Abstract
Purpose of review
To report recent data on the potential role of omega-3 fatty acids, in particular docosahexaenoic acid (DHA) and its derivatives, in the treatment of dry eye syndrome.
Recent findings
Dietary supplementation with polyunsaturated fatty acids (PUFAs) yields positive results in the improvement of dry eye signs and symptoms. Although several studies have shown this, evidence is still lacking as to which fatty acid or what combination constitutes the most effective treatment. Studies show that treatment with alpha-linoleic acid reduces dry eyeinduced inflammation. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) derivatives, particularly resolvin E1 (RvE1) and neuroprotectin D1 (NPD1), appear to be responsible for DHA’s anti-inflammatory effect. This is supported in a study where topical RvE1 resulted in decreased inflammation in a mouse dry eye model. Topical administration of pigment epithelium derived factor (PEDF) in combination with DHA accelerates the regeneration of corneal nerves after their damage during corneal surgery, promoting the return of sensitivity and reducing the signs of dry eye. This combined treatment also reduces objective signs of dry eye, such as rose bengal staining.
Summary
No firm recommendations can be made regarding optimal dietary supplementation of essential fatty acids that benefit dry eye patients. Based on animal data and preliminary human studies, DHA and its derivatives appear to be a safe, effective topical treatment for dry eye patients. This may result from their role in the resolution of inflammation and the regeneration of damaged corneal nerves.
Keywords: Dry eye, docosahexaenoic acid, omega-3 fatty acids, omega-6 fatty acids, corneal nerves, neuroprotectin D1
Introduction
The ocular surface is exposed to the external environment and is constantly challenged by injury, infections, chemical irritation, allergic reactions and dryness. The ocular surface unit is composed of the cornea, conjunctiva, lacrimal gland, lacrimal drainage system and the meibomian glands.
The cornea is the transparent part of the ocular surface that has the highest refractive power in the eye. It consists of three main layers. The most inner layer is the endothelium, a monolayer of cells in contact with the aqueous humor responsible for regulating fluid transport. Next is the stroma, a thick layer with almost quiescent keratocytes imbibed in a well-arranged matrix of collagen fibers. The outer layer is the epithelium, a 5-7 cell-thick fast-growing squamous epithelia. The cornea is devoid of blood vessels, which contributes to its transparency and relative immune privilege. The tear film, which is necessary for maintaining visual acuity, keeps the epithelium moist and smooth. One important characteristic of the cornea is its dense innervation with sensory nerve fibers derived from the ophthalmic division of the trigeminal nerve. These fibers enter the stroma in a radial form, divide into branches that penetrate the subepithelial layer, and finish as free nerve endings near the superficial layer of the cornea [1].
The conjunctiva covers the sclera and eyelids. It consists of an epithelium containing goblet cells that secrete mucins, which are important in the lubrication of the ocular surface [2], and an underlying connective tissue known as lamina propia, which is enriched with blood and lymphatic vessels. The meiobiam glands are distributed along the eyelid margin and produce and secrete the outer lipid layer of the tear film.
The lacrimal gland anatomically continues with the conjunctiva and contains branched tubules that end in secretory acini. It provides water, electrolytes and proteins to the aqueous layer of the tear film, which protects the ocular surface. The secretion of its components is under neural control in which activation of the afferent sensory nerves in the cornea is the first response [3]. The tear film is composed of three layers: the mucin layer, the aqueous layer and the lipid layer. The lacrimal drainage system contains the lacrimal puncta and canaliculi that connect to the lacrimal sac and through the nasolacrimal duct and into the nose. After bathing the ocular surface, tears are collected in the puncta to start the drainage.
Dry eye or dysfunctional tear syndrome is a multifunctional, extremely common disorder involving different components of the ocular surface. It affects between 15-20% of adults over 45 years old. This condition is associated with ocular discomfort, including burning, itching, foreign body sensation, pain, photophobia, and blurred vision. In severe cases, dry eye can lead to ocular complications such as corneal thinning, infections, non-healing epithelial defects, and scarring that can permanently impair vision.
