Abstract
An essential issue in perception is how the location of an object is estimated from tactile signals in the context of self-generated changes in sensor configuration. Here, we review the pathways and dynamics of neuronal signals that encode touch in the rodent vibrissa sensorimotor system. Rodents rhythmically scan an array of long, facial hairs across a region of interest. Behavioral evidence shows that these animals maintain knowledge of the azimuthal position of their vibrissae. Electrophysiological measurements have identified a reafferent signal of the azimuth that is coded in normalized coordinates, broadcast throughout primary sensory cortex and provides strong modulation of signals of vibrissa contact. Efferent signals in motor cortex report the range of the scan. Collectively, these signals allow the rodent to form a percept of object location.
Animals must determine the position of objects and other animals in their environment, far and near, as they navigate and search. The sense of distant objects requires the use of propagating signals, light to see, sound to hear, and for some animals the use of electrical disturbances (Kleinfeld et al., 2006; König and Luksch, 1998; Nelson and MacIver, 2006). Even the sense of smell involves detection at a distance as odorants are carried along plumes (Wachowiak, 2011). In all of these cases, animals can use stereopsis or an analogous variant to gauge the distance of objects to their body as well as their relative orientation. A different ethological problem arises when objects or conspecifics are close by, so that stereopsis is no longer effective. The perception of nearby objects is particularly acute with animals that track or borrow. Here, long pliable hairs, or in the case of insects long antennae, are used to probe the near environment. In many cases, the hairs or antennae are mobile so that a bilateral scan allows the animal to probe the entire region about its head and provides a shell of detection to keep the animals head from directly touching objects. The computational problem poised by the use of moving sensors in general, and long facial hairs in particular to sense nearby objects, is that sensation and motor control are intertwined. The perception of where an object is relative to the face of the animal requires that the contact of the hairs must be assessed relative to their changing position in space.
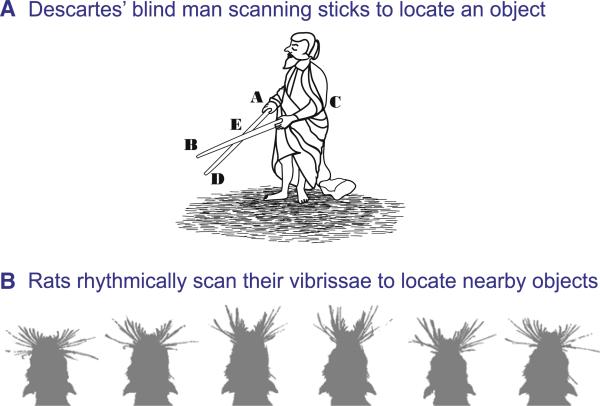
The problem of object localization with moving sensors was first discussed by Descartes (1637). With reference to a drawing of a blind man with walking sticks (Figure 1A), he notes “...when the blind man... turns his hand A towards E, or again his hand C towards E, the nerves embedded in that hand cause a certain change in his brain, and through this change his soul can know not only the place A or C but also all the other places located on the straight line AE or CE; in this way his soul can turn its attention to the objects B and D, and determine the places they occupy without in any way knowing or thinking of those which his hands occupy. Similarly, when our eye or head is turned in some direction, our soul is informed of this by the change in the brain which is caused by the nerves embedded in the muscles used for these movements.” Steps toward the solution of this neuronal computational problem are the focus of this review.
Figure 1. Rhythmic Exploratory Movements.
(A) Sketch of a blind man using a pair of sticks to scan the space in front of him for objects. Redrawn from Descartes (1637) by Jenny Groisman. (B) Successive frames as a rat whisks in air as it searches for a food tube. Frames were taken at 17 ms intervals, using darkfield illumination, and the images were individually thresholded. Adapted from Fee et al. (1997).
The rat vibrissa system, with its tactile hairs and their associated neuronal architecture, provides a prototype sensorimotor system (Figure 1B). For nearly a century, researchers have compiled behavioral evidence that the vibrissae are both sensors and effectors in a complex sensory system that is able to locate and identify objects (Brecht et al., 1997; Gustafson and Felbain-Keramidas, 1977). The pioneering work of Vincent (1912) indicated that rats use this system for detection of surfaces during navigation. More recent studies have shown that the vibrissae provide information about object distance (Shuler et al., 2001; Solomon and Hartmann, 2006), bilateral distance (Knutsen et al., 2006; Krupa et al., 2001), and orientation (Polley et al., 2005). Yet few of these behaviors inherently engaged the sensorimotor nature of the system, and rats are known to perform some tasks, such as vibration discrimination (Hutson and Masterton, 1986), with only passive vibrissa contacts. Thus it is critical to establish whether touch and motion are used in concert to form an “active perceptual system” (Gibson, 1962).
We review the current understanding of object location in the azimuthal plane by rodents, a specific sensorimotor task that incorporates elements of behavior, anatomy, and electro-physiology. This focus highlights the choices made by the rodent nervous system in the conditioning of sensory input signals, the formulation of motor control, and the choice of coordinate representation. Related work on schemes to use vibrissae to code object location in three dimensions have been discussed by Knutsen and Ahissar (2009). The overall neuroanatomy of the vibrissa sensorimotor system has been reviewed (Bosman et al., 2011; Kleinfeld et al., 1999), and different aspects of the system are the subject of extensive reviews (Ahissar and Zacksenhouse, 2001; Brecht, 2007; Castro-Alamancos, 2004; Deschênes et al., 2005; Diamond et al., 2008; Fox, 2008; Haidarliu et al., 2008; Hartmann, 2011; Jones and Diamond, 1995; Kleinfeld et al., 2006; Kublik, 2004; Mitchinson et al., 2011; Moore et al., 1999; O'Connor et al., 2009; Petersen et al., 2002) including an emphasis on vibrissa areas of cortex (Alloway, 2008; Brecht, 2007; Lübke and Feldmeyer, 2007; Petersen, 2007; Schubert et al., 2007; Swadlow, 2002).
Lessons from Behavior
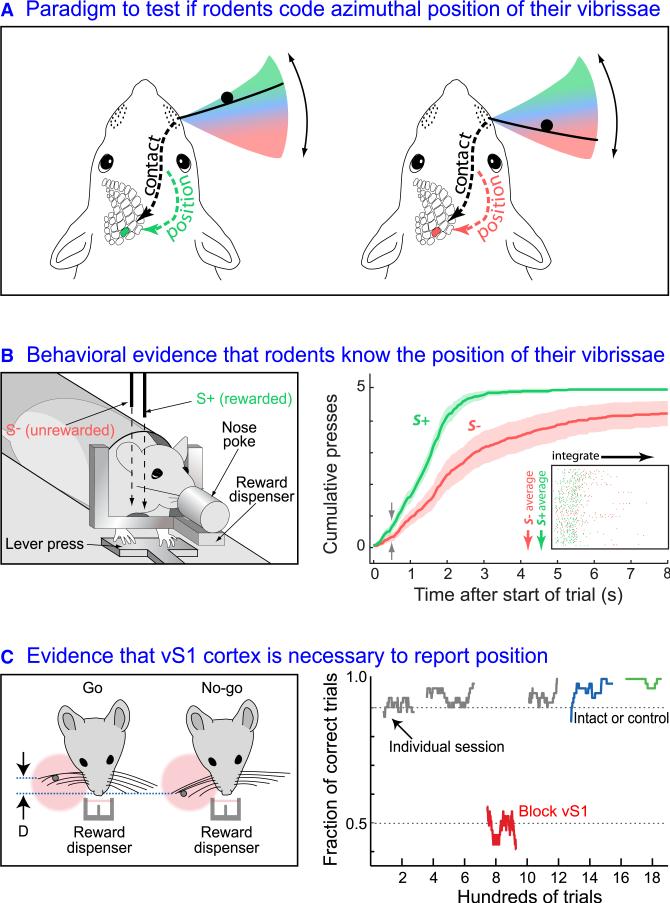
As a means to establish the vibrissa system as a model of choice for the study of sensorimotor control, it is essential to first determine if rodents have an internal representation of the position of their vibrissae. This question has been addressed through behavioral tasks, in which the animal must report the position of a pin relative to the face. As a practical matter, there are numerous algorithms that can allow an animal to approximate this task when the full complement of vibrissae are present. A clean paradigm is to test if an animal with a single vibrissa can determine the relative position of a pin within the azimuthal sweep of the vibrissa (Figure 2A). This form of experiment is realized through operant conditioning, in which a rat is trained to maintain a fixed posture and press a lever with a frequency that discriminates between a contact position that is rewarded (S+) versus one that is unreward (S–) (left panel and insert in right panel, Figure 2B). Mehta et al. (2007) found that rats can perform this discrimination task within a period of one or two whisks (right panel, Figure 2B). This implies that rats know the azimuthal position of their vibrissae. The results from related work, in which rats were trained to report the relative depth between two pins, suggests that azimuthal acuity is better than 6° (Knutsen et al., 2006).
Figure 2. Behavioral Evidence that Rats Code the Azimuth of Vibrissa Position.
(A) Cartoon of the computational problem: detection of an object with a moving sensor. An animal with one vibrissa is prompted to differentiate between one of two positions of an object, i.e., the black pin. When this task is performed with a single vibrissa the animal must code the position of the vibrissa in addition to contact with the pin. Adapted from Mehta et al. (2007).
(B) An experiment with free ranging rats that are self-constrained in a nose poke. The data demonstrate that rats can differentiate the position of a pin, lowered from above, using only a single vibrissa that is swept through space. The angular separation between pins was 15°. One of the two pins is paired with a reward and rodents respond by increasing their rate of lever pressing for the rewarded pin, S+, versus the unrewarded pin S–. Cumulative numbers of presses, plotted as mean with 95% confidence limits, are statistically different for the two cases 0.5 s after the start of the trial. The insert shows the raster plots for the first five lever presses for the rewarded and unrewarded presentations. Adapted from Mehta et al. (2007).
(C) An experiment with head-fixed mice. The data show that mice can detect the relative position of a pin using their vibrissae, with angular separations down to 6°, but that inactivation of contralateral vS1 cortex with injections of muscimol (red) removes this capability. Green trace is a control where saline is injected and blue trace is a control where vS1 cortex is injected with muscimol. Adapted from O'Connor et al. (2010a) with further analysis kindly made by Daniel H. O'Connor.
What is the role of cortex in this discrimination task? In particular, while rodents may be trained to discriminate object location, this process could occur at a subcortical level. This question was addressed by O'Connor et al. (2010a), who used head-fixed mice trained to discriminate among one of two positions of a pin (left panel, Figure 2C). Mice could perform this task with better than 90% discrimination at an acuity of less than 6°, albeit with a different strategy than found with the case for rats (Knutsen et al., 2006; Mehta et al., 2007). Here, rather than sweep their vibrissae, the animals tended to hold or slowly move their vibrissae near the site that one of the two pins was lowered. This difference aside, the ability to discriminate azimuthal location was lost when vibrissa primary sensory (vS1) cortex was shut down through an infusion of the GABAA agonist muscimol, and recovered upon wash out (right panel, Figure 2C). A potential caveat in this experiment is that inactivation of vS1 cortex can affect the ability of a rodent to whisk (Harvey et al., 2001; Matyas et al., 2010), so the transient loss in discrimination could reflect a motor rather than sensory defecit. In toto, behavioral data implies that the rodent vibrissa system is an valuable model to study the merge of sensor contact and position, and that vS1 cortex is likely to play a necessary role in computing the relative angle of touch.
