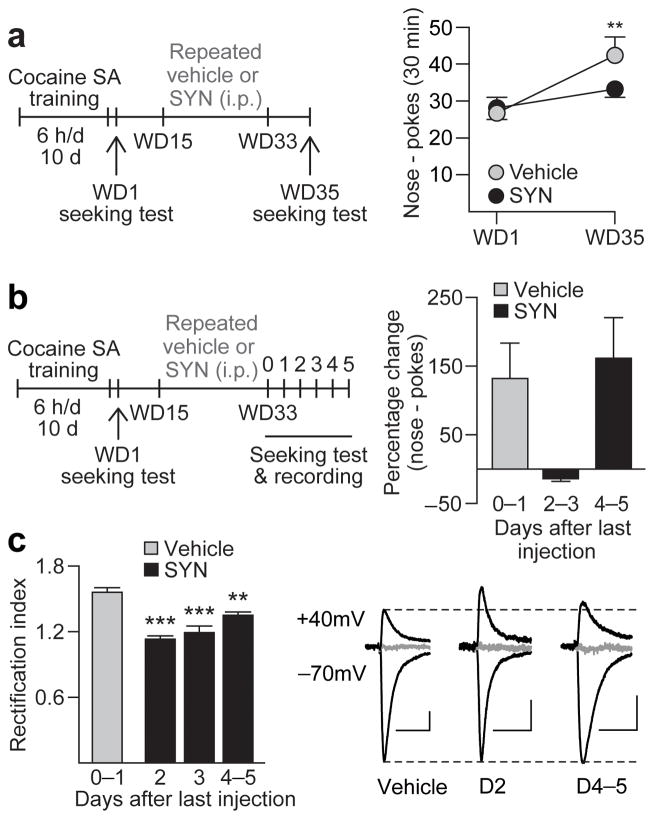
Given that surface expression of mGluR1 decreases at approximately the same withdrawal time as CP-AMPARs begin to accumulate (Fig. 3), we wondered whether potentiating mGluR1 transmission during this critical period would block CP-AMPAR accumulation and inhibit incubation of cocaine craving. Thus, after completion of cocaine self-administration training, rats were randomly assigned to receive vehicle or SYN119 injections every other day from WD15 through WD33. These rats were tested for cue-induced cocaine-seeking on WD1, before vehicle or SYN119 exposure, and again on WD35, two days after the last injection (Fig. 5a). Vehicle-treated rats exhibited increased seeking on WD35 compared to WD1, i.e., incubation occurred as expected (main effect of test day: F1,24=14.84, p<0.001). In contrast, rats treated with SYN119 during withdrawal did not exhibit incubation of cocaine-seeking between WD1 and WD35, indicating that enhancing mGluR1 transmission during cocaine withdrawal is sufficient to prevent incubation (Fig. 5a).
Figure 5. Repeated SYN119 injections during withdrawal interfere with CP-AMPAR accumulation and block incubation of cocaine craving.
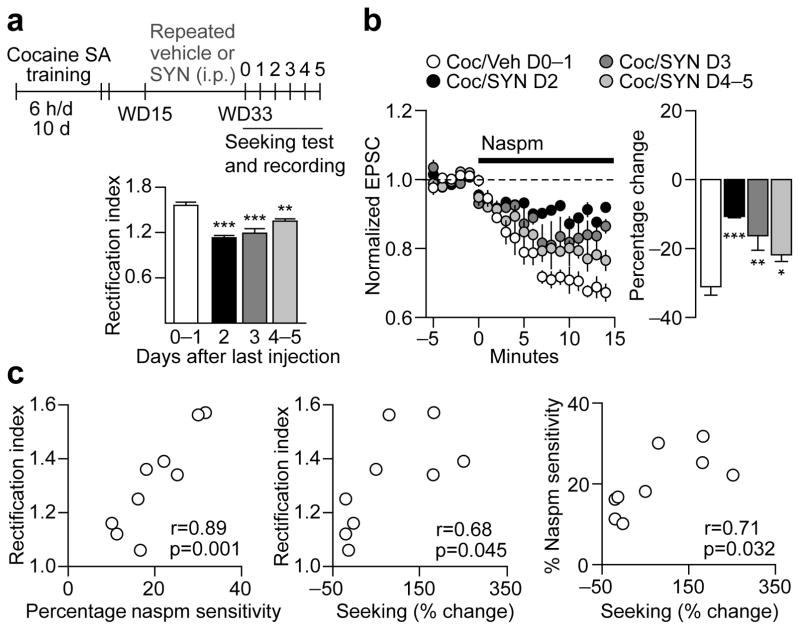
(a) Timeline summarizing the experimental design used to assess the effects of repeated SYN119 injections. Vehicle or SYN119 (10 mg/kg i.p.) was injected every other day from withdrawal day (WD) 15 to WD33 following cocaine self-administration (SA) (a, left). Repeated SYN119 injections blocked incubation of cocaine craving [a, right; data are shown as average number of nose-pokes (± s.e.m.) during seeking tests on WD1 and WD35; **p=0.005 versus WD1; n=13 rats per group]. (b,c) To determine the duration of SYN119-mediated attenuation of incubation and its mechanism, seeking tests and patch-clamp recordings were conducted at variable times after the last vehicle or SYN119 injection (b, left). SYN119-mediated reduction of cocaine-seeking (b, right; data expressed as percent change from WD1 test) and the RI (c, left) persisted 2–3 days. Representative traces (c, right) of EPSC-70mV, EPSC+40mV and the reversal potential (in gray, +5 mV) for cells from Repeated Vehicle, SYN Day 2 (D2), and SYN Day 4–5 (D4–5) groups (calibration bar=50 pA, 40 ms). Overall, the reversal potentials did not differ significantly between Vehicle- and SYN-treated groups (p=0.49). **p=0.002, ***p<0.001 versus vehicle controls; Repeated vehicle, n=6 cells/2 rats; SYN D2, n=4 cells/2 rats; SYN D3, n=4 cells/2 rats; SYN D4–5: n=6 cells/3 rats.
