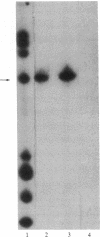
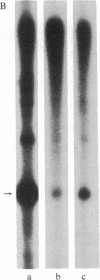
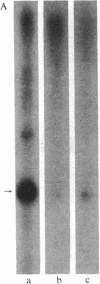
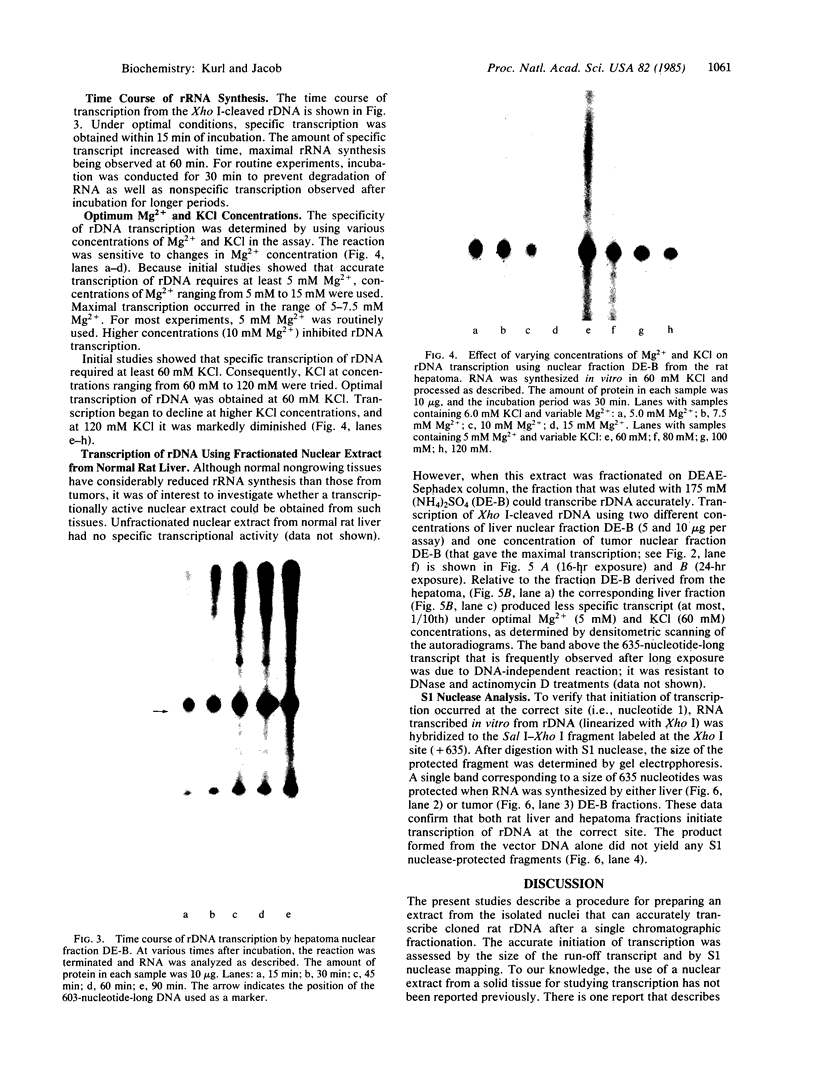
Abstract
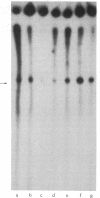
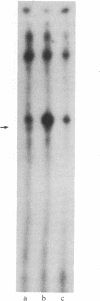
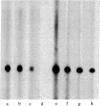
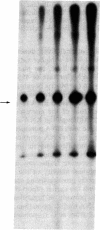
Nuclear extracts obtained from normal rat liver and from Morris hepatoma 3924A were fractionated by DEAE-Sephadex chromatography. The fraction eluted with 175 mM (NH4)2SO4 (DE-B), which contains greater than 90% of RNA polymerase I activity, supported accurate transcription of cloned rat rDNA. A similar fraction obtained from the cytosol had all of the factors required for rDNA transcription. However, its transcriptional activity was at most one-sixth that of the corresponding nuclear fraction, as determined by the amount of protein needed to produce a similar quantity of the transcript. Unfractionated nuclear or cytosol preparations did not yield an accurate transcript. Optimal KCl and magnesium concentrations for rDNA transcription were 60 mM and 5-7.5 mM, respectively. The extent of transcriptional activity was in the following order: hepatoma nuclear fraction DE-B greater than whole cell extract derived from rat mammary adenocarcinoma cells much greater than normal liver fraction DE-B. The hepatoma preparation produced at least 10 times the amount of transcript produced by the corresponding liver nuclear preparation. Transcriptional activity was proportional to the levels of RNA polymerase I and to the rate of rRNA synthesis in these tissues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duceman B. W., Jacob S. T. Transcriptionally active RNA polymerases from Morris hepatomas and rat liver. Elucidation of the mechanism for the preferential increase in the tumour RNA polymerase I. Biochem J. 1980 Sep 15;190(3):781–789. doi: 10.1042/bj1900781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duceman B. W., Rose K. M., Jacob S. T. Activation of purified hepatoma RNA polymerase I by homologous protein kinase NII. J Biol Chem. 1981 Nov 10;256(21):10755–10758. [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Isolation of transcription factors that discriminate between different promoters recognized by RNA polymerase II. Cell. 1983 Mar;32(3):669–680. doi: 10.1016/0092-8674(83)90053-3. [DOI] [PubMed] [Google Scholar]
- Financsek I., Mizumoto K., Muramatsu M. Nucleotide sequence of the transcription initiation region of a rat ribosomal RNA gene. Gene. 1982 May;18(2):115–122. doi: 10.1016/0378-1119(82)90109-3. [DOI] [PubMed] [Google Scholar]
- Grummt I. Specific transcription of mouse ribosomal DNA in a cell-free system that mimics control in vivo. Proc Natl Acad Sci U S A. 1981 Feb;78(2):727–731. doi: 10.1073/pnas.78.2.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S. T. Mammalian RNA polymerases. Prog Nucleic Acid Res Mol Biol. 1973;13:93–126. doi: 10.1016/s0079-6603(08)60101-4. [DOI] [PubMed] [Google Scholar]
