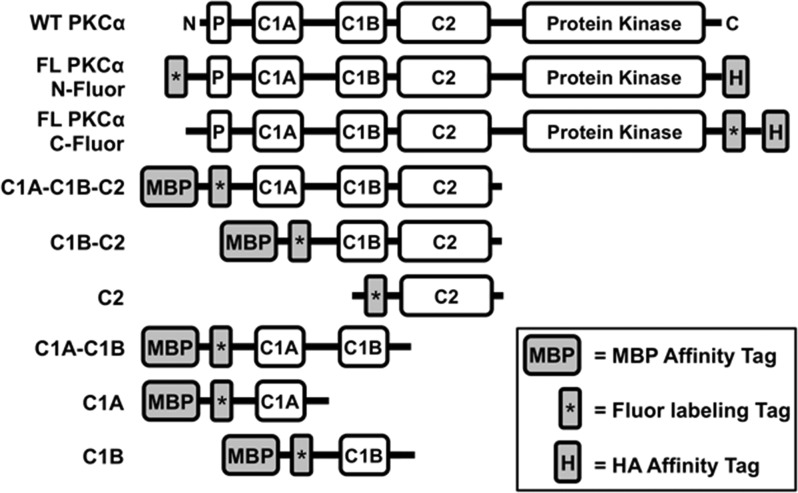
Figure 2.
Engineered full-length PKCα and truncation constructs used in this study. Shown are the domain layouts of the human PKCα constructs engineered for this study, and WT PKCα for comparison (native domains defined in Figure 1A). Each engineered construct contained both a fluorescent labeling tag (the 11-residue ybbr target peptide to which the Sfp enzyme covalently couples the fluor) and an affinity tag [the 9-residue HA peptide or the maltose-binding protein (MBP)]. Truncation borders were as follows: C1A–C1B–C2 (residues 26–294), C1B–C2 (residues 90–294), C2 (residues 157–294), C1A–C1B (residues 26–165), C1A (residues 26–100), and C1B (residues 90–165).