To determine whether repeated SYN119 treatment during withdrawal prevents incubation by opposing CP-AMPAR accumulation, rats received the same regimen of repeated SYN119 or vehicle (every other day, WD15-33) and were then tested for cue-induced cocaine-seeking at variable times after the last injection (0–5 days) (Fig. 5b). Immediately after each test, slices were prepared for whole-cell patch-clamp recording. After repeated vehicle treatment during withdrawal, rats displayed incubated cocaine-seeking compared to WD1 (Fig. 5b) and an elevated RI (Fig. 5c and Fig. 6a), indicating CP-AMPAR accumulation. Remarkably, repeated SYN119 treatment on WD15-33 blocked incubation and reduced the RI when these endpoints were assessed 2–3 days after the last SYN119 injection (group effect: F3,16=25.09, p<0.001; Fig. 5b,c and Fig. 6a). This attenuated RI was due to reduced CP-AMPAR-mediated transmission because naspm sensitivity was significantly reduced in these animals compared to “incubated rats” treated with vehicle (group effect: F3,15=11.17, p<0.001; Fig. 6b). A significant correlation was also observed between levels of cocaine-seeking, naspm sensitivity and CP-AMPAR transmission (Fig. 6c). However, 4–5 days after discontinuing repeated SYN119 injections, both seeking and CP-AMPAR levels recovered towards incubated levels (Fig. 5b,c and Fig. 6a,b). Thus, potentiation of mGluR1 transmission during withdrawal, via repeated systemic injections of an mGluR1 PAM, reduced cocaine craving by blocking CP-AMPAR accumulation in the NAc, an effect that lasted for ~2–3 days.
Figure 6. Correlations between effects of SYN119 on rectification index (RI), naspm sensitivity, and cue-induced cocaine-seeking.
(a) Timeline (SA, self-administration) and data demonstrating changes in RI (from Fig. 5) following repeated SYN119 treatment. (b) To further assess the contribution of CP-AMPARs to the changes in RI shown in (a), the CP-AMPAR antagonist naspm (100μM) was bath-applied for 15 min and EPSC amplitude was measured at a holding potential of -70mV (EPSC-70mV; data are normalized to pre-naspm baseline; b, left). As expected, naspm decreased EPSC-70mV by ~30% in cocaine rats that received repeated vehicle injections (b, right). In contrast, naspm sensitivity was significantly reduced in rats recorded 2–3 days after the last SYN injection (b, right), a time when the RI was also reduced (panel a and Fig. 5c) and cue-induced cocaine-seeking was below incubated levels (Fig. 5b). By 4–5 days following the last SYN injection, the RI and naspm sensitivity had returned to levels more similar to those observed in cocaine/vehicle (Coc/Veh) animals (a,b), as had cue-induced cocaine-seeking (Fig. 5b). ***p<0.001, **p=0.003, *p=0.04 versus Coc/Veh; Coc/Veh, n=5 cells/2 rats; SYN D2, n=4 cells/2 rats; SYN D3, n=4 cells/2 rats; SYN D4–5, n=6 cells/3 rats. (c) Analyses of correlations between cocaine-seeking (measured as percent change from active hole responses on WD1), RI and naspm sensitivity for all rats shown in Fig. 5b,c. As expected, naspm-sensitivity was highly correlated with RI values (c, left), indicating that an elevated RI is due to enhanced CP-AMPAR-mediated transmission. In addition, cocaine-seeking correlated with both RI and naspm sensitivity (c, middle and right).
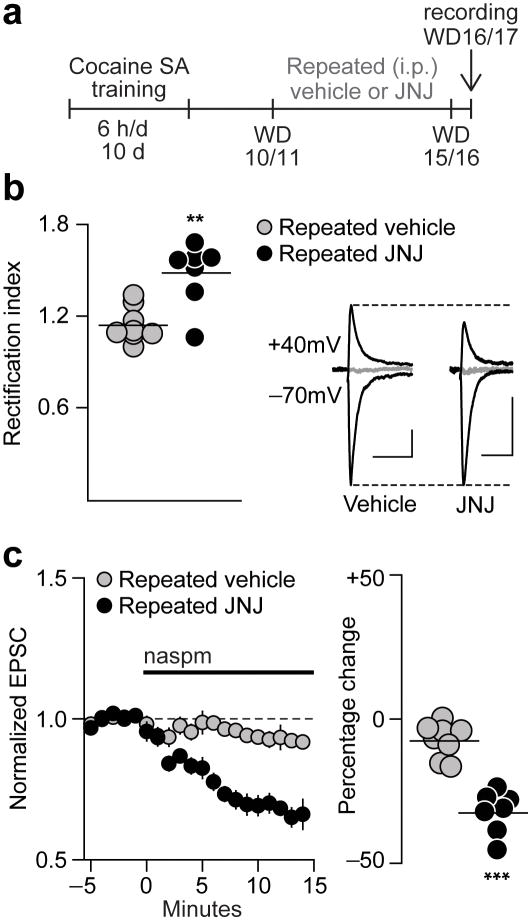
If mGluR1 normally exerts inhibitory tone on CP-AMPAR accumulation, then decreasing mGluR1 transmission very early in withdrawal should accelerate CP-AMPAR accumulation. To test this, rats that had self-administered cocaine received daily injections of an mGluR1 antagonist (JNJ16259685; 5 mg/kg, i.p.) or vehicle on WD10-15 (2 rats) or WD11-16 (1 rat), and patch-clamp recordings were performed 1 day after the last injection (WD16 or WD17) (Fig 7a). Normally, this is too early in withdrawal for detection of increased CP-AMPAR levels5. However, after repeated JNJ injections, recordings on WD16-17 revealed elevation of both the RI (F1,13=15.20, p=0.002) and naspm sensitivity (F1,13=52.12, p<0.001) (i.e., a profile identical to that of “incubated rats” recorded after >WD45) (Fig. 7b,c).