Dry eye can be caused by several underlying conditions and interlinked factors, but ocular surface inflammation and increased tear osmolarity are common features. These in turn cause epithelial damage and cell death, thus exacerbating inflammation and resulting in a vicious cycle. There are two types of dry eye, although clinically they are frequently encountered together: 1) aqueous insufficiency, in which the aqueous secretion from the lacrimal glands is reduced; and 2) evaporative dry eye, in which a deficient lipid layer results in an unstable tear film. Corneal nerves are also involved in the pathophysiology of dry eye. These nerves play a key role in maintaining a healthy ocular surface, and their damage leads to decreased tear secretion, decreased blink reflex, and dry eye symptoms, as seen in patients after refractive surgeries such as laser in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK).
Treatment for dry eye includes a variety of artificial tears and punctal plugs. Warm compresses and lid scrubs are used to improve meibomian gland dysfunction. Anti-inflammatory drugs help keep the inflammation under control. Steroids, however, have important side effects to be considered, and cyclosporine, although more suitable for long-term use than steroids, is not effective in every patient. Recently, research efforts have explored dietary modification, oral supplementation and even topical application of polyunsaturated fatty acids (PUFAs) as an alternative strategy for the treatment of dry eye. It is thought that PUFAs, omega-3 (n-3) and omega-6 (n-6), could act as natural anti-inflammatory agents, and may even improve the lipid layer composition of the tear film and increase tear secretion from the lacrimal gland. Most importantly, they could help regenerate damaged corneal nerves; there are no known effective treatments for this today. This review summarizes the most recent findings of polyunsaturated fatty acids, in particular docosahexaenoic acid and its derivatives, as a new strategy for the treatment of dry eye.
Polyunsaturated fatty acids and dry eye
PUFAs are precursors for the synthesis of a diverse class of oxygenate lipids. Oxygenation of the omega-6 arachidonic acid (AA) produces inflammatory eicosanoids such as tromboxanes, prostaglandins and leukotrienes. These derivatives induce pain, vasodilation and leukocyte recruitment. Dietary modification or supplementation could have a beneficial effect if the balance can be tilted toward the synthesis of anti-inflammatory mediators. For example, AA can be converted to anti-inflammatory lipoxins, which are involved in cornea repair after injury [4,5]. Aspirin acetylation of cyclooxygenase -2 (COX-2), the enzyme involved in prostaglandin synthesis, can redirect its activity to form epi-lipoxins, which also have anti-inflammatory and reparative properties [6,7]. A diet high in the omega-3 fatty acids alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA) can reduce the production of AA derivatives by competitive enzyme inhibition, thus enhancing metabolism of prostaglandin E3 series (PGE3) and resulting in reduced inflammation [8]. For this competitive inhibition to take place, it is thought that dietary intake of polyunsaturated fatty acids should have an n-6:n-3 ratio of at least 4:1 or lower.
The effect of PUFA’s in dry eye has been observed in several studies. Dietary supplementation of EPA and DHA reduced the increase of PGE1 and PGE2 levels in a rat dry eye model induced with scopolamine in the exorbital lacrimal gland. It also prevented the decrease in mucin production [9]. In a mouse dry eye model, topical administration of ALA resulted in a significant decrease in fluorescein corneal staining, and it was associated with a decrease in the number of CD11b+ cells as well as in the expression of interleukin 1 alpha (IL-1α) and tumor necrosis factor alpha (TNF-α) on the ocular surface [10]. This type of administration is interesting because it bypasses caloric intake and gastrointestinal side effects associated with oral supplementation. The effect could be attributed to elongation and desaturation products of ALA (such as EPA and DHA) or to their derivatives (such as resolvins and neuroprotectins).
In humans, a cross sectional study of 32,470 women showed that women with a higher omega-3 fatty acid intake in their diets had 68% less incidence of dry eye [11].
The specific omega-6 fatty acid linoleic acid (LA) and its product gamma linolenic acid (GLA) are other alternatives. In a recent study, supplementation with sea buckthorn oil (hippophae rhamnoides)(SB), which is high in omega-3 and the omega-6 fatty acid LA attenuated the increase in tear film osmolarity during the cold season and reduced symptoms in patients with dry eye [12].Gamma-linoleic acid treatment appeared beneficial in alleviating dry eye symptoms and improving overall contact lens comfort in patients suffering from contact lens associated dry eye [13]. In the same study, an increase in tear production was also observed. GLA and LA were also found to reduce ocular surface inflammation in patients with Sjogren’s syndrome [14]. Oral supplementation of LA and GLA along with eyelid hygiene has also been shown to improve symptoms and reduce eyelid margin inflammation in meibomian gland dysfunction more than either treatment alone [15]. This effect could be explained by the reduction of inflammatory AA products, where the dietary supplementation of LA and GLA results in the formation of less active prostanoids [16]. It is also possible that these fatty acids help normalize the melting point of meibomian secretion.
