Abstract
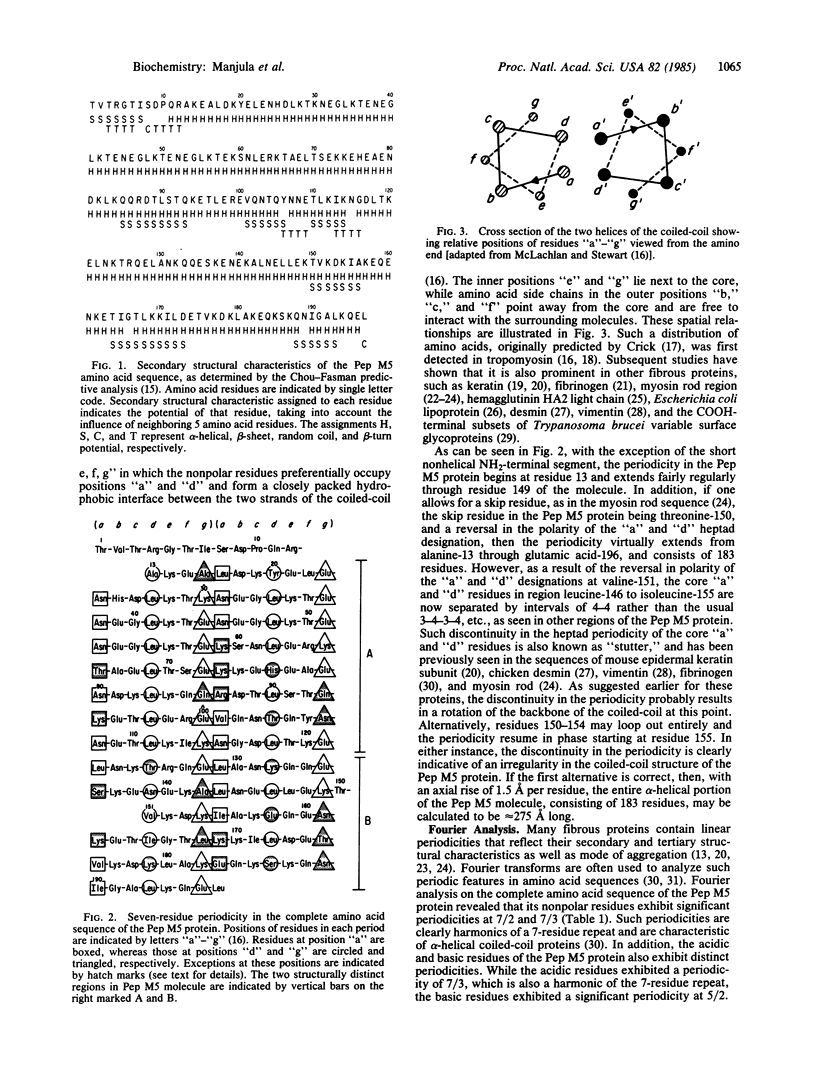
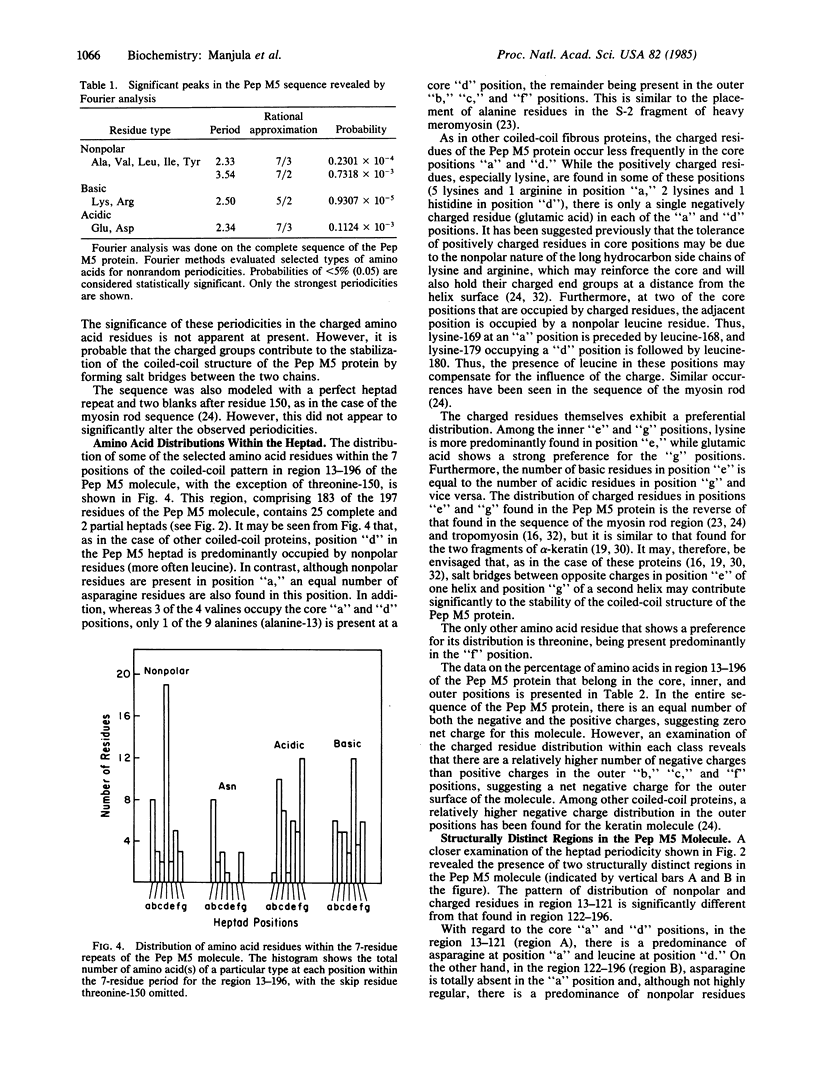
The complete amino acid sequence of Pep M5, a biologically active 197-residue fragment comprising nearly half of the group A streptococcal M5 protein, has structural features characteristic of an alpha-helical coiled-coil protein. Fourier analyses of the nonpolar residues show strong periodicities based on repeats of 7 residues (7/2 and 7/3). Except for the nonhelical NH2-terminal 12-residue segment, the 7-residue periodicity in the distribution of nonpolar residues extends through the remainder of the Pep M5 molecule, with some discontinuities and irregularities. The molecule contains two distinct regions that differ in the pattern of distribution of the nonpolar and charged residues. The 7-residue pattern "a, b, c, d, e, f, g" in region 13-121 is atypical in that position "a" is predominantly occupied by asparagine, rather than nonpolar residues. On the other hand, the periodicity in region 122-196 is more typical of that found in other coiled-coil proteins, such as the myosin rod region, keratin, desmin, and vimentin, rather than tropomyosin. Although the periodicity in nonpolar residues is not highly regular, the predominance of basic and acidic residues in the inner "e" and "g" positions, respectively, suggests that ionic interactions between chains may contribute significantly to the stability of the coiled-coil. The distribution of charged residues in the outer positions within the two regions of the molecule is also distinct. The NH2-terminal region carries a significantly higher net negative charge than the COOH-terminal region, suggesting that the former region may play an important role in some of the biological functions of the Pep M5 molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H., Seyer J. M., Dale J. B., Hasty D. L. Repeating covalent structure and protective immunogenicity of native and synthetic polypeptide fragments of type 24 streptococcal M protein. Mapping of protective and nonprotective epitopes with monoclonal antibodies. J Biol Chem. 1983 Nov 10;258(21):13250–13257. [PubMed] [Google Scholar]
- Beachey E. H., Seyer J. M., Kang A. H. Primary structure of protective antigens of type 24 streptococcal M protein. J Biol Chem. 1980 Jul 10;255(13):6284–6289. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Cohen C., Reinhardt B., Parry D. A., Roelants G. E., Hirsch W., Kanwé B. Alpha-helical coiled-coil structures of Trypanosoma brucei variable surface glycoproteins. Nature. 1984 Sep 13;311(5982):169–171. doi: 10.1038/311169a0. [DOI] [PubMed] [Google Scholar]