Figure 7. The onset of CP-AMPAR accumulation in the NAc is accelerated by decreasing mGluR1 tone during early withdrawal.
(a) Timeline (SA, self-administration). Following completion of cocaine self-administration training, rats received daily injections with vehicle or an mGluR1 antagonist (JNJ16259685; 5 mg/kg, i.p.) on WD10-15 (2 rats) or WD11-16 (1 rat) and patch-clamp recordings were performed 1 day after the last injection (WD16 or WD17). (b) Left: In rats treated with vehicle during withdrawal, the RI on WD16-17 is similar to saline controls, whereas it is elevated after repeated JNJ injections. Each circle represents one cell. Lines are means for each group. Right: Representative traces of EPSC-70mV, EPSC+40mV and the reversal potential (in gray, 0 mV) for cells from Cocaine/Vehicle (Vehicle) and Cocaine/JNJ (JNJ) groups (calibration bar=50 pA, 40 ms). Overall, the reversal potentials did not differ significantly between the groups (p=0.18). (c) Repeated JNJ injections also increased naspm sensitivity on WD16-17 compared to the Coc/Vehicle group. Data are shown as group average EPSC-70mV amplitude (± s.e.m.) before (5 min) and during (15 min) Naspm application (left) and as the average percentage change from baseline during the last 5 min of Naspm application (right; each circle represents one cell; lines are means for each group) ***p<0.001, **p=0.002 versus Vehicle; Vehicle, n=8 cells/3 rats; JNJ, n=7 cells/3 rats.
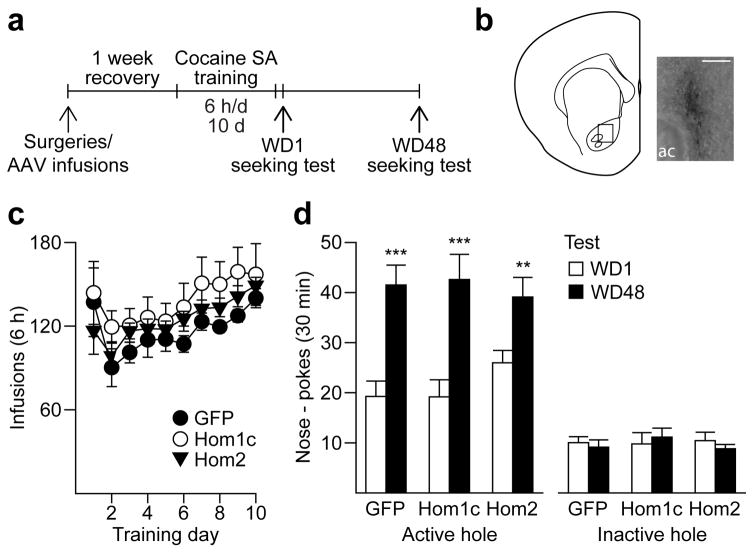
Prior studies in animal models of cocaine and alcohol addiction have shown that genetically altering long Homer expression in the NAc can mimic or disrupt drug-induced alterations in group I mGluR-mediated signaling pathways and modify behavioral responding to and or intake of the drug25,32–33. Since long Homer isoforms potentiate certain group I mGluR-mediated signaling pathways (see previous section), we assessed whether we could mimic the effect of repeated mGluR1 PAM treatment (Fig. 5) by over-expressing long Homers in the NAc during withdrawal (Fig. 8a–c). We used previously characterized adeno-associated viruses (AAV) to over-express Homer1c, Homer2 or GFP (control). We found that animals in all 3 groups demonstrated equivalent incubation of cocaine craving (main effect of test day, F1,25=47.47, p<0.001) (Fig. 8d). These results indicate that Homer over-expression does not mimic the effects of repeated mGluR1 potentiation during withdrawal and that enhancing mGluR1 transmission using a PAM is a more promising therapeutic strategy.
Figure 8. Homer over-expression in the NAc core does not affect incubation of cue-induced cocaine-seeking.
(a) Timeline (SA, self-administration). To assess the effects of Homer overexpression on incubation of cocaine craving, viruses (AAV-GFP, Homer1c or Homer2) were injected into the NAc core at the same time that animals underwent jugular catheterization surgery, allowing Homer expression to peak during early withdrawal. (b) Staining for the HA tag on cDNA-Homer1c verified localized overexpression in the NAc core (20X magnification; image taken ~3 months after virus injection; scale bar, 100μm; ac, anterior commissure). (c) Following ~1 week of recovery from surgeries, animals self-administered cocaine (0.5 mg/kg) for 6 h/day for 10 days. No difference in cocaine intake (average infusions obtained each day ± s.e.m.) was observed between the 3 groups. (d) All animals showed an increase in cue-induced cocaine-seeking on WD48 compared to WD1 (i.e., incubation), regardless of which virus infusion they received. ***p<0.001, **p=0.01, WD1 versus WD48 (ANOVA followed by least significant difference post-hoc comparisons); GFP, n=10 rats; Homer1c, n=8 rats; Homer2, n=10 rats.