Resolvins and Protectins
Recent studies have begun to shed light on the intrinsic mechanism involved in inflammation resolution. It is now thought that a tightly controlled and programmed mechanism is involved in the resolution of inflammatory processes. During the course of this process there is a switch from the production of pro-inflammatory lipid-derived mediators to anti-inflammatory pro-resolution mediators. Four new families of molecules have been identified with the use of lipidomic analysis: 1) lipoxins synthesized from AA, 2) resolvins and 3) protectins derived from omega-3 fatty acids (EPA and DHA), and 4) maresins. As a group, these molecules are endogenous, potent, local-acting mediators that limit PMN infiltration and promote macrophage clean up of the inflammation site, without generating immunosuppressive activity [6].
Given the positive effect of EPA and DHA in dry eye, it is logical to think that an important part of their effect has to do with the resolution of inflammation promoted by some of their products. The synthesis of resolvins and protectins from EPA and DHA is carried out by lipoxygenases. We know that human corneal epithelial cells express 15-lipoxygenase, which converts DHA into neuroprotectin D1 (NPD1), a lipid mediator that has been shown to have neuroprotective and anti-apoptotic activity [17]. Furthermore, topical application of NPD1 increases re-epithelialization in a mouse wound model [4]. Thus, it is possible that omega-3 fatty acids help reverse corneal epithelial damage associated with dry eye.
We have tested the derivative of EPA, resolvin E1 (RvE1), in a mouse model of dry eye. When applied topically, this compound increased tear flow, promoted a healthy epithelium, and decreased COX-2 expression and macrophage infiltration [18]. The synthetic analog of RvE1 (RX-100045) is being tested in a Phase 2 clinical trial for the treatment of chronic dry eye. Preliminary data of a 28-day, randomized, placebo-controlled, 232-patient trial showed dose-dependent and statistically significant improvements in dry-eye patients treated with RX-100045. The compound also appears to be well tolerated when applied topically (http://resolvyx.com/news-pubs/releases/082409). These studies demonstrate that stimulating resolution pathways could have an important role in the treatment of chronic inflammatory conditions, including dry eye.
DHA and corneal nerves
Damage to the corneal sensory nerves interrupts the cornea reflex arc. This decreases lacrimal function and the blink mechanism that allows tears to spread uniformly and then be cleared from the ocular surface to the nasolacrimal ducts. As a result, tear production and ocular surface sensitivity decrease, inducing dry eye. However, this is not the only mechanism that affects the ocular surface after corneal nerve damage. We know that keratocytes and corneal epithelial cells are innervated by nerve terminals, and those nerves (likely by the release of neuropeptides) are critical for maintaining cellular trophism [19]. Damage to the communication link between nerve terminals and corneal cells results in neurotrophic keratitis, a severe form of dry eye where the corneal epithelium becomes more susceptible to injury and, in severe cases, is unable to adequately heal.
The most common procedures for surgically correcting refractive errors in patients are PRK and LASIK. Both of these surgeries damage the corneal nerves, resulting in or exacerbating dry eye, which is particularly difficult to treat. Because the injury is relatively superficial, the corneal nerves eventually recover their sensitivity. However, several studies found that nerve recovery after surgery can take from one to several years [20-22]. When nerve injury is more severe, as in complete transection of corneal nerves during corneal transplantation or herpetic corneal infections, sensitivity does not typically return to normal levels [23]. As such, severe complications can occur, such as non-healing epithelial defects, thinning of the cornea, increased susceptibility to infectious keratitis, and even corneal perforation. Thus, there is a great need for finding therapeutic tools that accelerate recovery of corneal nerves.
