Abstract
Background:
Observational studies have found an increased risk of adverse effects such as hemorrhage, stroke, and increased mortality in patients taking selective serotonin reuptake inhibitors (SSRIs). The impact of prior use of these medications on outcomes in critically ill patients has not been previously examined. We performed a retrospective study to determine if preadmission use of SSRIs or serotonin norepinephrine reuptake inhibitors (SNRIs) is associated with mortality differences in patients admitted to the ICU.
Methods:
The retrospective study used a modifiable data mining technique applied to the publicly available Multiparameter Intelligent Monitoring in Intensive Care (MIMIC) 2.6 database. A total of 14,709 patient records, consisting of 2,471 in the SSRI/SNRI group and 12,238 control subjects, were analyzed. The study outcome was in-hospital mortality.
Results:
After adjustment for age, Simplified Acute Physiology Score, vasopressor use, ventilator use, and combined Elixhauser score, SSRI/SNRI use was associated with significantly increased in-hospital mortality (OR, 1.19; 95% CI, 1.02-1.40; P = .026). Among patient subgroups, risk was highest in patients with acute coronary syndrome (OR, 1.95; 95% CI, 1.21-3.13; P = .006) and patients admitted to the cardiac surgery recovery unit (OR, 1.51; 95% CI, 1.11-2.04; P = .008). Mortality appeared to vary by specific SSRI, with higher mortalities associated with higher levels of serotonin inhibition.
Conclusions:
We found significant increases in hospital stay mortality among those patients in the ICU taking SSRI/SNRIs prior to admission as compared with control subjects. Mortality was higher in patients receiving SSRI/SNRI agents that produce greater degrees of serotonin reuptake inhibition. The study serves to demonstrate the potential for the future application of advanced data examination techniques upon detailed (and growing) clinical databases being made available by the digitization of medicine.
There are questions in clinical medicine that cannot be answered through a traditional prospective randomized controlled trial (RCT). These types of issues tend to be complex, multifactorial, and context dependent in ways that exceed the constraints of traditional RCTs (eg, important factors may be lost upon exclusion of patients on the basis of age, disease, or medication use). One such issue is the effect of the long-term prior use of particular medications on outcomes during the course of ensuing conditions, such as the onset of critical illness. This type of clinical question is currently best addressed by the targeted analysis of large databases.
In a previous article, we described a system that uses clinical database networks to accumulate safety and efficacy evidence when drugs are used in wider, more diverse patient populations than those, typically, examined during premarket approval clinical studies.1 This is in accordance with the vision of a nationwide, data-driven learning system that monitors for ongoing safety signals after a new drug comes to market.2 In this article, using a public, deidentified clinical database, we report an analysis of patients admitted to the ICU who are receiving antidepressants—specifically, selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs).
The use of antidepressants, including SSRIs and SNRIs, has increased significantly in recent years. One in 10 Americans now takes an antidepressant; among women in their 40s and 50s, the figure is one in four.3,4 However, Mojtabai5 found that nearly two-thirds of a sample of 5,639 patients who had received a diagnosis of depression within the previous 12 months did not meet the Diagnostic and Statistical Manual of Mental Disorders criteria. Elderly patients were most likely to receive a misdiagnosis; six out of seven patients aged 65 years and older did not fit the criteria. The majority of the sample patients received prescription antidepressants, most for at least 2 years, and some took them for a decade or more. This unnecessary administration is of particular concern, as there is a growing body of literature reporting adverse effects with the long-term use of SSRIs and SNRIs.6,7 Furthermore, a substantial percentage of truly depressed people remain undiagnosed and untreated with appropriate medications.8
This study examines the effect of preadmission SSRI/SNRI use on mortality in critically ill patients. We are aware of the challenge in determining whether an association, if found in observational studies, is due to the underlying condition or the use of the medication. Clearly, the population receiving SSRIs and SSRNs is not a precise match with the population with true depression in view of the previously noted observations of both unnecessary and inadequate treatments with these agents. Therefore, our study is intended specifically to measure the impact of these particular agents on patient outcomes rather than the impact of depression, per se. With this in mind, we have observed that the literature suggests that antidepressants with different degrees of activity are often prescribed based on provider preference independent of the degree of depression (ie, the specific SSRI prescribed is more strongly influenced by provider preference than by the severity of the depression or other patient factors).9 We, therefore, examined whether the pharmacologic degree of serotonin reuptake was associated with ICU outcomes.
Materials and Methods
We conducted a retrospective cohort study using the Multiparameter Intelligent Monitoring in Intensive Care (MIMIC) II database. MIMIC II is a large database, freely available in the public domain, which includes information from electronic medical records of patients admitted to the ICUs at Beth Israel Deaconess Medical Center since 2001.10 The creation and use of the MIMIC database was approved by the institutional review boards of both Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology (IRB protocol 2001-P-001699/3).
All adult patient records in the database were screened for purposes of inclusion, with only the first hospital admission considered for analysis for those with multiple admissions. Patients were excluded if there was uncertainty regarding their pre-ICU admission medications or if they did not have an admitting Simplified Acute Physiology Score (SAPS) recorded. The exposure studied was documented use of SSRI, SNRI, or both immediately prior to ICU admission. Assessing SSRI/SNRI was the a priori primary outcome before any data had been extracted or analysis done. Preadmission use was defined by the presence of an SSRI/SNRI in the team-reconciled admission medication list in a patient’s discharge summary. Nonexposure was defined as the absence of any SSRI or SNRI on the admission medication list.