Basic Wiring
What are the neural pathways that support signals of vibrissa touch and position? We review the anatomy of the vibrissa sensorimotor system so that physiological measurements can be placed in the context of high level circuitry (Figure 3).
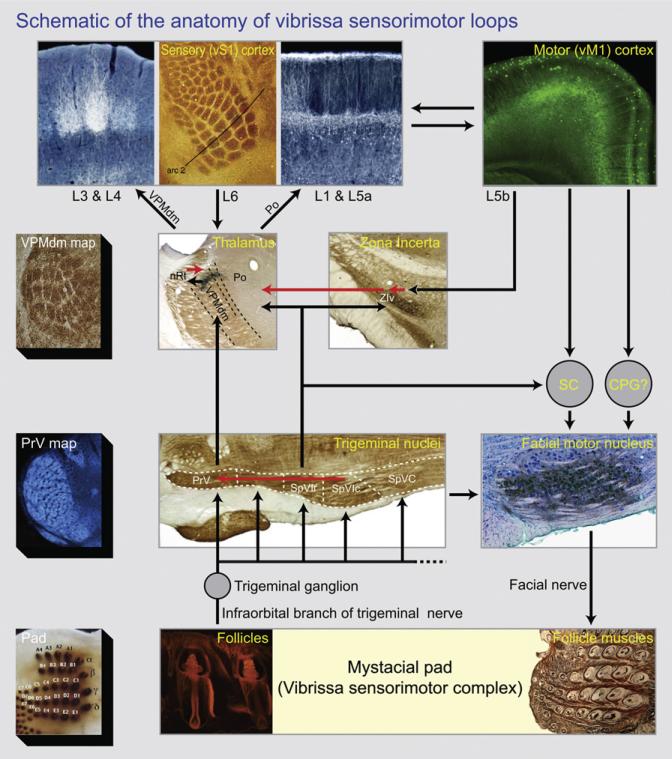
Figure 3. Schematic of the Anatomy of the Vibrissa Somatosensorimotor System.
Only the pathways from the vibrissae to the brainstem and up through neocortex are shown. Abbreviations: PrV, principal trigeminal nucleus; SpVIr and SpVIc, rostral and caudal divisions of spinal nucleus interpolaris, respectively; SpVC, spinal nucleus caudalis; VPMdm, dorsomedial aspect of the ventral posterior medial nucleus of dorsal thalamus; Po, medial division of the posterior group nucleus; nRt, nucleus reticularis; ZIv, ventral aspect of the zona incerta; CS, superior colliculus; and CPG, a yet to be described central pattern generator in the brainstem. Black arrows indicate excitatory projections while red arrows are inhibitory projections.
At the level of the periphery, the image of the mystacial pad is adapted from Haidarliu et al. (2010) (dorsal to the top and caudal to the right), the fluorescent microphotograph of the follicles was prepared by David W. Matthews following the advice of Fan Wang to use a mouse line in which red fluorescence protein expression is driven the promoter for parvalbumin (Hippenmeyer et al., 2005), and the microphotograph of the musculature was adapted from Haidarliu et al. (2010) (dorsal to the top and caudal to the right). At the level of the brainstem, the microphotograph of barrelettes in the PrV nucleus is from Martin Deschênes (coronal section), the microphoto-graph of the trigeminus is adapted from Furuta et al. (2008) (horizontal section, medial to the top and caudal to the right), and the microphotograph of the facial nucleus is from Harvey J. Karten (sagittal section, dorsal to the top and caudal to the right). At the level of the thalamus, the photomicrograph of the barreloids in the core region of VPMdm thalamus is adapted from Haidarliu and Ahissar (Haidarliu and Ahissar, 2001) (oblique section), the photomicrograph of multiple thalamic regions is from Martin Deschênes (coronal section, dorsal to the top and medial to the right), and the photomicrograph of zona incerta is adapted from Urbain and Deschênes (2007a) (coronal section, dorsal to the top and medial to the right). At the level of cortex, the photomicrographs of vS1 cortex in coronal section and the barrels in flattened horizontal section (rostral to the top and medial to the right) are from Martin Deschênes and that of vM1 cortex was prepared by Per Magne Knutsen using a mouse in which L5b neurons express green fluorescent protein (Arenkiel et al., 2007).
The basic layout of the sensorimotor system is one of nested loops (Kleinfeld et al., 1999). The follicles, which are both sensors through their support of vibrissae and effectors through their muscular drive, and the mystacial pad that supports the follicles form the common node in these loops. Afferent input is generated by shear or compression of mechanosensors in the follicles (Kim et al., 2011; Rice, 1993). The afferent signal propagates through primary sensory cells in the trigeminal ganglion, whose axons form the infraorbital branch of the trigeminal nerve. These cells make synaptic contacts onto neurons that lie within different nuclei of the trigeminus, all arranged in parallel. Of note is the one-to-one map of the input from the follicles onto the nucleus principalis (PrV) and the caudal division of the spinal nucleus interpolaris (SpVIc) (left column, Figure 3). A projection, but not one-to-one mapping, also occurs to the rostral division of nucleus interpolaris (SpVIr).
Two feedback loops in the brainstem condition the incoming sensory input. First, cells in nucleus SpVIc, which respond to an individual vibrissa, form inhibitory synapses onto neurons in nucleus PrV (red arrow in middle row, Figure 3). This feedback acts to spatially and temporally sharpen the response in a “center-surround” manner (Bellavance et al., 2010; Furuta et al., 2008). A second feedback pathway involves projections from the SpVI and SpVC trigeminal nuclei to the facial motoneurons, which independently drive motion of the follicle and that of the mystacial pad (Hill et al., 2008; Klein and Rhoades, 1985). This in turn leads to activation of the mystacial muscles and a forward thrust of the vibrissae upon contact (Nguyen and Kleinfeld, 2005; Sachdev et al., 2003). In principle, the latter feedback provides the animal with a means to distinguish between spikes in the trigeminus that are unrelated to contact, for which the thrust would push the vibrissae forward without the generation of additional spikes, and a true touch event, where the thrust enhances contact and can provide additional spikes.
The single projection from the trigeminal nucleus to the facial nucleus is paralleled by multiple polysynaptic pathways at the level of the brainstem and midbrain, e.g., the superior colliculus, and by pathways that extend through the forebrain (Kleinfeld et al., 1999; Figure 3); we focus on the latter. There are two major ascending pathways from the trigeminus. Projections from nucleus PrV ascend to the dorsal medial aspect of the ventral posterior medial (VPMdm) nucleus of dorsal thalamus, where they make a triplet of representations (Pierret et al., 2000; Urbain and Deschênes, 2007b; Veinante et al., 2000). The core region of this triplet is considered the primary afferent pathway and, as in the case of trigeminal nucleus PrV, this representation in VPMdm thalamus contains a one-to-one map of the input from the follicles (left column, Figure 3). Neurons in the core region of the VPMdm nucleus form a closed loop with inhibitory cells in nucleus reticularis (nRt), (red arrow in middle row, Figure 3) and further project to the middle layers, i.e., L3 and L4, of vibrissa primary sensory (vS1) cortex. The projections cluster into columns, commonly called barrels, that maintain the one-to-one relation with the spatial distribution of the vibrissae (top row, Figure 3).
The second set of ascending projections emanate from trigeminal nucleus SpVIr to the medial division of the posterior group (Po) nucleus of dorsal thalamus and involves both direct excitatory input from nucleus SpVIr as well as inhibitory input that comes indirectly via projections to the ventral aspect of the zona incerta (ZIv) (Barthó et al., 2002). The latter input is part of a forebrain loop in which activity in Po thalamus is modulated by projection neurons from vibrissa primary motor (vM1) cortex to ZIv, which inactivates an inhibitory input to Po thalamus (Urbain and Deschênes, 2007a) (back-to-back red arrows in middle row, Figure 3). Neurons in the Po nucleus project to the septa between columns and primarily form connections with dendrites on the surface and middle layers of vS1, i.e., L1 and L5a, in a pattern that appears complementary to that formed by input from VPMdm thalamus (top row, Figure 3). This is suggestive of different computational roles for inputs from VPMdm versus Po thalamus (Bureau et al., 2006; Meyer et al., 2010; Nakamura et al., 2009; Yu et al., 2006).
The classically described sensory and motor regions of cortex are highly interconnected, both at the level of the cortex itself and, as noted above, through subcortical interactions (Hoffer et al., 2003; top row, Figure 3) as well as feedback from cortex to thalamus. The highest level feedback loop in the vibrissa system is completed by descending projections from cortex to the vibrissa motoneurons in the facial motor nucleus. The dominant pathway of cortical activation of the vibrissae is from vM1 cortex (Berg et al., 2005; Brecht et al., 2004a, 2004b). This involves indirect connections through the superior colliculus and other midbrain as well as brainstem structures (Hattox et al., 2002; Miyashita et al., 1994) as well as a direct, albeit sparse projection (Grinevich et al., 2005; right column, Figure 3). A second pathway for activation of the vibrissae involves a projection from vS1 cortex (Matyas et al., 2010) that activates cells in trigeminal nuclei SpVI and SPVC. These, as also noted above, project to the facial nucleus (Erzurumlu and Killackey, 1979). The combined anatomical data suggests that the moto-neurons can be viewed as arbitrators of the control of motor output from different levels in the brain (Hattox et al., 2002), a role consistent with their electrotonically long dendrites and active currents (Nguyen et al., 2004).
Quantitative Description of Rhythmic Whisking
Whisking involves changes in vibrissa position that vary from the nominally 100 ms period of the whisking rhythm to one second changes in the envelope and midpoint of the motion (Berg et al., 2005; Carvell and Simons, 1995). Further, bouts of whisking may last tens of seconds, particularly as an animal searched for its home cage (Ganguly and Kleinfeld, 2004). This great range of time-scales serves as a diagnostic to understand how motor control is segregated between different brain circuits. For example, the frequency and amplitude of whisking are controlled by different neuromodulators (Pietr et al., 2010).
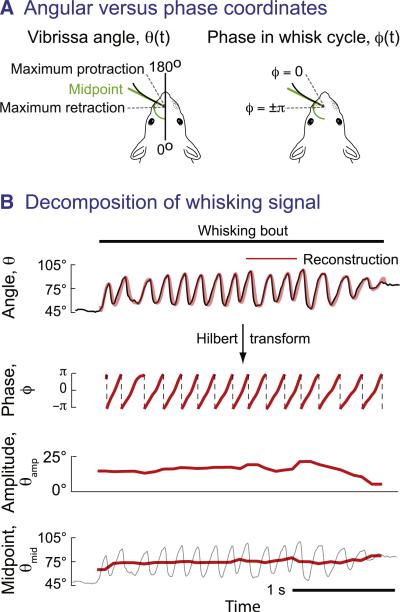
The near rhythmic form of the azimuthal angle of the vibrissae, θ(t), may be decomposed into rapidly and slowly varying waveforms by the Hilbert transformation (Figure 4A). This transform extracts a rapidly varying phase signal, denoted ϕ(t), that increases from –π to π radians on each whisk cycle regardless of slow variations in the amplitude of the whisking envelope, denoted θamp(t), and the midpoint of the motion, denoted, θmid(t) (Figure 4B). This allows the measured motion of the vibrissae to be expressed in the form
| (1) |
Figure 4. Decomposition of Rhythmic Whisking into a Varying Phase Component and Slowly Varying Envelope Parameters.
