Abstract
In this issue of Neuron, McElvain et al. demonstrate for the first time plasticity at the synapse between vestibular nerve afferents and their postsynaptic targets in the medial vestibular nuclei. This new type of plasticity, which is gated by inhibition, is well suited to drive motor learning during adaptation of the vestibulo-ocular reflex.
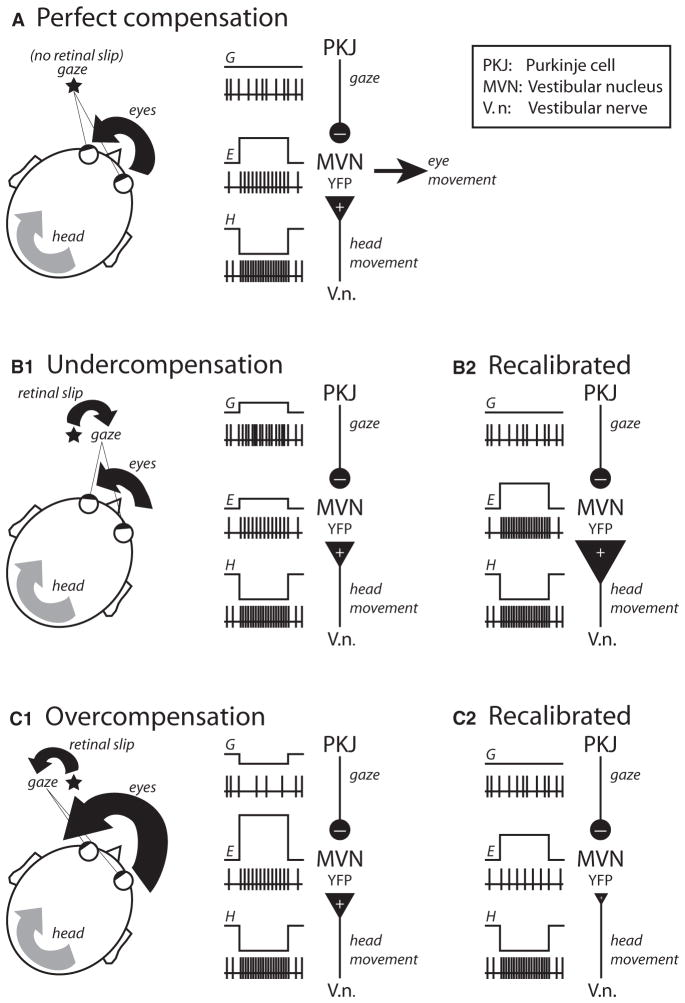
At first glance, the vestibulo-ocular reflex (VOR) appears to be just another rather uninteresting example of how the brain controls our movements. However, there are a number of reasons why the VOR has become one of the darling model systems in many research laboratories across the world. First, the function of the system and its general mode of operation are simple and have been well characterized. The VOR is used to maintain stable gaze on an object of interest even as the head is bobbing and bouncing around; without the VOR, visual acuity would deteriorate appreciably as a result of the slipping of the object’s image on the retina. To accomplish stable gaze, this simple reflex ensures that every head movement is quickly (in fewer than 15 ms) and precisely counterbalanced by the exact opposite movement of the eyes (Figure 1A). A second advantage of the VOR system is that the underlying neural circuitry mediating the normal reflex is known (Figure 1A). The input to the system, head movement, is sensed by the semicircular canals of the inner ear and sent via the vestibular nerve to neurons in the medial vestibular nuclei (VN). In turn, the VN generates a motor command for the compensatory eye movement, which is then conveyed via direct and indirect neural pathways to the motoneurons innervating the muscles that move the eyes. Knowledge about the neural circuit and about the basic operation of the reflex are good enough reasons to adopt the VOR as a model system for investigating how the brain controls movement; however, what makes the VOR so popular among neuroscientists, especially those interested in the neural basis of learning and memory, is that this simple reflex is modifiable by prior experience.
Figure 1.
The Miles and Lisberger Hypothesis
It was recognized very early on that the VOR must be constantly calibrated to ensure that the movement of the head is perfectly compensated by the exact same movement of the eyes in the opposite direction (Figure 1A). Consider the example in Figure 1B1, in which the eye muscles have weakened considerably (possibly because of aging), and as a result, the motor command generated by the VN is no longer able to compensate for the movement of the head (i.e., the eyes move less than they should, and gaze is not stable). In this case, an error signal indicating undercompensation will be generated, and this signal will drive adaptive processes somewhere in the brain such that ultimately, the output of the VN is increased and the VOR is once again perfectly calibrated (Figure 1B2). Adaptation of the VOR has now been demonstrated by a range of techniques in a variety of animals(Miles and Lisberger, 1981; Broussard and Kassardjian, 2004), and in all cases the activity of neurons in the VN has been shown to be adaptively modulated (increased output to correct for undercompensation, Figure 1B2; decreased output to correct for overcompensation, Figure 1C2). However, many fundamental questions remain and are currently under intense investigation—chief among them are questions about the nature of the error signals and about the molecular mechanisms of neural plasticity underlying adaptation.