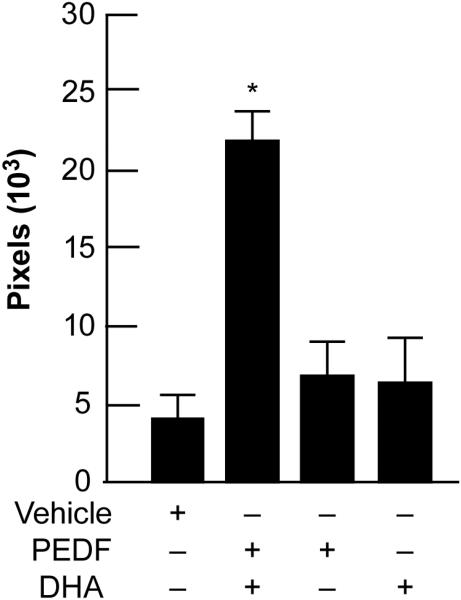
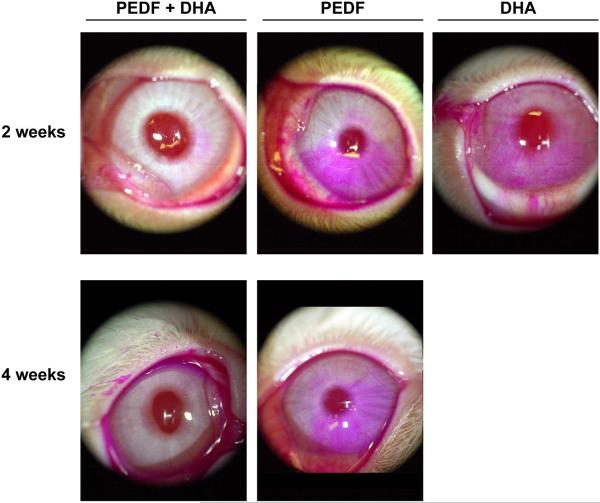
There are several growth factors that promote nerve regeneration, such as nerve growth factor (NGF), and corneas treated with NGF after LASIK surgery have experienced enhanced sensitivity [24]. In a PRK rabbit model, treatment with a combination of NGF and DHA stimulated nerve regeneration and corneal epithelial proliferation [25]. At that time, we postulated that the mechanism could be through the action of the DHA derivative NPD1, which has potent anti-inflammatory and anti-apoptotic properties [17]. Several growth factors activate NPD1 synthesis; the most active one, pigment epithelial derived factor (PEDF), is ten times more potent than NGF [26]. In more recent studies, we performed a lamellar keratectomy that severed corneal nerves at the level of the mid stroma in a rabbit model. The animals were treated with a combination of PEDF and DHA for six weeks. Confocal microscopy in vivo showed a 4-fold increase in the nerve area four weeks after treatment with PEDF and DHA compared to corneas treated with vehicle [27]. Treatment with DHA or PEDF alone did not induce an increase in nerve regeneration (Fig 1). Furthermore, staining the ocular surface with rose bengal, which stains damaged epithelial cells from the cornea and conjunctiva, showed a very clear ocular surface after two and four weeks of PEDF- and DHA-combined treatment, while PEDF or DHA alone resulted in strong diffuse staining (Fig 2). Patients with severe rose bengal staining produce less tears after sensory stimulation. Our results demonstrate that PEDF in combination with DHA stimulates rapid nerve regeneration and protects the epithelium.
Figure 1.
Quantification of corneal nerve area examined in vivo by confocal microscopy at four weeks after lamellar keratectomy. Treatment with pigment epithelial derived factor (PEDF) plus docosahexaenoic acid (DHA) showed a higher corneal nerve area when compared to animals treated either with PEDF, DHA or vehicle alone.
Figure 2.
Slit lamp photos showing rose bengal staining of devitalized rabbit corneal and conjunctival epithelial cells two and four weeks after lamellar keratectomy. Note the significantly decreased punctate staining in the cornea and conjunctiva of animals treated with pigment epithelial derived factor (PEDF) plus docosahexaenoic acid (DHA).
It is still unclear what the underlying mechanism is that makes the combination of PEDF plus DHA so successful in accelerating corneal nerve regeneration and reversing epithelial damage after injury. We have demonstrated by lipidomic analysis that NPD1 synthesis increases in animals treated with PEDF plus DHA [27]. In addition, we have shown that PEDF synthesis increases after epithelial injury in vitro and induces expression of 15-lipoxygenase [28], the enzyme that synthesizes NPD1 from DHA. Together, this evidence supports our hypothesis that NPD1 is involved in accelerating corneal nerve regeneration.