(A) Schematic of the angular parameters and their relation to phase in the whisk cycle.
(B) Top panel shows vibrissa position along with its reconstruction using a Hilbert transform. Lower panels show the phase, ϕ(τ), as calculated from the Hilbert transform, along with the amplitude, θamp, and midpoint, θmid, of the envelope calculated from individual whisk cycles. Broken vertical lines indicate wrapping of phase from π to –π radians. Adapted from (Hill et al., 2011a).
The reconstructed whisking waveform, , compares very well with the recorded motion (top line, Figure 4B). We interpret the slowly varying amplitude as the range of motion, the slowly varying midpoint as defining the region of interest, and the rapidly changing phase as the scan pattern of the vibrissae. Recall that phase is single valued and thus defines the position and direction of motion; the phase interval (–π, 0) corresponds to protraction and (0, π) to retraction. Lastly, individual vibrissae may have different midpoints, but the motion between vibrissae is highly correlated (Hill et al., 2011a).
Cortical Correlate of Vibrissa Position
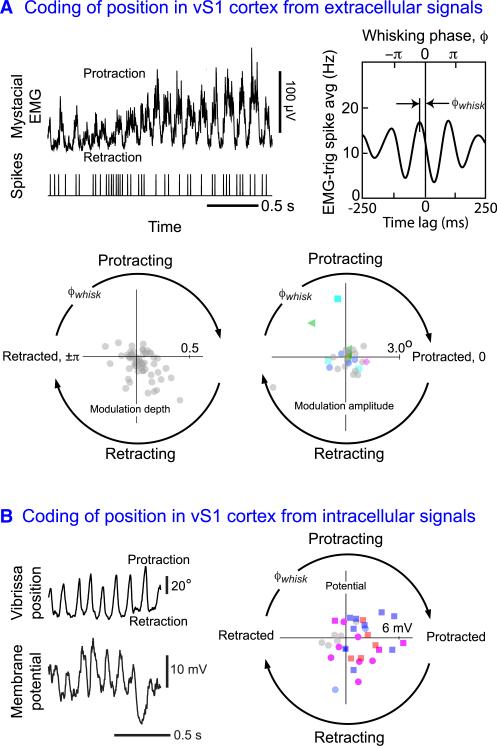
The necessity of vS1 cortex to perform a object localization task in the azimuthal plane (Figure 2C), as well as for other vibrissa-based tasks (Hutson and Masterton, 1986), raises the question of if and how vibrissa motion is represented in vS1 cortex. This was first addressed with free-ranging animals trained to whisk in air in search of a food tube (Fee et al., 1997; Figure 1B). Single units were recorded from microwires lowered throughout the depth of cortex, while vibrissae position was inferred from the electromyogram (EMG) of papillary muscles that drive the follicles (Figure 3). The EMG is a good surrogate of the phase and amplitude of whisking but not of the midpoint angle (Hill et al., 2011a; Figure 4A). The peak of the EMG signal corresponds to the most protracted position of the vibrissae and the valleys correspond to retraction. A quantitative relation between the spike trains and the EMG is determined from the cross-correlation of the spike arrival times with the times of the peaks of the EMG during each epoch of whisking (top row, Figure 5A). Statistically significant correlations were observed for about 60% of the units examined. The extent of the modulation of the spike rate by whisking is small, about 0.1 of the average rate. Subsequent work showed that similarly recorded units were distributed throughout all layers of cortex (Curtis and Kleinfeld, 2009).
Figure 5. Evidence that Neurons in Vibrissa Primary Sensory Cortex Code the Phase of Vibrissa Position.
(A) The top panel shows an example of extracellular recordings from vS1 cortex together with the electromyogram (EMG), a surrogate of vibrissa position. The EMG-triggered spike correlation shows that the two signals are correlated; this example shows a particularly strong correlation. The polar plots show the composite results for the EMG-triggered spike correlation from microwire recordings (left) and the spike-triggered motion correlation (right). Microwire recording adapted from Fee et al. (1997); layer specificity is unknown. Juxtacellular recordings adapted from de Kock and Sakmann (2009) and replotted on a linear scale; magenta diamonds correspond to L2/3 pyramidal cells, blue circles are L4 granular spiny neurons, cyan boxes are L5 slender tufted pyramids, green triangles are L5 thick tufted pyramids, orange circles are L6 pyramids, and gray circles have unknown layer specificity.
(B) Intracellular records from vS1 in an awake mouse as the animal whisk in air; this example shows a particularly strong modulation. The polar plot shows the EMG-triggered membrane potential average. Adapted from Crochet and Petersen (2006) and Gentet et al. (2010); we thank Carl C.H. Petersen for clarification of the data; yellow circles correspond to L2/3 pyramids, magenta circles and squares to L4 cells, gray circles have unknown superficial layer specificity, blue squares are L4 fast spiking inhibitory neurons, and red squares are regular spiking inhibitory neurons.
The peak of the correlation occurs at a phase that is different than the peak of protraction. This phase shift corresponds to the phase in the whisk cycle for which the rate of spiking is maximum and is referred to as the preferred whisking phase, or ϕwhisk. We observe that the preferred phase extends over all possible phases in the whisk cycle (lower left panel, Figure 5A) with a small but significant bias for relatively large amplitudes at the onset of retraction. A similarly broad distribution of phases, although without a bias in amplitudes, was found in measurements of the correlation between vibrissa position and spiking activity using head-fixed mice and juxtacelluar recording (de Kock and Sakmann, 2009). This extracellular procedure permits many of the neurons to be filled with dye and identified post hoc. Here, about 70% of the cells exhibited statistically significant albeit small modulation, corresponding to roughly 0.1 of the maximum whisking amplitude (lower right panel, Figure 5A), not inconsistent with the microwire results.
The relatively weak modulation of the spike rate by vibrissae position leaves open the question of whether the subthreshold potentials of neurons in vS1 cortex are strongly or weakly modulated by vibrissa position. Intracellular recording from the upper layers of vS1 cortex in head-fixed mice showed that the intracellular potentials are less variable as animals whisked compared to sessile periods and, critically, strongly modulated by changes in the position of the vibrissae (Crochet and Petersen, 2006; Gentet et al., 2010; left panel, Figure 5B). The modulation in voltage over a whisk cycle was 2 millivolts on average, which implies the convergence of many individual synaptic inputs. As with the case of extracellular recording, the preferred whisking phase, ϕwhisk, was distributed over all phases in the whisk cycle (right panel, Figure 5B). Further, the bias in the distribution found from the intracellular records for excitatory cells was consistent with that observed in the microwire data (cf lower left panel in Figure 5A and right panel in Figure 5B).
The composite result is that a majority of neurons throughout the depth of vS1 cortex report a signal that corresponds to the phase of the vibrissae in the whisk cycle. The tuning curves are broad, in the sense that the correlation between spike rates and whisking approximate a cosine curve (Figure 5A). The modulation of the spike rate by whisking is small for the vast majority of cells, although a small fraction of cells have a sufficiently deep modulation, and sufficiently high spike rate, to allow the phase in the whisk cycle to be predicted on a whisk by whisk basis (Fee et al., 1997; Kleinfeld et al., 1999). Even if the responses with deep modulation are discounted, the output from a population of cells with broad tuning and a continuous distribution of preferred phases can be used to estimate angular position with high accuracy (Hill et al., 2011a; Seung and Sompolinsky, 1993).
Origin of the Cortical Correlate: Peripheral Reafference versus Efference Copy in vS1 Cortex
There are two potential pathways for a signal that codes vibrissa position to reach vS1 cortex. One is by peripheral reafference, in which position is encoded along with contact by mechanosensors in the follicle. The peripheral coding of vibrissa position is analogous to proprioception. Here, as in proprioception, an overlapping set of pressure and stretch receptors may code both vibrissa position and touch (Berryman et al., 2006). This possibility implies that primary sensory neurons code vibrissa position in the absence of contact, and that this signal is relayed to vS1 cortex. It further implies that the fast modulation of neuronal signals in sV1 cortex will be eliminated if movement of the follicle is blocked as the animal attempts to whisk. The second of the two possible pathways to code vibrissa position within vS1 cortex is via an efference copy. Here, an internal brain pathway provides a facsimile of the command signal that is used to drive the vibrissa muscles. Such a signal could originate from a hypothesized brainstem pattern generator (CPG; Figure 3), perhaps relayed via vM1 cortex. In this case fast modulation of neuronal signals in vS1 cortex by whisking could be altered, but not eliminated, if whisking is blocked. Concepts from control theory suggest that both signals could be present in cortex as a means to compare actual versus intended vibrissa position (Ahissar et al., 1997; Kleinfeld et al., 2002).
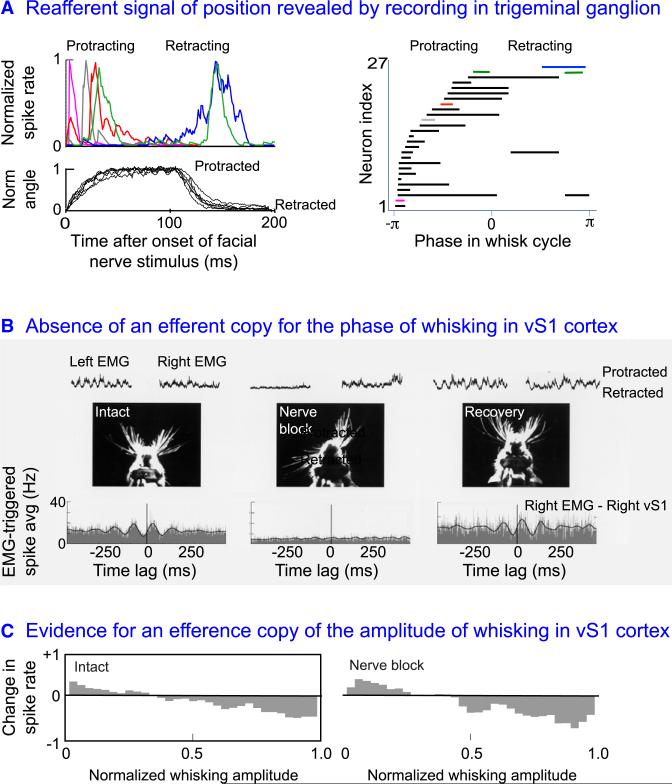
Recordings from primary sensory neurons during muscular activation of the follicle could distinguish between peripheral reafference and efference copy. Such recordings in the trigeminal ganglion are facilitated by the technique of fictive whisking, in which electrical stimulation of the facial nerve is used to rhythmically drive vibrissa motion in anesthetized animals (Brown and Waite, 1974; Zucker and Welker, 1969). Measurements of single-unit activity revealed a population of neurons in the trigeminal ganglion that spiked in response to a change in vibrissae position but not contact (Szwed et al., 2003). This established that muscular movement of the follicle alone is sufficient to drive spiking in primary sensory neurons. Further, different neurons spiked at different positions into the fictive whisk (Figure 6A). The histogram of spiking by different units covered the full range of protraction and part of retraction (Figure 6A). These data support a reafferent pathway that carries only reafferent signals of vibrissa position, as opposed to both position and touch signals. Yet details of the angle or phase response for different units are unlikely to reflect their response in the awake animal. The motor drive in fictive whisking consists only of protraction, as opposed to both retraction and protraction in awake animals (Berg and Kleinfeld, 2003). Further, the mechanics of the follicles are different for fictive whisking than when the follicle sinuses are gourged with blood in awake animals (Rice, 1993), so that the sensitivity of the receptors in the follicle to both self-motion and touch may be diminished in the anesthetized state.