DISCUSSION
In this study, we demonstrate that mGluR1 receptors in the NAc negatively regulate CP-AMPAR levels and thereby reduce cue-induced cocaine craving. First, we showed that intra-NAc infusions of the group I agonist DHPG or an mGluR1 PAM (Ro67-7476 or SYN119) attenuated the expression of incubated cue-induced cocaine-seeking. Next, in behavioral studies followed by patch-clamp recordings, we showed that acute systemic administration of SYN119 reduced the expression of incubated cocaine-seeking and removed CP-AMPAR transmission from NAc synapses. This protective effect persisted for ~1 day. Control experiments showed that the same SYN119 treatment that reduced incubation of cocaine-seeking was without effect on incubation of sucrose seeking. This is in agreement with a prior study34 indicating selectivity of GluA1-related adaptations for cocaine versus sucrose withdrawal. Based on these findings, we hypothesized that mGluR1 normally exerts inhibitory tone on CP-AMPAR levels in NAc synapses and that loss of mGluR1 tone during cocaine withdrawal enables CP-AMPAR accumulation. Indeed, we found that NAc mGluR1 surface expression was normal on WD14 but decreased by WD25 (i.e., just preceding CP-AMPAR accumulation), although associations with Homer proteins were apparently not altered. Restoring mGluR1 tone during this period by administering repeated, intermittent injections of SYN119 blocked CP-AMPAR accumulation and the incubation of cue-induced cocaine-seeking, effects which persisted 2–3 days after the last PAM injection. Conversely, pharmacological reduction of mGluR1 transmission during an early withdrawal period prior to the decrease in mGluR1 surface expression accelerated CP-AMPAR accumulation. Together, these results suggest that a recovering addict could take an mGluR1 PAM prior to an anticipated encounter with cocaine-associated cues and be afforded a reasonable period of protection. Although a more persistent effect would be desirable, it is revealing that both CP-AMPARs and craving recover to incubated levels within days of discontinuing PAM exposure. This indicates that mGluR1 tone dictates whether CP-AMPARs and incubated craving can emerge, but an undiscovered underlying mechanism must drive both phenomena. While suppressed during repeated mGluR1 PAM exposure, this mechanism re-engages within days of discontinuing SYN119 treatment.
In the same animals used for analysis of mGluR1 expression during withdrawal, mGluR5 surface expression was unchanged on WD14 and WD25; however, a modest decrease was observed on WD48, along with decreased association between mGluR5 and long Homer proteins measured by co-IP. These results provide a possible explanation for the impairment of mGluR5-dependent synaptic depression observed in the NAc of “incubated rats” at these late withdrawal times10. There are other reports of attenuated mGluR-LTD in the NAc after cocaine exposure35–38, but these studies used different cocaine regimens and withdrawal times, so our biotinylation and co-IP data are not necessarily applicable.
A large number of prior studies have suggested that anti-addictive effects in animal models can be achieved by blocking group I mGluRs (primarily mGluR5)39 rather than enhancing mGluR1 transmission as in the present study. However, none of these prior studies were conducted in animals that had undergone extended-access cocaine self-administration and prolonged withdrawal. Both conditions must be met for CP-AMPARs to accumulate in the NAc of adult rats. Thus, CP-AMPAR accumulation is not observed, regardless of withdrawal time, after experimenter-administered cocaine or limited-access cocaine self-administration8,11 or even after short withdrawals from extended-access cocaine self-administration5,6. In the absence of CP-AMPARs, no anti-addictive effects of mGluR1 PAMs would be anticipated. Instead, mGluR1 blockade may be beneficial (e.g., 40) by tapping into the same mechanisms achieved with mGluR5 blockade. In young animals, CP-AMPAR plasticity appears to be induced more readily7. However, based on our results in adult rodents, mGluR1 PAM-based therapies would selectively benefit addicts who have achieved abstinence and seek to sustain it, whereas different approaches would be preferred in other situations, such as when an addict is still using or just attempting to abstain39,41. We recognize liabilities and mixed clinical results obtained with previous glutamate-based addiction treatments41, but mGluR1 PAMs may represent an improvement due to their selectivity for a single target (vis-à-vis non-selective ion channel blockers such as gabapentin) and duration of effect (e.g., acamprosate must be taken 3 times/day).
While we have focused on effects of mGluR1 PAMs mediated within the NAc, their effects in the VTA would also be expected to benefit cocaine users. After many types of cocaine exposure (even a single injection), CP-AMPARs are inserted into synapses onto VTA dopamine neurons in exchange for lower conductance CI-AMPARs, resulting in synaptic potentiation13,14,42. This is reversed by acute mGluR1 stimulation, that is, CP-AMPAR transmission is decreased and CI-AMPAR transmission is enhanced, resulting in LTD7,12–14. Furthermore, the level of mGluR1 tone in the VTA determines the duration of CP-AMPAR elevation in the VTA, e.g., decreasing mGluR1 tone permits CP-AMPARs to persist for a longer time7. Importantly, mGluR1 receptors in the VTA also regulate CP-AMPAR plasticity downstream in the NAc. Thus, while a single cocaine injection is not sufficient to increase CP-AMPAR levels in the NAc shell of young mice, it becomes sufficient after interruption of coupling between VTA group I mGluRs and Homer1bc7. Combined with the present findings, these results predict that mGluR1 PAMs would oppose addiction-promoting plasticity in the VTA and NAc of human cocaine users. They would also have potential benefits with regard to cognitive deficits associated with cocaine use43.
It is intriguing that, when CP-AMPARs are present, mGluR1 receptors exhibit a unique ability to remove them from synapses. This is true not only in the VTA and NAc (above), but also in other brain regions44. For example, mGluR1 stimulation results in LTD by increasing CI-AMPAR and reducing CP-AMPAR synaptic transmission in cerebellar stellate cells15. In the lateral amygdala, mGluR1-LTD mediated by CP-AMPAR removal has been implicated in extinction of fear memories16. The cellular mechanisms underlying these effects of mGluR1 stimulation are best understood in the VTA, where strong evidence supports internalization of CP-AMPARs and translocation of CI-AMPARs from intracellular compartments to the synapse14. It is likely that a similar exchange occurs in the NAc10, although it may not be driven by increased GluA2 synthesis as seen in the VTA5. In stellate cells, lateral diffusion of CI-AMPARs into the synapse may contribute to enhanced CI-AMPAR transmission45.