- Dale J. B., Seyer J. M., Beachey E. H. Type-specific immunogenicity of a chemically synthesized peptide fragment of type 5 streptococcal M protein. J Exp Med. 1983 Nov 1;158(5):1727–1732. doi: 10.1084/jem.158.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Goldbaum D. M., Doolittle L. R. Designation of sequences involved in the "coiled-coil" interdomainal connections in fibrinogen: constructions of an atomic scale model. J Mol Biol. 1978 Apr 5;120(2):311–325. doi: 10.1016/0022-2836(78)90070-0. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Manjula B. N., Scott J. R. Streptococcal M6 protein expressed in Escherichia coli. Localization, purification, and comparison with streptococcal-derived M protein. J Exp Med. 1984 Apr 1;159(4):1083–1095. doi: 10.1084/jem.159.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A. Requirements for the opsonic activity of human IgG directed to type 6 group A streptococci: net basic charge and intact Fc region. J Immunol. 1983 Feb;130(2):896–902. [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein extracted by nonionic detergent. III. Correlation between immunological cross-reactions and structural similarities with implications for antiphagocytosis. J Exp Med. 1978 Jun 1;147(6):1771–1778. doi: 10.1084/jem.147.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. N. M proteins of group A streptococci. Bacteriol Rev. 1974 Mar;38(1):57–86. doi: 10.1128/br.38.1.57-86.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Kaufmann E., Weber K. Proteinchemical characterization of three structurally distinct domains along the protofilament unit of desmin 10 nm filaments. Cell. 1982 Aug;30(1):277–286. doi: 10.1016/0092-8674(82)90033-2. [DOI] [PubMed] [Google Scholar]
- Geisler N., Plessmann U., Weber K. Related amino acid sequences in neurofilaments and non-neural intermediate filaments. Nature. 1982 Apr 1;296(5856):448–450. doi: 10.1038/296448a0. [DOI] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. Electrophoretic mobility and N-acetyl neuraminic acid content of human normal and leukemic lymphocytes and granulocytes. Blood. 1970 Jan;35(1):12–22. [PubMed] [Google Scholar]
- Maizel J. V., Jr, Lenk R. P. Enhanced graphic matrix analysis of nucleic acid and protein sequences. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7665–7669. doi: 10.1073/pnas.78.12.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjula B. N., Acharya A. S., Mische S. M., Fairwell T., Fischetti V. A. The complete amino acid sequence of a biologically active 197-residue fragment of M protein isolated from type 5 group A streptococci. J Biol Chem. 1984 Mar 25;259(6):3686–3693. [PubMed] [Google Scholar]
- Manjula B. N., Fischetti V. A. Studies on group A streptococcal M-proteins: purification of type 5 M-protein and comparison of its amino terminal sequence with two immunologically unrelated M-protein molecules. J Immunol. 1980 Jan;124(1):261–267. [PubMed] [Google Scholar]
- Manjula B. N., Fischetti V. A. Tropomyosin-like seven residue periodicity in three immunologically distinct streptococal M proteins and its implications for the antiphagocytic property of the molecule. J Exp Med. 1980 Mar 1;151(3):695–708. doi: 10.1084/jem.151.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjula B. N., Mische S. M., Fischetti V. A. Primary structure of streptococcal Pep M5 protein: Absence of extensive sequence repeats. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5475–5479. doi: 10.1073/pnas.80.18.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A. D. Analysis of periodic patterns in amino acid sequences: collagen. Biopolymers. 1977 Jun;16(6):1271–1297. doi: 10.1002/bip.1977.360160609. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Karn J. Periodic features in the amino acid sequence of nematode myosin rod. J Mol Biol. 1983 Mar 15;164(4):605–626. doi: 10.1016/0022-2836(83)90053-0. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Stewart M. The 14-fold periodicity in alpha-tropomyosin and the interaction with actin. J Mol Biol. 1976 May 15;103(2):271–298. doi: 10.1016/0022-2836(76)90313-2. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Stewart M. Tropomyosin coiled-coil interactions: evidence for an unstaggered structure. J Mol Biol. 1975 Oct 25;98(2):293–304. doi: 10.1016/s0022-2836(75)80119-7. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D. The double helix coiled coil structure of murein lipoprotein from Escherichia coli. J Mol Biol. 1978 Jun 5;121(4):493–506. doi: 10.1016/0022-2836(78)90396-0. [DOI] [PubMed] [Google Scholar]
- Parry D. A. Analysis of the primary sequence of alpha-tropomyosin from rabbit skeletal muscle. J Mol Biol. 1975 Nov 5;98(3):519–535. doi: 10.1016/s0022-2836(75)80084-2. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Crewther W. G., Fraser R. D., MacRae T. P. Structure of alpha-keratin: structural implication of the amino acid sequences of the type I and type II chain segments. J Mol Biol. 1977 Jun 25;113(2):449–454. doi: 10.1016/0022-2836(77)90153-x. [DOI] [PubMed] [Google Scholar]
- Parry D. A. Structure of rabbit skeletal myosin. Analysis of the amino acid sequences of two fragments from the rod region. J Mol Biol. 1981 Dec 5;153(2):459–464. doi: 10.1016/0022-2836(81)90290-4. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Flicker P. F., Cohen C., Manjula B. N., Fischetti V. A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyer J. M., Kang A. H., Beachey E. H. Primary structural similarities between types 5 and 24 M proteins of Streptococcus pyogenes. Biochem Biophys Res Commun. 1980 Jan 29;92(2):546–553. doi: 10.1016/0006-291x(80)90368-x. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Rice R. H., Roop D. R., Trus B. L., Steven A. C. Complete amino acid sequence of a mouse epidermal keratin subunit and implications for the structure of intermediate filaments. Nature. 1983 Apr 28;302(5911):794–800. doi: 10.1038/302794a0. [DOI] [PubMed] [Google Scholar]
- Swanson J., Hsu K. C., Gotschlich E. C. Electron microscopic studies on streptococci. I. M antigen. J Exp Med. 1969 Nov 1;130(5):1063–1091. doi: 10.1084/jem.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C. W., Dopheide T. A. Influenza virus haemagglutinin. Structural predictions suggest that the fibrillar appearance is due to the presence of a coiled-coil. Aust J Biol Sci. 1980 Aug;33(4):441–447. doi: 10.1071/bi9800441. [DOI] [PubMed] [Google Scholar]