Sensitivity studies of animals treated with PEDF plus DHA after lamellar keratectomy demonstrated that corneal sensation recovers almost to baseline levels as early as six weeks after surgery, which correlates with the histology studies showing increased corneal nerve area in the treated animals compared to untreated controls [29]. This evidence demonstrates that a combination of PEDF plus DHA could be an effective treatment for dry eye, which develops secondary to corneal nerve damage, as is the case in post-refractive surgery patients.
Conclusions
The present review indicates that the role of PUFAs in the treatment of dry eye is still not completely understood, but increasing evidence supports their potential use for the treatment of this condition. Several studies point to DHA and its derivatives as potential steroid-spearing options that can reduce dry eye-related inflammation. Their effect in corneal nerve damage, resulting in accelerated nerve regeneration, is also an exciting finding that could be very important in the treatment of patients with neurotrophic keratitis and post-refractive surgery dry eye. Prospective human studies are still needed to confirm optimal dosing and method/s of administration, effectiveness and safety of these very promising new treatments for dry eye.
Key points.
Dry eye is a multifactorial condition.
EPA and DHA reduce dry eye inflammation.
Damage to corneal nerves induce dry eye.
Combination of DHA plus PEDF stimulates nerve regeneration.
Acknowledgements
This work was supported by a grant from Resolvyx Pharmaceuticals, Inc., a Translational Research Initiative grant from the Louisiana State University Health Sciences Center, New Orleans (MSC, HEPB), and the National Institutes of Health, National Eye Institute grants EY004928 and EY019465 (HEPB).
Supported by a grant from Resolvyx Pharmaceuticals, Inc., LSUHSC Translational Research Initiative grant (MSC, HEPB), and NIH/NEI grants EY004928 and EY019465 (HEPB).
References
Papers of particular interest published with the annual period of review have been highlighted as:
* of special interest
** of outstanding interest
- 1*.He J, Bazan NG, Bazan HE. Mapping the entire human corneal nerve architecture. Exp Eye Res. 2010;91:367–74. doi: 10.1016/j.exer.2010.07.007. This study describes the architecture of human corneal nerves in a complete immunohistochemical reconstruction of whole corneas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Govindarajan B, Gipson IK. Membrane-tethered mucins have multiple functions in the ocular surface. Exp Eye Res. 2010;90:655–663. doi: 10.1016/j.exer.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dartt D. Neural regulation of lacrimal gland secretory processes:relevance in dry eye diseases. Prog Ret Eye Res. 2009;28:155–177. doi: 10.1016/j.preteyeres.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gronert K, Maheswari N, Khan N, et al. A role for the mouse 12/15 lipoxigenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280:15267–78. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- 5.Kenchegowda S, Bazan NG, Bazan HE. EGF stimulates lipoxin A4 synthesis and modulates repair in corneal epithelial cells through ERK and p38 activation. Invest Ophthal Vis Sci. 2010 doi: 10.1167/iovs.10-6199. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation. To resolve or not? Am J Pathol. 2010;177:1576–91. doi: 10.2353/ajpath.2010.100322. Excellent review on the role of EPA and DHA derivatives in the resolution of inflammatory processes that may be of significance in dry eye related inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakazu AH, He J, Bazan HEP. Aspirin-triggered lipoxin A4 (epi-LXA4) increases the preservation time of human endothelium in Optisol-GS. ARVO Meeting Abstract. 2010;51:2824. [Google Scholar]
- 8.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71(suppl.):S343–8. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 9.Viau S, Maire MA, Pasquis B, Gregoire S, Acar N, Bron AM, Bretillion L, Creuzot-Garcher, Joffre C. Efficacy of a 2-month dietary supplementation with polyunsaturated fatty acids in dry eye induced by scopolamine in rat model. Graefes Arch Clin Exp Ophthalmol. 2009;247:1039–1050. doi: 10.1007/s00417-009-1080-z. [DOI] [PubMed] [Google Scholar]