Research on these questions about the neural basis of VOR adaptation has emphasized ideas about the role of inhibitory Purkinje cells in the vestibulocerebellum (Figure 1). Perhaps the most influential of these ideas, with roots that can be traced back to the Marr and Albus hypothesis of cerebellar learning, was developed in the early 1970s by Masao Ito (Ito, 2002). In this theory, Purkinje cells, whose axons inhibit the VN, are themselves the site of neural plasticity; visually related error signals about the image of the object slipping in the retina cause long-term synaptic changes that ultimately modify Purkinje cell activity in a way that recalibrates the reflex (decreased output to correct for under-compensation; increased output to correct for overcompensation). There has been much support for this hypothesis, with perhaps the strongest and most direct evidence coming from the discovery of cerebellar long-term depression (cLTD) and potentiation (cLTP) in Purkinje cells, and the finding that this form of plasticity is under the control of climbing fibers that can be activated by retinal slip (Ito, 2002).
Ito’s hypothesis was the only game in town for many years, but in the 1980s a new theory about the neural basis of VOR adaptation was proposed by Miles and Lisberger (Miles and Lisberger, 1981). The trigger for their revolutionary hypothesis was the discovery that Purkinje cell activity in awake-behaving monkeys provides a signal that is related to gaze (Figure 1) (Lisberger and Fuchs, 1978). In the new formulation, Purkinje cells are not the site of plasticity; instead, they send a gaze-related error signal to the VN, driving long-term changes there. For undercompensation (Figure 1B), gaze is in the same direction as the head movement, and the resulting increase in Purkinje cell activity would induce long-term potentiation (vLTP) of vestibular nerve synapses in the VN; for overcompensation (Figure 1C), gaze is in the opposite direction as the head movement, and the resulting decrease in Purkinje cell activity would induce long-term depression (vLTD) of the vestibular synapses. Computational studies and careful analysis of Purkinje cell and VN activity after VOR adaptation have provided indirect support for the Miles and Lisberger hypothesis. What has been missing from the picture, until now, is any evidence of a cellular mechanism of plasticity in the VN.
In this issue of Neuron, McElvain et al. demonstrate for the first time that vestibular nerve synapses on the VN are plastic (McElvain et al., 2010). What makes these results particularly exciting is the discovery that this form of synaptic plasticity is under the control of postsynaptic membrane potential, in a manner that is fully compatible with the rule proposed for VOR adaptation by Miles and Lisberger 30 years ago. Pairing vestibular nerve stimulation with hyperpolarization (which in vivo could be mediated by increased Purkinje cell inhibition of the VN) results in vLTP (illustrated by the big vestibular nerve synapse in Figure 1B2); vestibular nerve stimulation in the absence of hyperpolarization results in vLTD (small vestibular nerve synapse in Figure 1C2). Futhermore, McElvain et al. show that vLTP and vLTD cause bidirectional, linear changes in the postsynaptic firing response of the VN. Overall, the properties of this new form of plasticity seem to be perfectly suited for mediating the changes in VN firing that have been observed during VOR adaptation for both under- and overcompensatory eye movements (Figures 1B2 and 1C2).
The findings of McElvain et al. close the chapter on the long-standing question of whether vestibular nerve synapses onto the VN are plastic, and under what conditions. But just as one chapter is closed, others are beginning to open, offering a glimpse of what may lie ahead.
At the molecular level, for example, there remain questions about the generality of the results with regard to synaptic plasticity at other sites that, like the VN, are targets of Purkinje cell axons. Indeed, plasticity in the deep cerebellar nuclei (CN), the target of all Purkinje cells outside of the vestibular system, has been well characterized and shares many features with plasticity in the VN, including its non-Hebbian nature and the role that inhibition plays in setting the appropriate conditions for the induction of LTD and LTP (Pugh and Raman, 2009). But there are distinctions as well; although calcium is a key player in gating synaptic plasticity in both the CN and the VN, there are clear differences in the precise manner in which inhibition modulates calcium levels in the two nuclei and in the dynamic patterns of calcium signals that are required for the induction of plasticity (McElvain et al., 2010; Person and Raman, 2010). Further work is necessary to determine whether these differences are indicative of an underlying heterogeneity in function or simply reflect independent molecular solutions to achieve the same goal in separate motor systems (i.e., bidirectional motor learning). In this context it is interesting to note that the rule for plasticity initially hypothesized by Miles and Lisberger for the VN has been successfully incorporated into recent theories of non-vestibular, cerebellar-dependent motor learning tasks that include a site of plasticity in the CN (Medina et al., 2000).