- Jacob S. T., Steele W. J., Busch H. Turnover of 45 S RNA of regenerating liver and Walker tumor. Cancer Res. 1967 Jan;27(1):52–60. [PubMed] [Google Scholar]
- Leonard T. B., Jacob S. T. Alterations in DNA-dependent RNA polymerase I and II from rat liver by thioacetamide: preferential increase in the level of chromatin-associated nucleolar RNA polymerase IB. Biochemistry. 1977 Oct 4;16(20):4538–4544. doi: 10.1021/bi00639a032. [DOI] [PubMed] [Google Scholar]
- Lin Y. C., Bullock L. P., Bardin C. W., Jacob S. T. Effect of medroxyprogesterone acetate and testosterone on solubilized RNA polymerases and chromatin template activity in kidney from normal and androgen-insensitive (Tfm/Y) mice. Biochemistry. 1978 Oct 31;17(22):4833–4838. doi: 10.1021/bi00615a034. [DOI] [PubMed] [Google Scholar]
- Lin Y. C., Rose K. M., Jacob S. T. Evidence for the nuclear origin of RNA polymerases identified in the cytosol: release of enzymes from the nuclei isolated in isotonic sucrose. Biochem Biophys Res Commun. 1976 Sep 7;72(1):114–120. doi: 10.1016/0006-291x(76)90968-2. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Sollner-Webb B. Transcription of mouse rRNA genes by RNA polymerase I: in vitro and in vivo initiation and processing sites. Cell. 1981 Nov;27(1 Pt 2):165–174. doi: 10.1016/0092-8674(81)90370-6. [DOI] [PubMed] [Google Scholar]
- Rose K. M., Ruch P. A., Morris H. P., Jacob S. T. RNA polymerases from a rat hepatoma. Partial purification and comparison of properties with corresponding liver enzymes. Biochim Biophys Acta. 1976 Apr 15;432(1):60–72. doi: 10.1016/0005-2787(76)90041-1. [DOI] [PubMed] [Google Scholar]
- Rose K. M., Stetler D. A., Jacob S. T. Protein kinase activity of RNA polymerase I purified from a rat hepatoma: probable function of Mr 42,000 and 24,600 polypeptides. Proc Natl Acad Sci U S A. 1981 May;78(5):2833–2837. doi: 10.1073/pnas.78.5.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblum L. I., Reddy R., Cassidy B. Transcription initiation site of rat ribosomal DNA. Nucleic Acids Res. 1982 Nov 25;10(22):7345–7362. doi: 10.1093/nar/10.22.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdel E. M., Jacob S. T. Mechanism of early effect of hydrocortisone on the transcriptional process: stimulation of the activities of purified rat liver nucleolar RNA polymerases. Biochem Biophys Res Commun. 1971 Nov 5;45(3):707–715. doi: 10.1016/0006-291x(71)90474-8. [DOI] [PubMed] [Google Scholar]
- Weil P. A., Luse D. S., Segall J., Roeder R. G. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. doi: 10.1016/0092-8674(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Wu G. J. Adenovirus DNA-directed transcription of 5.5S RNA in vitro. Proc Natl Acad Sci U S A. 1978 May;75(5):2175–2179. doi: 10.1073/pnas.75.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]