Figure 6. Evidence that the Cortical Reference Signal of the Phase of Vibrissa Position, but Not the Amplitude, Is Derived from Peripheral Reafference.
(A) Unit records from the trigeminal ganglion obtained from an anesthetized rat in which the facial nerve was shocked, for an interval of 100 ms at a repetition rate of 5 Hz, to drive fictive whisking in air. Left, the normalized firing rate of five units during one whisking cycle, along with the concurrent normalized azimuthal deflection of the vibrissae. Right, the response as a function of spatial phase for all units; spatial phase is the relative azimuthal angle between fully retracted, i.e., ±π, and fully protracted, i.e., 0. The line segments for each unit indicate when the spike rate was between 50% and 100% of its maximum value. Adapted from Szwed et al. (2003); we thank Ehud Ahissar for kindly replotting the data.
(B) Single unit record from vS1 cortex in a rat that was whisking in air in search of a food tube, together with the simultaneously recorded ipsilateral (right) and contralateral (left) electromyogram (EMG), which act as a surrogate of vibrissa position. We observe a strong correlation with the motor nerve intact, similar to that in Figure 5A, and a loss of correlation upon block of the whisking, using a nerve cuff flushed with Lidocaine, on the contralateral face. The correlation reappears after the block is resolved. These data show that the phasic variation in spike rate depends on self motion. Adapted from Fee et al. (1997).
(C) The relation of the spike rate for the same unit in (B) to the amplitude of whisking. This slowly evolving signal shows a higher spike rate for small amplitude as opposed to large amplitude whisks; the data is normalized as EMG rather than actual vibrissa position was measured. We observe that the relation between spike rate and amplitude is unchanged during the nerve block, which implies that neurons in vS1 cortex receive an efference copy of this signal. Adapted from Fee et al. (1997).
Measurements from neurons in the trigeminal ganglion in awake animals are difficult as the ganglion lies in a cranial fossa. Reports from two laboratories provide evidence that different units will spike in different phases of the whisking cycle (Khatri et al., 2009; Leiser and Moxon, 2007). However, these same units invariably respond to touch as well. While this speaks against the possibility of a solely reafferent pathway, technical considerations suggest that the unit data contained contributions from more than one neuron (Hill et al., 2011b). Even if reafference and touch are coded by the same cells, the respective signals may be separated by differences in their spectral content for the case of repetitive contact; whisking occurs at low frequencies while punctate contact events lead to high frequency components in the spike response.
We return to the issue of efference copy. The test for signaling along this pathway makes use of two special aspects of whisking. First, there is exceptionally high coherence between whisking on both sides of the face. Second, the sensory nerve and the motor nerve are separate (Figure 3), so that motion can be blocked without affecting the receptors. This allows vibrissa motion on the ipsilateral side of the face to be used as a positional reference when motion of the vibrissae on the contraleral side is transiently blocked. These advantages were exploited, using the EMG as a surrogate to determine the phase and amplitude of vibrissa motion (Fee et al., 1997). Transient blockage of the contralateral facial nerve leads to loss of the correlation between spiking and the rhythmic component of the EMG on the intact side (Figure 6B). This implies that the phasic reference of vibrissa position is signaled through peripheral reafference, i.e., the rat “listens” to its own motion. In contrast, transient blockage of the contralateral facial nerve does not affect the correlation between the spike rate and the slowly varying amplitude of whisking (Fee et al., 1997; Figure 6C). This implies that the amplitude of whisking, which is weakly reported in vS1 cortex, is derived from an internal brain signal.
Coding the Range of Whisking
In the absence of information about the amplitude or midpoint of the whisk, the azimuthal position is left unspecified. Where is the additional information coded? Motivated by the internal generation of the amplitude signal of whisking (Figure 6C), a report of an overall increase in the spike rate of units in vM1 cortex concurrent with whisking (Carvell et al., 1996), and the extensive connectivity of vM1 with vS1 cortex (Hoffer et al., 2005; Figure 3), we turn our attention to this region of the brain.
Measurements of the relation between spiking in vM1 cortex and parameters of rhythmic whisking (Figure 4) were performed with both free-ranging and head fixed rats trained to whisk in air (Figure 1B; Hill et al., 2011a). Single units were recorded from microwires lowered throughout the depth of cortex, while vibrissa position was measured with videography. Spike trains from single unit data were found to be correlated with all aspects of whisking. Of particular note, about two-thirds of the units were modulated by the slow variations in the amplitude, θamp, and midpoint, θmid, of whisking (Figure 7). This representation persists after transection of the sensory nerve, i.e., the infraorbital branch of the trigeminal nerve (Figure 3), indicating an efferent source of the signal. Thus, the amplitude and midpoint of whisking are either generated in vM1 cortex or relayed to vM1 cortex from another brain area. A recent analysis of multiunit data supports the notion of amplitude coding by neurons in vM1 cortex (Friedman et al., 2011). Given the extensive connectivity between vS1 and vM1 cortices (Hoffer et al., 2005), a likely possibility is that the internally generated amplitude signal described in vS1 cortex (Fee et al., 1997) is relayed from vM1 cortex.
Figure 7. Evidence that Neurons in vM1 Cortex Report the Amplitude and Midpoint of Rhythmic Whisking.
Firing rate profiles for two example units in vM1 cortex as a function of slowly varying parameters, i.e., amplitude and midpoint, of vibrissa motion (Figure 4). The left and middle columns are profiles of units that show different relative modulation. Each plot is calculated by dividing the distribution of the respective signal at spike time by the distribution of that signal over the entire behavioral session. Green lines are fits from a smoothing algorithm along with the 95% confidence band. The right column shows composite data across units and illustrates that, on average, the rate is unaffected by whisking, consistent with the presence of units that both increase (green) and decrease (red) their rate with increasing angle; blue dots correspond to a nonmonotonic change. Adapted from Hill et al. (2011a).
Cortical Representation of Touch
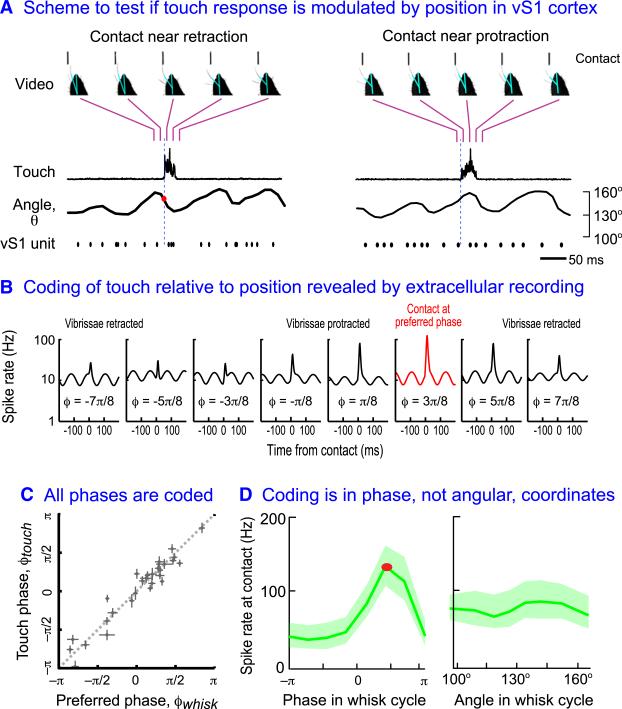
We now come to the crux issue and ask if neurons in vS1 cortex code touch conditioned on vibrissa position, i.e., on peripheral reafference. Such conditioning would imply that neurons in vS1 cortex contain the information necessary to report the location of an object that makes contact with a single vibrissa. These cells could therefore underlie the animal's ability to report object position (Knutsen et al., 2006; Mehta et al., 2007; O'Connor et al., 2010a; Figure 2). In principle, neurons can code both touch and position independently. The critical test of whether touch and reafferent signals are merged in vS1 cortex is if the strength of the touch response depends on where the vibrissae are in the whisk cycle. The experimental realization involved recording single units in vS1 cortex while rodents contacted a sensor for a liquid reward. Both free ranging and body fixed animals were used in a paradigm designed to ensure that the animals contacted the sensor at all possible positions in the whisk cycle across a different set of trials (Figure 8A). This in turn ensured that the strength of the contact response for each unit could be determined as a function of position and, with further analysis (Figure 4), as a function of phase in the whisk cycle.
Figure 8. Evidence that Neurons in vS1 Cortex Encode Contact with an Object Relative to the Phase of the Vibrissae in the Whisk Cycle.
(A) The scheme used to measure the spike response of units in vS1 cortex as animals rhythmically whisk first in air then whisk to touch a contact sensor. Vibrissa position is determined from videography while contact is determined via displacement of the sensor. A critical aspect of this measurement is that touch need to be recorded across all phases of the whisk cycle, as shown here for the cases of retraction (left) and protraction (right). Adapted from Curtis and Kleinfeld (2009).
(B) Histograms of the smoothed, trial-averaged instantaneous spiking relative to the time of contact. The data was parsed according to the phase in the whisk cycle with a phase interval of π/4 radians. The instantaneous spike rate is plotted on a logarithmic scale to highlight the underlying rhythmic modulation by whisking, as well as the increase in spiking upon contact. Note that the contact event is greatest at the particular phase φ = 3π/8 (red trace and corresponding dot in (A). This data shows that the touch response is strongly modulated by the phase in the whisk cycle. Adapted from Curtis and Kleinfeld (2009).
(C) Scatter plot of the preferred phase for free whisking versus the preferred phase for touch. Shown are mean values plus 95% confidence intervals for the estimates of either phase. All phases are represented, as in free whisking (Figure 5A), and the data is consistent with φtouch = φwhisk. Adapted from Curtis and Kleinfeld (2009).
(D) The left plot shows the peak values of the touch response from the fits to each of the eight intervals of the touch responses in (B). The uncertainly represents the 95% confidence interval. A smooth curve through this data defines the phase of maximal touch response, denoted φtouch (red dot and corresponding dot in (A). The right plot is the same data parsed according to the angular position of the vibrissa upon contact. The angle is measured relative to the midline of the animal's head. Unlike the case for phase, there is no significant tuning for angle. Adapted from Curtis and Kleinfeld (2009).