- 10.Rashid S, Jin Y, Ecoiffier T, Barabino S, et al. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126:219–225. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- 11.Milijanovic B, Rivedi KA, Dana MR, et al. Relationship between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye. Am J Clin Nutr. 2005;82:887–93. doi: 10.1093/ajcn/82.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Larmo PS, Jarvinen RL, Sertala NL, et al. Oral Sea Buckthorn Oil Attenuates Tear Film Osmoloarity and Symptoms in Individuals with Dry Eye. J Nutr. 2010;140:1462–8. doi: 10.3945/jn.109.118901. Interesting paper using oral sea buchthorn oil that appears to be effective in reducing tear osmolarity, one of the main inflammation triggers in dry eye. [DOI] [PubMed] [Google Scholar]
- 13.Kokke KH, Morris JA, Lawrenson JG. Oral omega-6 fatty acid treatment in contact lens associated dry eye. Contact Lens & Anterior Eye. 2008;31:141–6. doi: 10.1016/j.clae.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Aragona P, Bucolo C, Spinella R, et al. Systemic Omega-3 Essential Fatty Acid Treatment and PGE1 Tear Content in Sjogren’s syndrome Patients. Invest Ophthalmol Vis Sci. 2005;46:4474–9. doi: 10.1167/iovs.04-1394. [DOI] [PubMed] [Google Scholar]
- 15.Pinna A, Piccinini P, Carta F. Effect of oral linoleic and gamma-linolenic acid on mibomian gland dysfunction. Cornea. 2007;26:260–4. doi: 10.1097/ICO.0b013e318033d79b. [DOI] [PubMed] [Google Scholar]
- 16.Wu D, Meydani M, Leka LS, et al. Effect of dietary supplementation with black currant seed oil on the immune response of healthy subjects. Am J Clin Nutr. 1999;70:536–543. doi: 10.1093/ajcn/70.4.536. [DOI] [PubMed] [Google Scholar]
- 17**.Bazan NG. Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection. Prostaglandins Leuko Essent Fatty Acids. 2009;81:205–211. doi: 10.1016/j.plefa.2009.05.024. Updated review describing effects of DHA derivatives, in particular neuroprotectin D1 (NPD1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Li N, He J, Scwartz CE, et al. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther. 2010;26:431–439. doi: 10.1089/jop.2010.0019. The first study to show the effectiveness of RvE1 in a desiccating animal model of dry eye. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 20.Battat L, Macri A, Dursun D, Pflugfelder SC. Effects of laser in situ keratomileusis ontear production, clearance, and the ocular surface. Ophthalmology. 2001;108:1230–1235. doi: 10.1016/s0161-6420(01)00623-6. [DOI] [PubMed] [Google Scholar]
- 21.Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140:1059–1064. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Lee BH, McLaren JW, Erie JC, Hodge DO, Bourne WM. Reinnervation in the cornea after LASIK. Invest Ophthalmol Vis Sci. 2002;43:3660–3664. [PubMed] [Google Scholar]
- 23*.Gallar J, Tervo TM, Neira W, et al. Selective changes in human corneal sensation associated with herpes simplex virus keratitis. Invest Ophthalmol and Vis Sci. 2010;51:4516–22. doi: 10.1167/iovs.10-5225. Interesting paper showing selectivity of nerve damage to mechano and polymodal nociceptors terminals in herpetic infections. [DOI] [PubMed] [Google Scholar]
- 24.Joo ML, Yuhan KR, Hyon JY, et al. The effect of nerve growth factor on corneal sensitivity after laser in situ keratomileusis. Arch Ophthalmol. 2004;122:1338–41. doi: 10.1001/archopht.122.9.1338. [DOI] [PubMed] [Google Scholar]
- 25.Esquenazi S, Bazan HE, Bui V, et al. Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2005;46:3121–3127. doi: 10.1167/iovs.05-0241. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee PK, Marcheselli VL, Barreiro S, et al. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci USA. 2007;104:13152–13157. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Cortina MS, He J, Li N, et al. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest Ophthalmol Vis Sci. 2010;51:804–10. doi: 10.1167/iovs.09-3641. This study describes one of the first treatments to be effective in accelerating corneal nerve regeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li N, Kenchegowda S, Cortina MS, Bazan HEP. Pigment epithelium derived factor increases after corneal epithelial injury and stimulates the expression of 15-LOX1. ARVO Meeting Abstract. 2009;50:6304. [Google Scholar]
- 29.Cortina MS, He J, Li N, Bazan HEP. PEDF plus DHA promotes recovery of corneal sensitivity after experimental surgery. ARVO Meeting Abstract. 2010;51:2856. [Google Scholar]