At the cellular level, it will be important to determine whether the properties of plasticity in vitro resemble the behavioral properties of motor learning. For example, adaptation of the VOR is bidirectional and “reversible.” The gain of the system will be turned up by increasing VN output if eye movements are undercompensating for the head movement, but if conditions change and the eyes start overcompensating, adaptation will be reversed by decreasing VN output and turning the gain of the system down. Turning the gain of the system up and down can be repeated as many times as necessary. However, it is not known yet whether vLTP and vLTD reverse each other at the molecular level, for example by increasing and decreasing the expression of a particular receptor at the synapse between the vestibular nerve and the VN. This does not mean that to contribute to bidirectional adaptation of the VOR, the molecular mechanisms for vLTP and vLTD must reverse each other; but finding that they do not would certainly point to other sites of plasticity that could then collaborate with synaptic changes in the VN to mediate different aspects of the behavioral adaptation.
At the systems level, the burning question is deciphering how error signals present during VOR adaptation might engage mechanisms of plasticity in the VN and elsewhere (Boyden et al., 2004), and how these distributed neural changes might work together to help recalibrate the reflex and improve motor performance. In this regard, previous work indicates that short-term adaptation of the VOR may be accomplished by using mechanisms of plasticity within the cerebellar cortex, including cLTD and cLTP, whereas long-term consolidation of the adaptation may be mediated by plasticity in the VN (Broussard and Kassardjian, 2004). It is not known whether during normal adaptation of the VOR, plasticity in the VN requires first the induction of plasticity in the cerebellar cortex, as suggested by the trigger-and-storage model of cerebellar motor learning (Medina et al., 2002). An alternative, supported by recent work in mice (van Alphen and De Zeeuw, 2002) and monkeys (Ke et al., 2009), and more in line with the original Miles and Lisberger proposal, is that plasticity in the VN can proceed independently of synaptic changes in Purkinje cells, albeit at a slower rate. Analyzing the patterns of Purkinje cell activity that are present at different stages during VOR adaptation, and comparing them with the patterns of inhibition required to induce vLTP and vLTD in the VN in vitro, will go a long way toward resolving this key issue.
Clearly, many questions remain, but one thing is for certain: addressing these fundamental issues, and many others, is possible now thanks to the work of McElvain et al. Their findings, which have revealed synaptic plasticity in the VN, and uncovered the mechanisms and rules under which it operates, represent an extraordinary accomplishment, one that gets us closer to achieving a full mechanistic understanding of how the brain adapts and perfects movements by cooking plasticity with inhibition in the VN. What a great recipe!
References
- Boyden ES, Katoh A, Raymond JL. Annu Rev Neurosci. 2004;27:581–609. doi: 10.1146/annurev.neuro.27.070203.144238. [DOI] [PubMed] [Google Scholar]
- Broussard DM, Kassardjian CD. Learn Mem. 2004;11:127–136. doi: 10.1101/lm.65804. [DOI] [PubMed] [Google Scholar]
- Ito M. Ann N Y Acad Sci. 2002;978:273–288. doi: 10.1111/j.1749-6632.2002.tb07574.x. [DOI] [PubMed] [Google Scholar]
- Ke MC, Guo CC, Raymond JL. Nat Neurosci. 2009;12:1171–1179. doi: 10.1038/nn.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG, Fuchs AF. J Neurophysiol. 1978;41:733–763. doi: 10.1152/jn.1978.41.3.733. [DOI] [PubMed] [Google Scholar]
- McElvain LE, Bagnall MW, Sakatos A, du Lac S. Neuron. 2010;68:763–775. doi: 10.1016/j.neuron.2010.09.025. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Nores WL, Ohyama T, Mauk MD. Curr Opin Neurobiol. 2000;10:717–724. doi: 10.1016/s0959-4388(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Medina JF, Repa JC, Mauk MD, LeDoux JE. Nat Rev Neurosci. 2002;3:122–131. doi: 10.1038/nrn728. [DOI] [PubMed] [Google Scholar]
- Miles FA, Lisberger SG. Annu Rev Neurosci. 1981;4:273–299. doi: 10.1146/annurev.ne.04.030181.001421. [DOI] [PubMed] [Google Scholar]
- Person AL, Raman IM. Neuron. 2010;66:550–559. doi: 10.1016/j.neuron.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. Trends Neurosci. 2009;32:170–177. doi: 10.1016/j.tins.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen AM, De Zeeuw CI. Eur J Neurosci. 2002;16:486–490. doi: 10.1046/j.1460-9568.2002.02094.x. [DOI] [PubMed] [Google Scholar]