A majority of neurons in L4 and L5a exhibit a prompt response to self-induced contact (Crochet and Petersen, 2006; Curtis and Kleinfeld, 2009; O'Connor et al., 2010b), not unlike that observed in experiments with mechanical stimulation of a vibrissa in an anesthetized preparation (Armstrong-James et al., 1992; Armstrong-James and George, 1988; Simons, 1978). The strength of the contact response as a function of the phase in the whisk cycle was found for eight different phase intervals of π/4 radians. Consider the example of Figure 8B. The instantaneous rate varies by nearly a factor of three across the whisk cycle and, in this example, peaked near the start of protraction from the retracted position. In general, 85% of the units with a prompt touch response showed strong conditioning of the touch response by phase in the whisk cycle. The consensus data indicates that the preferred phases for touch, denoted ϕtouch, matches the preferred phase for whisk, i.e., ϕtouch y ϕwhisk (Figure 8C). Thus the spike rate upon contact is nominally proportional to a nonlinear function, such as cos [ϕ(t) – ϕwhisk]. These data show that vS1 cortex codes touch contingent upon position in the whisk cycle.
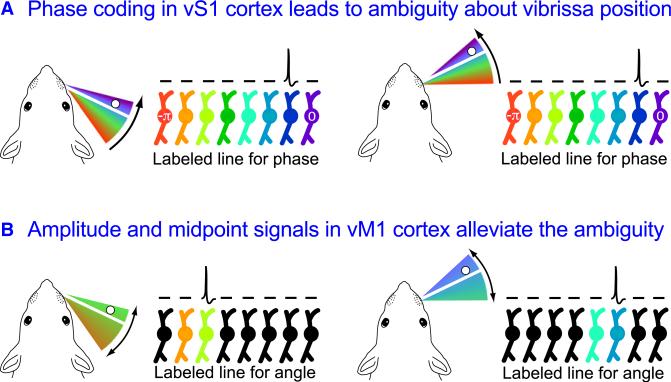
Resolving the Ambiguity of Phase Coordinates
We now return to the topic of the coordinate system used to code vibrissae motion (Figure 4A). The videographic analysis of vibrissa movement allowed the instantaneous spike rate upon contact to be plotted against either phase or actual azimual angle (Figure 8A). Across the entire population of single units in which the contact response was significantly dependent on phase, only 10% showed a significant dependence of contact on angular position (Curtis and Kleinfeld, 2009). Thus, we conclude that neurons in vS1 cortex predominantly code the phase, and not the angle, of vibrissa position. However, the representation of vibrissa contact solely in terms of phase is ambiguous (Figure 9A). When a neuron in vS1 cortex that encoded vibrissa touch produces a spike, or a group of such neurons spike, the available information only specifies the value of the phase at that point in time, i.e., ϕ(t) = ϕtouch at the time of the spike.
Figure 9. Ambiguity Posed by Coding in Phase Coordinates.
(A) The dilemma posed by coding only the phase of contact of a vibrissa with an object (white dot). Whisking at two different midpoints can lead to the same pattern of neuronal activation in vS1 cortex. For simplicity, the labeled line of neurons corresponds only to the (–π, 0) interval of phases during protraction. Black dot indicates the activated neuron. Panel inspired by Fairhall et al. (2001).
(B) Unambiguous coding of vibrissa azimuthal position in terms of angle. Each neuron in the labeled line, presumably in deep layers of vS1 cortex or in vM1 cortex, now responds to a unique angle, as opposed to phase as in (A). Colored neurons are those potentially activated for the indicated range of whisking, while the spike labels the particular neuron activated by contact. Panel inspired by Fairhall et al. (2001).
The combination of the coding of the rapid phase signal of touch, which predominates the neuronal response in vS1, and the slowly varying envelope signals that predominate the response in vM1 cortex, allows the azimuthal angle of contact of a vibrissa to be unambiguously established (Equation 1; Figure 9B). The locus and mechanism for reforming angle from the phase, amplitude and midpoint components is presently unknown. Yet these neuronal signals comprise the neurological correlate for behavioral data that show that rodents can discriminate the angle of a pin relative to their face (Mehta et al., 2007; O'Connor et al., 2010a; Figure 2).
Synopsis
We have summarized work on a common neuronal computation, computing sensory input relative to the orientation of the body. The model behavior involves the vibrissa sensorimotor system of rodents (Figure 1B), where an animal touches an object while rhythmically scanning its vibrissae and must determine where touch occurred relative to its head (Figure 2). The computation appears to be performed in primary sensory cortex (Figure 3) and makes use of a strong, nonlinear interaction in which a weak reafferent signal of vibrissa position (Figure 5) modulates a strong touch signal (Figure 8). Further, the sensory branch of the vibrissa system makes use of coding in normalized coordinates, where the spatial range of sensory coding adapts to the physical range of motion spanned by motor control of the vibrissae (Figure 9).
Are the results reviewed here applicable for nonrhythmic yet spatially localized scanning of the vibrissae? The rapidly varying component of the motion, the phase ϕ(t), is an inherently rhythmic quantity that also describes the relative range of vibrissa motion. In this latter sense, the description of vibrissa motion in terms of a rapidly varying phase and a slowly varying amplitude and midpoint, θamp and θmid, are valid descriptions of vibrissa motion under conditions of non-rhythmic as well as rhythmic whisking and complies with the tendency of rodents to limit the spatial extent of whisking in a task dependent manner (Crochet and Petersen, 2006; Gentet et al., 2010; Knutsen et al., 2006; Mehta et al., 2007; O'Connor et al., 2010a). The interpretation of phase as a spatial variable also suggests why different sensory (Crochet and Petersen, 2006; Curtis and Kleinfeld, 2009; de Kock and Sakmann, 2009; Fee et al., 1997; Gentet et al., 2010; Szwed et al., 2003; Figures 5 and 6) as well as motor neurons (Hill et al., 2011a) have a multiplicity of preferred phases, when, for a purely rhythmic system, only a single phase reference is required.
Next Steps
Numerous open issues remain within the rubric of object location by the vibrissa system per se. We consider a select set of these solely as a means to spark discussion about future experiments.
First and foremost, what is the cortical circuitry involved in the detection of contact in the azimuthal plane? The contact response is conditioned on vibrissa position in the whisk cycle (Figure 8B). The nonlinearity that governs this process is primarily confined to layers L4 and L5a (Curtis and Kleinfeld, 2009; O'Connor et al., 2010b), which receive direct input from VPMdm thalamus (Figure 3). One possibility is that the touch signal is modulated by shunting inhibition that is driven by reafference (Curtis and Kleinfeld, 2009), although the present data does not support this hypothesis (Gentet et al., 2010). A second possibility involves a strong nonlinear dependence of the gain (Lundstrom et al., 2009), i.e., spike rate versus input current, of cells that report vibrissa touch. Another aspect of this question concerns the readout of the response. This is likely to involve L5b projection neurons, whose prolonged response after touch (Curtis and Kleinfeld, 2009) is consistent with their hypothesized role as integrators of local and long-range cortical signals (London and Häusser, 2005). Experiments to address these questions will undoubtedly involve cell-based circuit analysis procedures (Arenkiel and Ehlers, 2009; O'Connor et al., 2009).
What is the nature of the transduction that governs touch? The largest obstacle to progress is that the mechanosensors in the follicle are uncharacterized, with the exception of the Merkel receptors (Hasegawa et al., 2007). Identification of the receptors and their connections through the trigeminal ganglion will bear on our understanding of the multiple representations of vibrissa input across different brainstem trigeminal nuclei (Figure 3). Does each nucleus receive input from all types of mechanoreceptors, as implied from the results of studies with individually filled trigeminal ganglion cells (Shortland et al., 1995, 1996)? Or rather do different nuclei predominantly represent different receptor types? These questions may be considered part of a larger effort to identify all mechanosensors involved in somatosensation (Bautista and Lumpkin, 2011; Luo et al., 2009). Second, the mechanics of the follicle need to be analyzed. The mechanoreceptors are arranged in a stereotypic pattern of rings and sheets (Mosconi et al., 1993). How are these activated by self motion versus touch to the vibrissa? How are these arranged, or connected, so that primary afferents are active only at a single phase in the whisk cycle (Figure 6A), as opposed to two phases if velocity of position was directly coded?
Are there separate pathways for the touch signal versus reafferent signals of vibrissa position? The current data support the role of neurons in VPMdm thalamus as the main pathway for touch signaling (Khatri et al., 2004, 2010; Masri et al., 2008; Simons and Carvell, 1989; Yu et al., 2006) and as a conduit for changes in vibrissa position (Khatri et al., 2010; Masri et al., 2008; Yu et al., 2006). However, there is discord as to the nature of signaling through Po thalamus (Masri et al., 2008; Yu et al., 2006), particularly whether this pathway supports a pure position signal or is even activated by afferent, as opposed to corticofugal, inputs (Diamond et al., 1992). The resolution of this issue bears on the representation of proprioception in the vibrissa system, as well as the laminar distribution of the reafferent signal in vS1 cortex (Figure 3). Finally, it is critical to determine whether or not conditioning of the touch signal by changes in vibrissa position occurs first in thalamus and is merely reported to vS1 cortex.
Where and how does the presumed conversion of phase to angular position take place? The circuitry for this coordinate transformation (Equation 1) is unknown. As alluded to earlier, the answer is likely to involve the interaction between vS1 and vM1 cortices (Figures 3 and 9). Related questions concern the form of the neuronal code for the location of an object that has been contacted. Do individual neurons code the azimuthal angle as a scalar quantity, or rather is the code in terms of the output of a population of motor units that control the heading of the animal toward or away from the object? Experimental progress on these fronts will involve first, measuring the motor modulation of the contact response in vM1 cortex and second, examining the interaction between specifically labeled neurons in both cortices and the output from these areas; recent work supports the feasibility of the latter approach (Mao et al., 2011; Mittmann et al., 2011; Sato and Svoboda, 2010).
We now turn to basic questions about the control of vibrissa motion. Rhythmic whisking can occur in the absence of sensory feedback and in the absence of high-level control (Berg and Kleinfeld, 2003; Gao et al., 2001; Welker, 1964). Where is the hypothesized pattern generator for rhythmic whisking (CPG in Figure 3)? When and how does this rhythm synchronize with other orofacial rhythms, such as breathing and licking (Travers, 1995)? At a more abstract level, how is the motor pattern altered by ongoing pattern of sensory input? For example, contact of the vibrissae with an extended object can lead to turning of the head and an asymmetry of whisking, so that the range of whisking is shortened on one side of the face and extended on the other side to match the angle of the turn (Mitchinson et al., 2007; Towal and Hartmann, 2006). This phenomena appears analogous to the vestibulo-ocular reflex, which suggests that such control is governed by feedback at the level of the brainstem. Yet, does cortical processing play a role in this contact response? Progress on sensory control of motor programs is in need of sophisticated yet rapidly learned behavior paradigms, perhaps involving object recogniton (Brecht et al., 1997).
