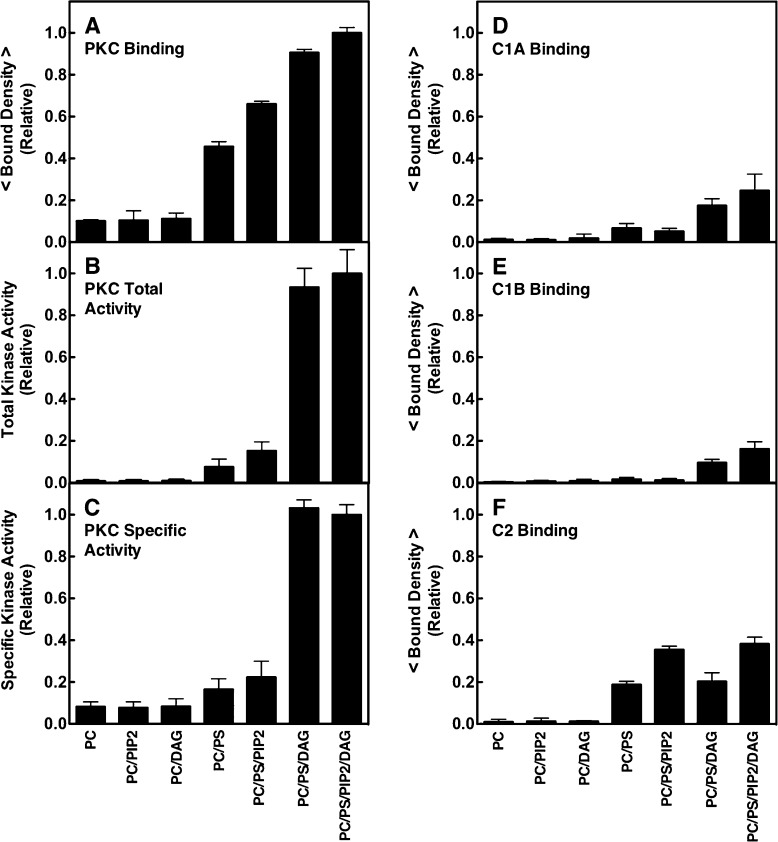
Figure 3.
Dependence of PKCα membrane binding and kinase activation, and domain membrane binding, on lipid composition. (A and D–F) Single-molecule TIRF quantitation of binding of constructs to supported lipid bilayers, normalized to the same total protein concentration (1 pM). A given construct was added to the imaging chamber containing the indicated supported bilayer (Table 1), and then the density of fluorescent protein binding per unit area was quantitated. Each average was determined from 20 temporally isolated frames from three separate movie streams in at least five separate experiments (n ≥ 15). The free Ca2+ concentration was 6 μM in a physiological buffer (Materials and Methods). (B) Total kinase activity of fluorescently labeled PKCα measured by a modified PepTag (Promega) assay (Materials and Methods). The enzyme was activated by membranes containing the same lipid compositions as the binding density measurements, and PKC-specific phosphorylation of the PepTag target peptide was quantitated by electrophoresis. Each condition was repeated twice in duplicate (n = 4) and separate experiments using varying PKCα and/or lipid concentrations confirmed that enzyme saturation did not occur. (C) Specific activity of PKCα determined by the ratio of kinase activity to binding density under each bilayer condition. In all experiments, T = 22 ± 0.5 °C. In this and subsequent figures, an average parameter determined from purely single molecule data is indicated by < parameter >.