In light of evidence that D1 receptor- and D2 receptor-expressing MSN in the NAc may contribute differently to cocaine-related behaviors46,47, it may seem surprising that we observe CP-AMPAR accumulation in nearly all MSN in the NAc core of “incubated rats”11 and present results. An important consideration is that CP-AMPAR accumulation in the NAc is first detected ~1 month after the last cocaine self-administration session. Based on this delay, we propose that CP-AMPAR accumulation is more likely to reflect withdrawal-dependent changes in glutamate transmission onto MSN (for example, a decrease in transmission that triggers homeostatic plasticity) as opposed to differential activation of D1 and D2 receptors during the period of cocaine self-administration5. Nevertheless, while our recordings establish that nearly all MSN in the NAc core exhibit CP-AMPAR plasticity, it remains to be determined if CP-AMPARs accumulate at most spines or whether they are selectively positioned to respond to particular afferents. Very recently, it was shown that CP-AMPAR insertion during incubation leads to unsilencing of cocaine-generated silent synapses in the amygdala-accumbens projection48. It will be important to determine if this occurs in other pathways, and how mGluR1 and silent synapse-based reorganization interact to shape excitatory synaptic transmission in the NAc.
In conclusion, administration of mGluR1 PAMs to “incubated rats” reduced cue-induced cocaine craving by inhibiting CP-AMPAR-mediated synaptic transmission in the NAc. Furthermore, repeated mGluR1 PAM injections during a critical period of withdrawal opposed the decrease in surface mGluR1 that normally occurs, thus maintaining mGluR1-mediated inhibitory control over CP-AMPAR accumulation and delaying incubation. These results suggest that mGluR1 PAMs could be used by recovering addicts to control cue-induced craving and prolong abstinence. This has major translational significance as no treatment is presently available that provides such protection.
METHODS
Subjects and surgery
Adult male Sprague-Dawley rats (Harlan) weighing 250–275 g (~8 weeks old) upon arrival were housed on a reverse 12 h light:dark cycle (lights off at 7:00 AM). They were acclimated to the animal facility for ~7 days before undergoing surgery for intravenous catheter implantation as described previously6,8,9,10,11. Following surgery, rats were singly housed. For studies combining intracranial injection with drug self-administration, rats were implanted with guide cannulae positioned 1.5 mm above the NAc core subregion6. During the recovery phase (~7 days) and subsequent self-administration training phase, catheters were flushed every 24–48 h with 0.9% sterile saline and the antibiotic cefazolin (15 mg, i.v.; Webster Veterinary Supply). Behavioral testing began within ~1 hour of the start of the dark cycle. All procedures were performed according to the USPHS Guide for Care and Use of Laboratory Animals, and were approved by the Rosalind Franklin University of Medicine and Science Institutional Animal Care and Use Committee.
Drugs
Cocaine HCl was obtained from the National Institute on Drug Abuse and dissolved in 0.9% saline. The selective mGluR1 positive allosteric modulator (PAM) SYN119 (9H-Xanthene-9-carboxylic acid (4-trifluoromethyl-oxazol-2-yl)-amide), also referred to as compound 14a49 or Ro071140121, was synthesized by EAG labs. For systemic administration, SYN119 was dissolved in vehicle consisting of 20% 2-hydroxypropyl-β-cyclodextrin (#332593, Sigma-Aldrich) in 0.9% saline. For patch-clamp recordings or intracranial injection, SYN119 and Ro67-7476 (kindly provided by Dr. Jeffrey Conn, Vanderbilt University) were dissolved in 100% DMSO as a stock solution (stored at −20 °C) and used at a final concentration of ≤ 0.05% DMSO. The aCSF for intracranial injections contained (in mM) 145 NaCl, 2.7 KCl, 1.0 MgCl2, 1.2 CaCl2, 2.0 NaH2PO4, and 2.0 Na2HPO4. DHPG (Sigma-Aldrich) was dissolved in this same aCSF solution for intracranial injection studies. JNJ16259685 (Tocris) was dissolved in the same vehicle as SYN119.
Cocaine self-administration training
Rats were randomly assigned to self-administer cocaine (0.5 mg/kg/infusion in a 100 μl/kg volume over 3 sec) or saline for 10 days (6 h/day) under a fixed ratio 1 schedule as described previously6,8,9,10,11. Briefly, self-administration chambers (MED Associates) were located in sound-attenuating cabinets and were equipped with two holes (active and inactive) located on opposite sides of the chamber, approximately 2 cm above the floor. Nose-poking in the active hole activated the pump and delivered an infusion of saline or cocaine paired with a 20-sec light cue located inside the nose hole and a 20-sec time-out period. Nose-poking in the inactive hole was without consequence. For controls in the electrophysiology experiments, we used a combination of rats that self-administered saline and “control” rats that were treated identically except that they were not exposed to the self-administration chambers. Instead, after recovery from catheter implantation surgery, they were brought to holding cages away from their colony room for 6 h/day for 10 days, thus receiving the same surgery, recovery, time away from the colony and handling as self-administration groups. Our previous work has shown that NAc AMPAR transmission in these animals is identical to that observed in saline self-administering controls9 and in drug-naïve rats (unpublished findings).