Finally, it is important to redress our focus on signaling in thalamocortical pathways to the exclusion of feedback through basal ganglia as well as subcortical loops formed by pontinecerebellar and collicular pathways. The involvement of basal ganglia in whisking is largely uncharted, as only sensory responses in anesthetized animals have been reported (Pidoux et al., 2011). Cerebellar projection cells respond to vibrissa input (Bosman et al., 2010) and cerebellar output can affect the timing in vM1 cortex (Lang et al., 2006), but again there is no composite understanding. The situation is more advanced for the case of the superior colliculus, which receives direct vibrissa input via a trigeminotectal pathway (Killackey and Erzurumlu, 1981; Figure 3), indirect input via a corticotectal pathway through vS1 and vM1 cortices (Alloway et al., 2010; Miyashita et al., 1994; Wise and Jones, 1977) and can drive whisking as well (Hemelt and Keller, 2008). Recording in awake free ranging animals show that cells in the colliculus respond to vibrissa touch (Cohen and Castro-Alamancos, 2010), while experiments that used fictive whisking with anesthetized animals show that cells can respond to movement in the absence of contact (Bezdudnaya and Castro-Alamancos, 2011). It remains to be determined if the colliculus contains neurons that report touch conditioned on the position of the vibrissae and, if so, how these interact with the computation of touch in cortex.
Conclusions
The vibrissa system is a particularly powerful proving ground to establish basic circuitry for sensorimotor control. The relatively stereotyped whisking motion, the separation of sensory and motor signals on different nerves, and the accessibility of the system for electrophysiological study allow for fine experimental control. How general are these results? Essential aspects of sensation, such as balance with the vestibular system, seeing through the visual system, or touch through the somatosensory system, all make use of moving sensors and must solve an analogous problem to that discussed for the case of the rodent vibrissa sensorimotor system. This problem has been well studied for the case of vestibular control (Cullen et al., 2011; Green and Angelaki, 2010), but has gained accelerating interest for the cases of other sensory modalities, in part from the advent of automated behavioral procedures (Dombeck et al., 2007; Perkon et al., 2011), new tools to record intracellular (Lee et al., 2006) and multicellular (Sawinski et al., 2009) activity from behaving animals, and tools for targeted optical stimulation (Gradinaru et al., 2007). Even within the field of primate vision, traditionally performed with behaving animals, early work on pathways for the control of gaze (Richmond and Wurtz, 1980) has been revitalized (Sommer and Wurtz, 2002) under the rubric of “active vision” (Wurtz et al., 2011). We argue that the rodent sensorimotor system can be a cornerstone for the impact of neuroscience in areas of motion control that range from algorithm design for robotics to insights into normal and dysfunctional aspects of human motor activities.
ACKNOWLEDGMENTS
This review is dedicated to Prof. Wallace I. Welker, late of the University of Wisconsin, whose prescient studies taught us to view the circuitry of the vibrissa system in light of the behavioral strategies of rodents, and whose papers remind us that computations in the vibrissa system start and end at the brainstem. We thank our colleagues Ehud Ahissar, Matthew E. Diamond, Adrienne L. Fairhall, Jeffrey C. Magee, Bert Sakmann, Haim Sompolinsky, and Karel Svoboda, and members of their respective laboratories, for discussions that shaped this review, Ehud Ahissar, Harvey J. Karten, Charles F. Stevens, the anonymous reviewers, and especially Jeffrey D. Moore for comments on the submitted version, and the Canadian Institutes of Health Research (grant MT-5877), the National Institutes of Health (grant NS058668), and the US-Israeli Binational Foundation (grant 2003222) for their support.
REFERENCES
- Ahissar E, Zacksenhouse M. Temporal and spatial coding in the rat vibrissal system. Prog. Brain Res. 2001;130:75–87. doi: 10.1016/s0079-6123(01)30007-9. [DOI] [PubMed] [Google Scholar]
- Ahissar E, Haidarliu S, Zacksenhouse M. Decoding temporally encoded sensory input by cortical oscillations and thalamic phase comparators. Proc. Natl. Acad. Sci. USA. 1997;94:11633–11638. doi: 10.1073/pnas.94.21.11633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloway KD. Information processing streams in rodent barrel cortex: the differential functions of barrel and septal circuits. Cereb. Cortex. 2008;18:979–989. doi: 10.1093/cercor/bhm138. [DOI] [PubMed] [Google Scholar]
- Alloway KD, Smith JB, Beauchemin KJ. Quantitative analysis of the bilateral brainstem projections from the whisker and forepaw regions in rat primary motor cortex. J. Comp. Neurol. 2010;518:4546–4566. doi: 10.1002/cne.22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel BR, Ehlers MD. Molecular genetics and imaging technologies for circuit-based neuroanatomy. Nature. 2009;461:900–907. doi: 10.1038/nature08536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-James M, George MJ. Influence of anesthesia on spontaneous activity and receptive field size of single units in rat Sm1 neocortex. Exp. Neurol. 1988;99:369–387. doi: 10.1016/0014-4886(88)90155-0. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J. Neurophysiol. 1992;68:1345–1358. doi: 10.1152/jn.1992.68.4.1345. [DOI] [PubMed] [Google Scholar]
- Barthó P, Freund TF, Acsády L. Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur. J. Neurosci. 2002;16:999–1014. doi: 10.1046/j.1460-9568.2002.02157.x. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Lumpkin EA. Perspectives on: information and coding in mammalian sensory physiology: probing mammalian touch transduction. J. Gen. Physiol. 2011;138:291–301. doi: 10.1085/jgp.201110637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavance MA, Demers M, Deschênes M. Feedforward inhibition determines the angular tuning of vibrissal responses in the principal trigeminal nucleus. J. Neurosci. 2010;30:1057–1063. doi: 10.1523/JNEUROSCI.4805-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RW, Kleinfeld D. Rhythmic whisking by rat: retraction as well as protraction of the vibrissae is under active muscular control. J. Neurophysiol. 2003;89:104–117. doi: 10.1152/jn.00600.2002. [DOI] [PubMed] [Google Scholar]
- Berg RW, Friedman B, Schroeder LF, Kleinfeld D. Activation of nucleus basalis facilitates cortical control of a brain stem motor program. J. Neurophysiol. 2005;94:699–711. doi: 10.1152/jn.01125.2004. [DOI] [PubMed] [Google Scholar]
- Berryman LJ, Yau JM, Hsiao SS. Representation of object size in the somatosensory system. J. Neurophysiol. 2006;96:27–39. doi: 10.1152/jn.01190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdudnaya T, Castro-Alamancos MA. Superior colliculus cells sensitive to active touch and texture during whisking. J. Neurophysiol. 2011;106:332–346. doi: 10.1152/jn.00072.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman LWJ, Koekkoek SK, Shapiro J, Rijken BFM, Zandstra F, van der Ende B, Owens CB, Potters JW, de Gruijl JR, Ruigrok TJ, De Zeeuw CI. Encoding of whisker input by cerebellar Purkinje cells. J. Physiol. 2010;588:3757–3783. doi: 10.1113/jphysiol.2010.195180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman LWJ, Houweling AR, Owens CB, Tanke N, Shevchouk OT, Rahmati N, Teunissen WHT, Ju C, Gong W, Koekkoek SKE, et al. Anatomical pathways involved in generating and sensing rhythmic whisker movements. Front. Integr. Neurosci. 2011 doi: 10.3389/fnint.2011.00053. Published online October 3, 2011. 10.3389/fnint.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M. Barrel cortex and whisker-mediated behaviors. Curr. Opin. Neurobiol. 2007;17:408–416. doi: 10.1016/j.conb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behav. Brain Res. 1997;84:81–97. doi: 10.1016/s0166-4328(97)83328-1. [DOI] [PubMed] [Google Scholar]
- Brecht M, Krauss A, Muhammad S, Sinai-Esfahani L, Bellanca S, Margrie TW. Organization of rat vibrissa motor cortex and adjacent areas according to cytoarchitectonics, microstimulation, and intracellular stimulation of identified cells. J. Comp. Neurol. 2004a;479:360–373. doi: 10.1002/cne.20306. [DOI] [PubMed] [Google Scholar]
- Brecht M, Schneider M, Sakmann B, Margrie TW. Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature. 2004b;427:704–710. doi: 10.1038/nature02266. [DOI] [PubMed] [Google Scholar]
- Brown AWS, Waite PME. Responses in the rat thalamus to whisker movements produced by motor nerve stimulation. J. Physiol. 1974;238:387–401. doi: 10.1113/jphysiol.1974.sp010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau I, von Saint Paul F, Svoboda K. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol. 2006;4:e382. doi: 10.1371/journal.pbio.0040382. 10.1371/journal.pbio.0040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Task- and subject-related differences in sensorimotor behavior during active touch. Somatosens. Mot. Res. 1995;12:1–9. doi: 10.3109/08990229509063138. [DOI] [PubMed] [Google Scholar]
- Carvell GE, Miller SA, Simons DJ. The relationship of vibrissal motor cortex unit activity to whisking in the awake rat. Somatosens. Mot. Res. 1996;13:115–127. doi: 10.3109/08990229609051399. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog. Neurobiol. 2004;74:213–247. doi: 10.1016/j.pneurobio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Castro-Alamancos MA. Behavioral state dependency of neural activity and sensory (whisker) responses in superior colliculus. J. Neurophysiol. 2010;104:1661–1672. doi: 10.1152/jn.00340.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S, Petersen CCH. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat. Neurosci. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Brooks JX, Jamali M, Carriot J, Massot C. Internal models of self-motion: computations that suppress vestibular reafference in early vestibular processing. Exp. Brain Res. 2011;210:377–388. doi: 10.1007/s00221-011-2555-9. [DOI] [PubMed] [Google Scholar]
- Curtis JC, Kleinfeld D. Phase-to-rate transformations encode touch in cortical neurons of a scanning sensorimotor system. Nat. Neurosci. 2009;12:492–501. doi: 10.1038/nn.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kock CP, Sakmann B. Spiking in primary somatosensory cortex during natural whisking in awake head-restrained rats is cell-type specific. Proc. Natl. Acad. Sci. USA. 2009;106:16446–16450. doi: 10.1073/pnas.0904143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descartes R. P.J. Olscamp (trans.) Hackett Publsihing Compant; Indianapolis, IN: 1637. Discourse on Methods, Optics, Geometry, and Meteorology. [Google Scholar]
- Deschênes M, Timofeeva E, Lavallée P, Dufresne C. The vibrissal system as a model of thalamic operations. Prog. Brain Res. 2005;149:31–40. doi: 10.1016/S0079-6123(05)49003-2. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Budway MJ, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus: dependence on the barrel field cortex. J. Comp. Neurol. 1992;319:66–84. doi: 10.1002/cne.903190108. [DOI] [PubMed] [Google Scholar]