Tests for cue-induced cocaine-seeking
Rats were divided into groups matched for average number of infusions and active lever responses during training. Any rats that did not learn to self-administer cocaine and/or had faulty catheters were excluded from the study (8 rats). Rats were returned to the self-administration chambers on the specified withdrawal day and tested for 30 or 60 min under extinction conditions, i.e., nose-pokes in the active hole resulted in presentations of the light cue previously paired with cocaine infusions but no cocaine. The number of responses in the previously active hole was used as a measure of cocaine-seeking or craving. For studies in which vehicle or PAM was injected intracranially prior to a test for cue-induced cocaine-seeking (Fig. 1), we used a between-group design. Rats underwent withdrawal in their home cage for 45 or more days and were then injected with vehicle, DHPG (0.25 nmol/0.5 μl/side; 500 μM), Ro67-7476 (0.005 nmol/0.5 μl/side; 10 μM) or SYN119 (0.005 nmol/0.5 μl/side; 10 μM) into the NAc core 10 min before the test session (60 min) as described previously6; during the 10-min post-injection period, they were returned to their home cage. In most cases, rats underwent two seeking tests over a two week period, with at least 4 days between intracranial injections. Vehicle and drug injection groups were counterbalanced in such studies. Only rats with bilateral placements in the NAc core were included in the final analysis. As a result, 10 animals were excluded. For studies that evaluated the effect of systemic SYN119 treatment (Fig. 2), a within-subject design was used. Rats were tested for cue-induced cocaine-seeking (30 min) twice – first on WD1 (no treatment was administered) and again on or after WD45. Twenty minutes prior to this second seeking test, rats received a systemic vehicle or SYN119 (10 mg/kg, i.p.) injection; during the 20 min post-injection period, they were returned to their home cage. This 20 min period was determined based on the half-life of SYN119 (~55 min)49 such that the animals would be tested during the period when the drug should be most effective. For experiments in which SYN119 was administered repeatedly during withdrawal (Figs. 5 and 6; see next section for repeated treatment paradigm), rats were tested for cue-induced cocaine-seeking (30 min) on WD1 (no treatment was administered) and again 0–5 days following the last repeated SYN119 or vehicle injection.
Repeated vehicle, SYN119 or JNJ16259685 during withdrawal
Rats received an injection of SYN119 (10 mg/kg, i.p.) or vehicle every other day between WD15 and WD33, resulting in a total of 10 injections. These injections were performed in home cages during the dark cycle. In behavioral experiments, cue-induced cocaine-seeking tests were performed 2 days following the last repeated SYN or vehicle injection, i.e., WD35 (Fig. 5a). For experiments in which seeking tests were followed by patch-clamp recordings (Fig. 5b,c), treatments were staggered such that only one rat would complete the injection protocol on each recording day. Thus, several rats received 1–3 additional SYN119 or vehicle injections (total of 10–13 injections, every other day) so that they could be recorded on specific days after discontinuing SYN119 or vehicle treatment. In another cohort of rats (Fig. 7), daily injections of either vehicle or the mGluR1 antagonist JNJ16259685 (5 mg/kg, i.p.) were performed on WD10-16, and the presence of CP-AMPAR transmission was determined in patch-clamp recordings conducted 1 day after the last injection, a withdrawal time prior to the normal onset of CP-AMPAR accumulation5.
Biotinylation and co-immunoprecipitation
As described previously6,9, a punch was used to obtain bilateral NAc samples consisting primarily of core (total wet weight of ~15 mg). Lateral shell is also included but this is not believed to compromise the detection of effects relevant to CP-AMPAR accumulation because CP-AMPARs accumulate in both subregions during incubation6,7,11. NAc samples were biotinylated and processed to separate biotinylated proteins bound to NeutrAvidin beads (bound material; surface-expressed proteins) from the non-biotinylated (unbound) material9. An aliquot of starting material (NAc homogenate) from the same animals was used for co-immunoprecipitation (IP) experiments to measure changes in the association between mGluR1 or mGluR5 and Homer proteins. As described previously6,23, 3 μg of antibody (mGluR1a, 2031, PhosphoSolutions or mGluR5, AB5675, Millipore) was incubated overnight at 4 °C with protein A agarose slurry. The pellet containing antibody-coated beads was collected by centrifugation, washed in PBS and incubated overnight at 4 °C with 100 μg of NAc tissue. The agarose-bound antibody was then pelleted by centrifugation. This process produced two fractions: bound (pellet) and unbound (supernatant). The bound fraction was dissolved in 2X Laemmli sample buffer with 100mM DTT (using a volume equal to that of the input tissue volume). The process was repeated to maximize recovery.
Immunoblotting
All samples were processed and used for immunoblotting as described previously6,9,23. For immunoblotting of biotinylated tissue, the following primary antibodies were used: mGluR1 (1:2,000, 610965, BD Biosciences), mGluR5 (1:20,000, AB5675, Millipore), Homer1bc (1:20,000, sc-20807, Santa Cruz), and Homer2ab (1:500, sc-8924, Santa Cruz). For immunoblotting after IP, the same primary antibodies were used with two exceptions: mGluR1a (1:3,000; 2031-mGluR1a, PhosphoSolutions) and Homer2 (1:350, H00009455-B01P, Abnova). A higher concentration of Homer1bc (1:2,000) antibody was also used. In addition, we employed anti-rabbit or mouse IgG light-chain specific secondary antibodies (211-032-171 or 115-035-174, Jackson Immuno Research Laboratories) for co-IP studies so that the heavy chain (~50 kDa) did not obscure the Homer band (~45kDa). For mGluR antibodies, the mGluR1a and mGluR5 dimer band (~350 kDa) was analyzed because it represents the functional pool of these receptors50. Validation is provided on the supplier’s website for all antibodies utilized, as well as on Antibodypedia for Homer1bc (Santa Cruz) and Homer2 (Abnova). Immunoblots were analyzed with TotalLab (Life Sciences Analysis Essentials) as described previously6,9,11,23. Data were excluded only if there were bubbles/imperfections in the band which interfered with its analysis. The number of bands excluded varied for each antibody, fraction (surface or total), and time-point. The range is as follows: WD14, 0-2 bands; WD25, 0-4 bands; and WD48, 0-3 bands.