- Diamond ME, von Heimendahl M, Knutsen PM, Kleinfeld D, Ahissar E. ‘Where’ and ‘what’ in the whisker sensorimotor system. Nat. Rev. Neurosci. 2008;9:601–612. doi: 10.1038/nrn2411. [DOI] [PubMed] [Google Scholar]
- Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Killackey HP. Efferent connections of the brainstem trigeminal complex with the facial nucleus of the rat. J. Comp. Neurol. 1979;188:75–86. doi: 10.1002/cne.901880107. [DOI] [PubMed] [Google Scholar]
- Fairhall AL, Lewen GD, Bialek W, de Ruyter Van Steveninck RR. Efficiency and ambiguity in an adaptive neural code. Nature. 2001;412:787–792. doi: 10.1038/35090500. [DOI] [PubMed] [Google Scholar]
- Fee MS, Mitra PP, Kleinfeld D. Central versus peripheral determinants of patterned spike activity in rat vibrissa cortex during whisking. J. Neurophysiol. 1997;78:1144–1149. doi: 10.1152/jn.1997.78.2.1144. [DOI] [PubMed] [Google Scholar]
- Fox K. Barrel Cortex. Cambridge University Press; Cambridge: 2008. [Google Scholar]
- Friedman WA, Zeigler HP, Keller A. Vibrissae motor cortex unit activity during whisking. J. Neurophysiol. 2011 doi: 10.1152/jn.01132.2010. in press. Published online October 12, 2011. 10.1152/jn.00659.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T, Timofeeva E, Nakamura K, Okamoto-Furuta K, Togo M, Kaneko T, Deschênes M. Inhibitory gating of vibrissal inputs in the brainstem. J. Neurosci. 2008;28:1789–1797. doi: 10.1523/JNEUROSCI.4627-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Kleinfeld D. Goal-directed whisking increases phase-locking between vibrissa movement and electrical activity in primary sensory cortex in rat. Proc. Natl. Acad. Sci. USA. 2004;101:12348–12353. doi: 10.1073/pnas.0308470101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Bermejo R, Zeigler HP. Whisker deafferentation and rodent whisking patterns: behavioral evidence for a central pattern generator. J. Neurosci. 2001;21:5374–5380. doi: 10.1523/JNEUROSCI.21-14-05374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CCH. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron. 2010;65:422–435. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Gibson JJ. Observations on active touch. Psychol. Rev. 1962;69:477–491. doi: 10.1037/h0046962. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, Deisseroth K. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J. Neurosci. 2007;27:14231–14238. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AM, Angelaki DE. Internal models and neural computation in the vestibular system. Exp. Brain Res. 2010;200:197–222. doi: 10.1007/s00221-009-2054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich V, Brecht M, Osten P. Monosynaptic pathway from rat vibrissa motor cortex to facial motor neurons revealed by lentivirus-based axonal tracing. J. Neurosci. 2005;25:8250–8258. doi: 10.1523/JNEUROSCI.2235-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson JW, Felbain-Keramidas SL. Behavioral and neural approaches to the function of the mystacial vibrissae. Psychol. Bull. 1977;84:477–488. [PubMed] [Google Scholar]
- Haidarliu S, Ahissar E. Size gradients of barreloids in the rat thalamus. J. Comp. Neurol. 2001;429:372–387. doi: 10.1002/1096-9861(20010115)429:3<372::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Haidarliu S, Yu C, Rubin N, Ahissar E. Lemniscal and extralemniscal compartments in the VPM of the rat. Front. Neuroanat. 2008;2:4. doi: 10.3389/neuro.05.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidarliu S, Simony E, Golomb D, Ahissar E. Muscle architecture in the mystacial pad of the rat. Anat. Rec. (Hoboken) 2010;293:1192–1206. doi: 10.1002/ar.21156. [DOI] [PubMed] [Google Scholar]
- Hartmann MJ. A night in the life of a rat: vibrissal mechanics and tactile exploration. Ann. N Y Acad. Sci. 2011;1225:110–118. doi: 10.1111/j.1749-6632.2011.06007.x. [DOI] [PubMed] [Google Scholar]
- Hattox AM, Priest CA, Keller A. Functional circuitry involved in the regulation of whisker movements. J. Comp. Neurol. 2002;442:266–276. doi: 10.1002/cne.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Bermejo R, Zeigler HP. Discriminative whisking in the head-fixed rat: optoelectronic monitoring during tactile detection and discrimination tasks. Somatosens. Mot. Res. 2001;18:211–222. doi: 10.1080/01421590120072204. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Abbott S, Han B-X, Qi Y, Wang F. Analyzing somatosensory axon projections with the sensory neuron-specific Advillin gene. J. Neurosci. 2007;27:14404–14414. doi: 10.1523/JNEUROSCI.4908-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemelt ME, Keller A. Superior colliculus control of vibrissa movements. J. Neurophysiol. 2008;100:1245–1254. doi: 10.1152/jn.90478.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DN, Bermejo R, Zeigler HP, Kleinfeld D. Biomechanics of the vibrissa motor plant in rat: rhythmic whisking consists of triphasic neuro-muscular activity. J. Neurosci. 2008;28:3438–3455. doi: 10.1523/JNEUROSCI.5008-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DN, Curtis JC, Moore JD, Kleinfeld D. Primary motor cortex reports efferent control of vibrissa position on multiple time scales. Neuron. 2011a;72:344–356. doi: 10.1016/j.neuron.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DN, Mehta SB, Kleinfeld D. Quality metrics to accompany spike sorting of extracellular signals. J. Neurosci. 2011b;31:8699–8705. doi: 10.1523/JNEUROSCI.0971-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer ZS, Hoover JE, Alloway KD. Sensorimotor corticocortical projections from rat barrel cortex have an anisotropic organization that facilitates integration of inputs from whiskers in the same row. J. Comp. Neurol. 2003;466:525–544. doi: 10.1002/cne.10895. [DOI] [PubMed] [Google Scholar]
- Hoffer ZS, Arantes HB, Roth RL, Alloway KD. Functional circuits mediating sensorimotor integration: quantitative comparisons of projections from rodent barrel cortex to primary motor cortex, neostriatum, superior colliculus, and the pons. J. Comp. Neurol. 2005;488:82–100. doi: 10.1002/cne.20579. [DOI] [PubMed] [Google Scholar]
- Hutson KA, Masterton RB. The sensory contribution of a single vibrissa's cortical barrel. J. Neurophysiol. 1986;56:1196–1223. doi: 10.1152/jn.1986.56.4.1196. [DOI] [PubMed] [Google Scholar]
- Jones EG, Diamond IT. Cerebral Cortex: The Barrel Cortex of Rodents. Vol. 11. Springer; New York: 1995. [Google Scholar]
- Khatri V, Hartings JA, Simons DJ. Adaptation in thalamic barreloid and cortical barrel neurons to periodic whisker deflections varying in frequency and velocity. J. Neurophysiol. 2004;92:3244–3254. doi: 10.1152/jn.00257.2004. [DOI] [PubMed] [Google Scholar]
- Khatri V, Bermejo R, Brumberg JC, Keller A, Zeigler HP. Whisking in air: Encoding of kinetmatics by trigeminal ganglion neurons in awake rats. J. Neurophysiol. 2009;101:836–886. doi: 10.1152/jn.90655.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri V, Bermejo R, Brumberg JC, Zeigler HP. Whisking in air: encoding of kinematics by VPM neurons in awake rats. Somatosens. Mot. Res. 2010;27:111–120. doi: 10.3109/08990220.2010.502381. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Erzurumlu RS. Trigeminal projections to the superior colliculus of the rat. J. Comp. Neurol. 1981;201:221–242. doi: 10.1002/cne.902010207. [DOI] [PubMed] [Google Scholar]
- Kim JN, Koh KS, Lee E, Park SC, Song WC. The morphology of the rat vibrissal follicle-sinus complex revealed by three-dimensional computer-aided reconstruction. Cells Tissues Organs. 2011;193:207–214. doi: 10.1159/000319394. [DOI] [PubMed] [Google Scholar]
- Klein BG, Rhoades RW. Representation of whisker follicle intrinsic musculature in the facial motor nucleus of the rat. J. Comp. Neurol. 1985;232:55–69. doi: 10.1002/cne.902320106. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Berg RW, O'Connor SM. Anatomical loops and their electrical dynamics in relation to whisking by rat. Somatosens. Mot. Res. 1999;16:69–88. doi: 10.1080/08990229970528. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Sachdev RNS, Merchant LM, Jarvis MR, Ebner FF. Adaptive filtering of vibrissa input in motor cortex of rat. Neuron. 2002;34:1021–1034. doi: 10.1016/s0896-6273(02)00732-8. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr. Opin. Neurobiol. 2006;16:435–444. doi: 10.1016/j.conb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Knutsen PM, Ahissar E. Orthogonal coding of object location. Trends Neurosci. 2009;32:101–109. doi: 10.1016/j.tins.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Knutsen PM, Pietr M, Ahissar E. Haptic object localization in the vibrissal system: behavior and performance. J. Neurosci. 2006;26:8451–8464. doi: 10.1523/JNEUROSCI.1516-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König P, Luksch H. Active sensing—closing multiple loops. Z. Naturforsch., C, J. Biosci. 1998;53:542–549. doi: 10.1515/znc-1998-7-808. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Matell MS, Brisben AJ, Oliveira LM, Nicolelis MAL. Behavioral properties of the trigeminal somatosensory system in rats performing whisker-dependent tactile discriminations. J. Neurosci. 2001;21:5752–5763. doi: 10.1523/JNEUROSCI.21-15-05752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kublik E. Contextual impact on sensory processing at the barrel cortex of awake rat. Acta Neurobiol. Exp. (Warsz.) 2004;64:229–238. doi: 10.55782/ane-2004-1508. [DOI] [PubMed] [Google Scholar]
- Lang EJ, Sugihara I, Llinás R. Olivocerebellar modulation of motor cortex ability to generate vibrissal movements in rat. J. Physiol. 2006;571:101–120. doi: 10.1113/jphysiol.2005.102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, Manns ID, Sakmann B, Brecht M. Whole-cell recordings in freely moving rats. Neuron. 2006;51:399–407. doi: 10.1016/j.neuron.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Leiser SC, Moxon KA. Responses of trigeminal ganglion neurons during natural whisking behaviors in the awake rat. Neuron. 2007;53:117–133. doi: 10.1016/j.neuron.2006.10.036. [DOI] [PubMed] [Google Scholar]
- London M, Häusser M. Dendritic computation. Annu. Rev. Neurosci. 2005;28:503–532. doi: 10.1146/annurev.neuro.28.061604.135703. [DOI] [PubMed] [Google Scholar]
- Lübke J, Feldmeyer D. Excitatory signal flow and connectivity in a cortical column: focus on barrel cortex. Brain Struct. Funct. 2007;212:3–17. doi: 10.1007/s00429-007-0144-2. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Famulare M, Sorensen LB, Spain WJ, Fairhall AL. Sensitivity of firing rate to input fluctuations depends on time scale separation between fast and slow variables in single neurons. J. Comput. Neurosci. 2009;27:277–290. doi: 10.1007/s10827-009-0142-x. [DOI] [PubMed] [Google Scholar]
- Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD. Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron. 2009;64:841–856. doi: 10.1016/j.neuron.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao T, Kusefoglu D, Hooks BM, Huber D, Petreanu L, Svoboda K. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron. 2011;72:111–123. doi: 10.1016/j.neuron.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri R, Bezdudnaya T, Trageser JC, Keller A. Encoding of stimulus frequency and sensor motion in the posterior medial thalamic nucleus. J. Neurophysiol. 2008;100:681–689. doi: 10.1152/jn.01322.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas F, Sreenivasan V, Marbach F, Wacongne C, Barsy B, Mateo C, Aronoff R, Petersen CC. Motor control by sensory cortex. Science. 2010;330:1240–1243. doi: 10.1126/science.1195797. [DOI] [PubMed] [Google Scholar]
- Mehta SB, Whitmer D, Figueroa R, Williams BA, Kleinfeld D. Active spatial perception in the vibrissa scanning sensorimotor system. PLoS Biol. 2007;5:e15. doi: 10.1371/journal.pbio.0050015. 10.1371/journal.pbio.0050015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HS, Wimmer VC, Hemberger M, Bruno RM, de Kock CP, Frick A, Sakmann B, Helmstaedter M. Cell type-specific thalamic innervation in a column of rat vibrissal cortex. Cereb. Cortex. 2010;20:2287–2303. doi: 10.1093/cercor/bhq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchinson B, Martin CJ, Grant RA, Prescott TJ. Feedback control in active sensing: rat exploratory whisking is modulated by environmental contact. Proc. Biol. Sci. 2007;274:1035–1041. doi: 10.1098/rspb.2006.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchinson B, Grant RA, Arkley K, Rankov V, Perkon I, Prescott TJ. Active vibrissal sensing in rodents and marsupials. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:3037–3048. doi: 10.1098/rstb.2011.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittmann W, Wallace DJ, Czubayko U, Herb JT, Schaefer AT, Looger LL, Denk W, Kerr JN. Two-photon calcium imaging of evoked activity from L5 somatosensory neurons in vivo. Nat. Neurosci. 2011;14:1089–1093. doi: 10.1038/nn.2879. [DOI] [PubMed] [Google Scholar]
- Miyashita E, Keller A, Asanuma H. Input-output organization of the rat vibrissal motor cortex. Exp. Brain Res. 1994;99:223–232. doi: 10.1007/BF00239589. [DOI] [PubMed] [Google Scholar]
- Moore CI, Nelson SB, Sur M. Dynamics of neuronal processing in rat somatosensory cortex. Trends Neurosci. 1999;22:513–520. doi: 10.1016/s0166-2236(99)01452-6. [DOI] [PubMed] [Google Scholar]
- Mosconi TM, Rice FL, Song MJ. Sensory innervation in the inner conical body of the vibrissal follicle-sinus complex of the rat. J. Comp. Neurol. 1993;328:232–251. doi: 10.1002/cne.903280206. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Narumi T, Tsutsui K-I, Iijima T. Difference in the functional significance between the lemniscal and paralemniscal pathways in the perception of direction of single whisker stimulation examined by muscimol microinjection. Neurosci. Res. 2009;64:323–329. doi: 10.1016/j.neures.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Nelson ME, MacIver MA. Sensory acquisition in active sensing systems. J. Comp. Physiol. Sensory Neural. Behav. Physiol. 2006;192:573–586. doi: 10.1007/s00359-006-0099-4. [DOI] [PubMed] [Google Scholar]
- Nguyenf Q-T, Kleinfeld D. Positive feedback in a brainstem tactile sensorimotor loop. Neuron. 2005;45:447–457. doi: 10.1016/j.neuron.2004.12.042. [DOI] [PubMed] [Google Scholar]
- Nguyen Q-T, Wessel R, Kleinfeld D. Developmental regulation of active and passive membrane properties in rat vibrissa motoneurones. J. Physiol. 2004;556:203–219. doi: 10.1113/jphysiol.2003.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DH, Huber D, Svoboda K. Reverse engineering the mouse brain. Nature. 2009;461:923–929. doi: 10.1038/nature08539. [DOI] [PubMed] [Google Scholar]
- O'Connor DH, Clack NG, Huber D, Komiyama T, Myers EW, Svoboda K. Vibrissa-based object localization in head-fixed mice. J. Neurosci. 2010a;30:1947–1967. doi: 10.1523/JNEUROSCI.3762-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DH, Peron SP, Huber D, Svoboda K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron. 2010b;67:1048–1061. doi: 10.1016/j.neuron.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Perkon I, Kosir A, Itskov PM, Tasic J, Diamond ME. Unsupervised quantification of whisking and head movement in freely moving rodents. J. Neurophysiol. 2011;105:1950–1962. doi: 10.1152/jn.00764.2010. [DOI] [PubMed] [Google Scholar]
- Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Petersen RS, Panzeri S, Diamond ME. Population coding in somatosensory cortex. Curr. Opin. Neurobiol. 2002;12:441–447. doi: 10.1016/s0959-4388(02)00338-0. [DOI] [PubMed] [Google Scholar]
- Pidoux M, Mahon S, Deniau J-M, Charpier S. Integration and propagation of somatosensory responses in the corticostriatal pathway: an intracellular study in vivo. J. Physiol. 2011;589:263–281. doi: 10.1113/jphysiol.2010.199646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierret T, Lavallée P, Deschênes M. Parallel streams for the relay of vibrissal information through thalamic barreloids. J. Neurosci. 2000;20:7455–7462. doi: 10.1523/JNEUROSCI.20-19-07455.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietr MD, Knutsen PM, Shore DI, Ahissar E, Vogel Z. Cannabinoids reveal separate controls for whisking amplitude and timing in rats. J. Neurophysiol. 2010;104:2532–2542. doi: 10.1152/jn.01039.2009. [DOI] [PubMed] [Google Scholar]
- Polley DB, Rickert JL, Frostig RD. Whisker-based discrimination of object orientation determined with a rapid training paradigm. Neurobiol. Learn. Mem. 2005;83:134–142. doi: 10.1016/j.nlm.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Rice FL. Structure, vascularization, and innervation of the mystacial pad of the rat as revealed by the lectin Griffonia simplicifolia. J. Comp. Neurol. 1993;337:386–399. doi: 10.1002/cne.903370304. [DOI] [PubMed] [Google Scholar]
- Richmond BJ, Wurtz RH. Vision during saccadic eye movements. II. A corollary discharge to monkey superior colliculus. J. Neurophysiol. 1980;43:1156–1167. doi: 10.1152/jn.1980.43.4.1156. [DOI] [PubMed] [Google Scholar]
- Sachdev RNS, Berg RW, Champney G, Kleinfeld D, Ebner FF. Unilateral vibrissa contact: changes in amplitude but not timing of rhythmic whisking. Somatosens. Mot. Res. 2003;20:163–169. doi: 10.1080/08990220311000405208. [DOI] [PubMed] [Google Scholar]
- Sato TR, Svoboda K. The functional properties of barrel cortex neurons projecting to the primary motor cortex. J. Neurosci. 2010;30:4256–4260. doi: 10.1523/JNEUROSCI.3774-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawinski J, Wallace DJ, Greenberg DS, Grossmann S, Denk W, Kerr JND. Visually evoked activity in cortical cells imaged in freely moving animals. Proc. Natl. Acad. Sci. USA. 2009;106:19557–19562. doi: 10.1073/pnas.0903680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Kötter R, Staiger JF. Mapping functional connectivity in barrel-related columns reveals layer- and cell type-specific microcircuits. Brain Struct. Funct. 2007;212:107–119. doi: 10.1007/s00429-007-0147-z. [DOI] [PubMed] [Google Scholar]
- Seung HS, Sompolinsky H. Simple models for reading neuronal population codes. Proc. Natl. Acad. Sci. USA. 1993;90:10749–10753. doi: 10.1073/pnas.90.22.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortland PJ, DeMaro JA, Jacquin MF. Trigeminal structure-function relationships: a reevaluation based on long-range staining of a large sample of brainstem a beta fibers. Somatosens. Mot. Res. 1995;12:249–275. doi: 10.3109/08990229509093661. [DOI] [PubMed] [Google Scholar]
- Shortland PJ, Demaro JA, Shang F, Waite PME, Jacquin MF. Peripheral and central predictors of whisker afferent morphology in the rat brainstem. J. Comp. Neurol. 1996;375:481–501. doi: 10.1002/(SICI)1096-9861(19961118)375:3<481::AID-CNE10>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Shuler MG, Krupa DJ, Nicolelis MA. Bilateral integration of whisker information in the primary somatosensory cortex of rats. J. Neurosci. 2001;21:5251–5261. doi: 10.1523/JNEUROSCI.21-14-05251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J. Neurophysiol. 1978;41:798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformation in the rat vibrissa/barrel system. J. Neurophysiol. 1989;61:311–330. doi: 10.1152/jn.1989.61.2.311. [DOI] [PubMed] [Google Scholar]
- Solomon JH, Hartmann MJ. Biomechanics: robotic whiskers used to sense features. Nature. 2006;443:525. doi: 10.1038/443525a. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Thalamocortical control of feed-forward inhibition in awake somatosensory ‘barrel’ cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:1717–1727. doi: 10.1098/rstb.2002.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwed M, Bagdasarian K, Ahissar E. Encoding of vibrissal active touch. Neuron. 2003;40:621–630. doi: 10.1016/s0896-6273(03)00671-8. [DOI] [PubMed] [Google Scholar]
- Towal RB, Hartmann MJ. Right-left asymmetries in the whisking behavior of rats anticipate head movements. J. Neurosci. 2006;26:8838–8846. doi: 10.1523/JNEUROSCI.0581-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB. Oromotor nuclei. In: Paxinos G, editor. The Rat Nervous System. Second Edition Academic Press; San Diego, CA: 1995. pp. 239–255. [Google Scholar]
- Urbain N, Deschênes M. Motor cortex gates vibrissal responses in a thalamocortical projection pathway. Neuron. 2007a;56:714–725. doi: 10.1016/j.neuron.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Urbain N, Deschênes M. A new thalamic pathway of vibrissal information modulated by the motor cortex. J. Neurosci. 2007b;27:12407–12412. doi: 10.1523/JNEUROSCI.2914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Jacquin MF, Deschênes M. Thalamic projections from the whisker-sensitive regions of the spinal trigeminal complex in the rat. J. Comp. Neurol. 2000;420:233–243. doi: 10.1002/(sici)1096-9861(20000501)420:2<233::aid-cne6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Vincent SB. The function of the vibrissae in the behavior of the white rat. Behavior Monographs. 1912;1:7–81. [Google Scholar]
- Wachowiak M. All in a sniff: olfaction as a model for active sensing. Neuron. 2011;71:962–973. doi: 10.1016/j.neuron.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing of the albino rat. Behaviour. 1964;12:223–244. [Google Scholar]
- Wise SP, Jones EG. Cells of origin and terminal distribution of descending projections of the rat somatic sensory cortex. J. Comp. Neurol. 1977;175:129–157. doi: 10.1002/cne.901750202. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, McAlonan K, Cavanaugh J, Berman RA. Thalamic pathways for active vision. Trends Cogn. Sci. (Regul. Ed.) 2011;15:177–184. doi: 10.1016/j.tics.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Derdikman D, Haidarliu S, Ahissar E. Parallel thalamic pathways for whisking and touch signals in the rat. PLoS Biol. 2006;4:e124. doi: 10.1371/journal.pbio.0040124. 10. 1371/journal.pbio.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker E, Welker WI. Coding of somatic sensory input by vibrissae neurons in the rat's trigeminal ganglion. Brain Res. 1969;12:138–156. doi: 10.1016/0006-8993(69)90061-4. [DOI] [PubMed] [Google Scholar]