Electrophysiology
All electrophysiological procedures were conducted as previously described10,11. Briefly, 300 μm-thick coronal brain slices containing the NAc were cut and incubated in 95% O2-5% CO2 oxygenated artificial CSF (aCSF; in mM: 122.5 NaCl, 20 glucose, 25 NaHCO3, 2.5 KCl, 0.5 CaCl2, 3 MgCl2, 1 NaH2PO4, 1 ascorbic acid) for at least 1 h (32–34 °C). For recordings, CaCl2 was increased to 2.5 mM, MgCl2 was reduced to 1 mM and 0.1 mM picrotoxin was added along with 0.05 mM (2R)-amino-5-phosphonopentanoate (APV). All recordings were conducted at 32–34 °C using patch pipettes (6–8 MΩ) filled with a cesium-based/spermine-containing solution (in mM: 140 CsCl, 10 HEPES, 2 MgCl2, 5 NaATP, 0.6 NaGTP, 2 QX-314, 0.1 spermine) in voltage-clamp mode. A bipolar tungsten stimulating electrode placed ~300 μm from the recording site was used to elicit EPSC in NAc MSNs. Only neurons that exhibited a stable synaptic response at the −70 mV holding potential (<15% variability in EPSC-70mV amplitude) during 15 min of baseline recording were included. In the present study, both the rectification index (RI = [EPSC-70mV/(− 70 − Erev)]/[EPSC+40mV/(+ 40 − Erev)]) and the sensitivity to the CP-AMPAR antagonist naspm (100 μM) were assessed across treatment groups to determine the contribution of CP-AMPARs to synaptic transmission. Typically, 2 sets of depolarizations were applied to assess the reversal potential of the evoked response and the amplitude of the EPSC+40mV during baseline and following bath application of SYN119.
AAV construction, infusion, and behavioral testing
Recombinant adeno-associated viruses (rAAV) were constructed as described previously32,33,51,52. Briefly, AAVs carrying equal ratios of AAV1 and AAV2 capsid proteins were used to express either hemagglutinin (HA)-tagged Homer1c or Homer2 or enhanced green fluorescent protein (GFP) under the control of the chicken β-actin promoter. Prior studies using these viruses have shown that transgene expression is first observed after ~7 days, reaches maximal levels by ~3 weeks post-infection, and then persists32,33,51,52. Therefore, as shown in the timeline in Fig. 8a, we injected the viruses (AAV-GFP as a control, Homer1c or Homer2) into the NAc core on the same day as the jugular catheterization surgery so that Homer expression would peak during early withdrawal (WD1 is ~17 days post-infection). Rats received stereotaxic injections of virus (0.5 μl/side) into the NAc core over 5 min. Injectors were left in place for 5 min. Approximately 1 week later, rats began cocaine self-administration training (6 h/day for 10 days). Rats were tested for cue-induced cocaine-seeking on WD1 and again on WD48. At the end of the experiment, AAV transfection was verified by immunostaining for the HA tag and for GFP32,33,52.
Statistical analyses
The data from the extinction tests for cue-induced cocaine-seeking were analyzed using repeated measures Analysis of Variance (ANOVA) with treatment (vehicle or SYN119) as the between-subjects factor and withdrawal day (1, >34) as the within-subjects factor, followed by Sheffé post hoc comparisons, unless otherwise specified. For electrophysiological studies in which SYN119 was bath-applied, data were analyzed using a 2-way repeated measures ANOVA with drug exposure (saline, cocaine) as the between-subjects factor and time (before or after drug application) as the within-subjects factor, followed by least significant difference post hoc comparisons. Naspm sensitivity was assessed using a paired t-test (5 min of baseline versus last 5 min of napsm application). For electrophysiological studies conducted after in vivo SYN119 or JNJ16259685 administration, data were analyzed using a 1-way ANOVA followed by a Dunnett’s test (versus vehicle group) when appropriate. Pearson correlation calculations were used for correlational analysis of electrophysiological and behavioral data. For biochemical studies, independent sample t-tests were used to assess group differences (cocaine versus saline) in protein levels. All t-tests were two-tail with the exception of those conducted for Fig. 1d, Fig. 3c (WD48, mGluR1 total and mGluR5 surface) and Fig. 4b (WD48, mGluR5/Homer2). Data were normally distributed in all cases, as assessed using the Kolmogorov-Smirnov test. Data collection and analysis were not performed blind to the conditions of the experiments. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in our previous studies6,8,9,10,11 and other publications in the field30,32,34,52.
Supplementary Material
Acknowledgments
This work was supported by US Public Health Service grants DA009621 (M.E.W. and K.Y.T.), DA015835 (M.E.W.), DA029099 (M.E.W.), DA024355 (M.F.O.), Rosalind Franklin University of Medicine and Science (K.Y.T.), and postdoctoral National Research Service Award DA030844 (J.A.L.).
Footnotes
AUTHOR CONTRIBUTIONS
J.A.L., K.Y.T. and M.E.W. were responsible for overall study design. J.A.L. and M.M. conducted the biochemical experiments. A.F.S. conducted the electrophysiological experiments, with help from E.F.B. Surgeries, self-administration training and behavioral testing were performed by J.A.L., with help from C.T.W., X.L., K.A.F. and T.L., except the incubation of sucrose craving study which was performed by A.L.L. K.K.S. provided viral vectors and advice on viral vector experiments. M.F.O. provided SYN119, advised on in vivo SYN119 experiments, and designed and supervised sucrose incubation studies. J.A.L., A.F.S., K.Y.T. and M.E.W. analyzed the data. J.A.L., K.Y.T. and M.E.W. wrote the paper.
References
- 1.Pickens CL, et al. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reichel CM, Bevins RA. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr Drug Abuse Rev. 2009;2:184–194. doi: 10.2174/1874473710902020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 5.Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how and why? Frontiers Molec Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conrad KL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mameli M, et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 8.Purgianto A, et al. Different adaptations in AMPA receptor transmission in the nucleus accumbens after short versus long access cocaine self-administration regimens. Neuropsychopharmacology. 2013;38:1789–1797. doi: 10.1038/npp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrario CR, et al. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61:1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCutcheon JE, et al. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci. 2011;31:14536–14541. doi: 10.1523/JNEUROSCI.3625-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellone C, Lüscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:280–1288. doi: 10.1111/j.1460-9568.2005.03979.x. [DOI] [PubMed] [Google Scholar]
- 13.Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 14.Mameli M, Balland B, Luján R, Lüscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- 15.Kelly L, Farrant M, Cull-Candy SG. Synaptic mGluR activation drives plasticity of calcium-permeable AMPA receptors. Nat Neurosci. 2009;12:593–601. doi: 10.1038/nn.2309. [DOI] [PubMed] [Google Scholar]
- 16.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;331:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008;184:435–477. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- 19.Milton AL, Everitt BJ. The persistence of maladaptive memory: Addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev. 2012;36:1119–1139. doi: 10.1016/j.neubiorev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Vanderschuren LJ, Ahmed SH. Animal studies of addictive behavior. Cold Spring Harb Perspect Med. 2013 doi: 10.1101/cshperspect.a011932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ngomba RT, et al. Protective role for type-1 metabotropic glutamate receptors against spike and wave discharges in the WAG/Rij rat model of absence epilepsy. Neuropharmacology. 2011;60:1281–1291. doi: 10.1016/j.neuropharm.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 23.Reimers JM, Milovanovic M, Wolf ME. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2011;1367:223–233. doi: 10.1016/j.brainres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellone C, Mameli M, Lüscher C. In utero exposure to cocaine delays postnatal synaptic maturation of glutamatergic transmission in the VTA. Nat Neurosci. 2011;14:1439–1446. doi: 10.1038/nn.2930. [DOI] [PubMed] [Google Scholar]
- 25.Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol. 2008;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao B, et al. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 27.Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR. Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. J Neurosci. 2000;20:7238–7245. doi: 10.1523/JNEUROSCI.20-19-07238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kammermeier PJ, Worley PF. Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effects. Proc Natl Acad Sci USA. 2007;104:6055–6060. doi: 10.1073/pnas.0608991104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kammermeier PJ. Endogenous Homer proteins regulate metabotropic glutamate receptor signaling in neurons. J Neurosci. 2008;28:8560–8567. doi: 10.1523/JNEUROSCI.1830-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben-Shahar O, et al. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu JH, et al. Preso1 dynamically regulates group I metabotropic glutamate receptors. Nat Neurosci. 2012;6:836–844. doi: 10.1038/nn.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szumlinksi KK, et al. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 33.Szumlinski KK, et al. Homer isoforms differentially regulate cocaine-induced neuroplasticity. Neuropsychopharmacology. 2006;31:768–777. doi: 10.1038/sj.npp.1300890. [DOI] [PubMed] [Google Scholar]
- 34.Edwards S, Bachtell RK, Guzman D, Whisler KN, Self DW. Emergence of context-associated GluR1 and ERK phosphorylation in the nucleus accumbens core during withdrawal from cocaine self-administration. Addiction Biology. 2011;16:450–457. doi: 10.1111/j.1369-1600.2010.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fourgeaud L, et al. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moussawi K, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nature Neurosci. 2010;13:1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang CC, et al. Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci. 2011;31:4194–41203. doi: 10.1523/JNEUROSCI.5239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dravolina OA, Danysz W, Bespalov AY. Effects of group I metabotropic glutamate receptor antagonists on the behavioral sensitization to motor effects of cocaine in rats. Psychopharmacology. 2006;187:297–404. doi: 10.1007/s00213-006-0440-1. [DOI] [PubMed] [Google Scholar]
- 41.Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2012;100:801–810. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olive MF. Cognitive effects of Group I metabotropic glutamate receptor ligands in the context of drug addiction. Eur J Pharmacol. 2010;639:47–58. doi: 10.1016/j.ejphar.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loweth J, Tseng KY, Wolf ME. Using metabotropic glutamate receptors to modulate cocaine’s synaptic and behavioral effects: mGluR1 finds a niche. Curr Opin Neurobiol. 2013;23:500–506. doi: 10.1016/j.conb.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gardner SM, et al. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 46.Lobo MK, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bock R, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee BR, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013 Sep 29; doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vieira E, et al. Fluorinated 9H-xanthene-9-carboxlic acid oxazol-2-yl-amides as potent, orally available mGlu1 receptor enhances. J Bioorg Med Chem Lett. 2009;19:1666–1669. doi: 10.1016/j.bmcl.2009.01.108. [DOI] [PubMed] [Google Scholar]
- 50.Jingami H, Nakanishi S, Morikawa K. Structure of the metabotropic gluatame receptor. Curr Opin Neurobiol. 2003;13:271–278. doi: 10.1016/s0959-4388(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 51.Klugmann M, Szumlinski KK. Targeting Homer genes using adeno-associated viral vector: Lessons learned from behavioural and neurochemical studies. Behav Pharmacol. 2008;19:485–500. doi: 10.1097/FBP.0b013e32830c369f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lominac KD, et al. Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J Neurosci. 2005;25:11586–11594. doi: 10.1523/JNEUROSCI.3764-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.