The study outcome was in-hospital mortality among the entire patient cohort. This outcome was also analyzed across patient subsets and by specific drug type. We used 0.05 as the family-wise error rate for subgroup analyses. Because there is a family of hypotheses to be tested, Holm’s stepdown procedure was used to control the false-positive rate.11 It is a more powerful method than the Bonferroni procedure but does not increase the chances of a false positive. As we used a closed test (hypotheses are rejected in sequential order starting from the global), these analyses are protected against type 1 error inflation. We used families of hypotheses instead of having each subgroup analysis stand on its own, given that this data analysis is exploratory, rather than confirmatory. A cumulative Mann-Kendall trend test was used to test for the existence of a trend in hospital mortality with respect to the level of serotonin reuptake inhibition.12,13 The degree of serotonin reuptake inhibition used is based on those reported by Tatsumi et al.14
We also examined two prespecified falsification hypotheses. As noted by Prasad and Jena,15 prespecified falsification hypotheses can provide intuitive safeguards when examining observational data. To determine if the association between SSRIs and mortality is an artifact of the dataset, another hypothesis that could not be true was tested. If such an association was found to be statistically significant, the association between SSRIs and mortality would likely be similarly spurious. Our falsification hypotheses were that two other chosen admission medications (stool softeners and calcium supplements) would not be associated with in-hospital mortality. We examined these medication types in both the general cohort and in smaller subsets of the population where use was more common.
Data regarding each patient’s age, sex, SAPS,16 laboratory values, vital signs, International Classification of Diseases, Ninth Revision (ICD-9) diagnoses, and disease-related group were extracted. Medical comorbidities were represented by the Elixhauser scores for 30 comorbidities as calculated from the ICD-9 codes.17 Diagnoses and comorbidities used for subset analysis were likewise defined by the ICD-9 diagnoses. All ICD-9 diagnoses noted for the hospital admission were considered equally. The mean, maximum, minimum, and SDs of three vital signs (heart rate, BP, and temperature) were obtained over the first 24 h of admission. The first values of routine laboratory results were also extracted, including hemoglobin, partial thromboplastin time, platelet count, and troponin-T level. Vital signs data were usually reported once per hour, whereas routine laboratory data frequency varied per patient.
Patient variables and outcomes in the groups with and without SSRI/SNRI use were compared using the Wilcoxon rank-sum test for continuous variables and a χ2 test for categorical variables. Elixhauser scores were combined into a single weighted score using the method described by van Walraven et al18 and used as a covariate. Multivariate logistic regression was performed to test the relationship between SSRI/SNRI use and the study outcome, adjusted for confounding variables.
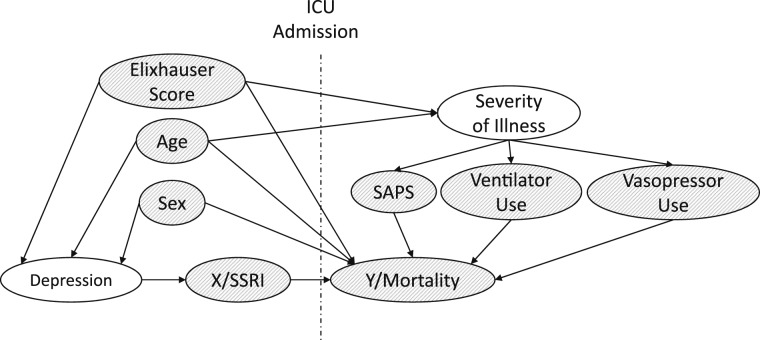
Covariates included in the models were age, sex, SAPS, Elixhauser score, ventilator use, and vasopressor use. Using a graph to represent causal effects between variables is one way to understand if bias could be reduced or increased by conditioning on specific covariates. In Figure 1, we describe the causal model connecting pre-ICU admission SSRI use with other confounders and modifier effects.19 In causal directed acyclic graphs, arrows between variables indicate causation, whereas unconnected variables have no direct causal association. We followed the steps described by Pearl19 to ensure that the statistical model including only the chosen covariates will minimize the bias of the estimate of X (SSRI) on Y (mortality). All data processing and modeling was performed using MATLAB R2011a (The MathWorks, Inc).
Figure 1.
Directed acyclic graph describing the relationship between covariates and the outcome. Note that preadmission and postadmission factors are separated by the labeled vertical line. This graph depicts our model of the observed causal relations connecting pre-ICU admission SSRI use with other confounders and modifier effects. These connections are represented by the arrow lines, with the arrow positioned specifically at the impacted factor. For example, age may have an impact on the preadmission occurrence of depression as well as on the severity of illness and mortality after admission. The figure represents a visual display of our data-driven examination of possible causal factors on mortality (Y) in addition to the causal factor of SSRI use (X). Observed variables are shaded, whereas unobserved variables are clear. Age, sex, and Elixhauser score were used as the confounding variables for SSRI use, because they share a “back door” path that needs to be adjusted for. Other ICU-specific modifier effects, such as SAPS, ventilator use, and vasopressor use, are not confounding but are strongly tied to the outcome Y (mortality) in the ICU setting and are, therefore, adjusted for in the model. Note that the precise vertical or horizontal positions of the variables do not imply more or less quantitative impact (eg, severity of illness is not a more impactful factor than the three grouped below it). The positioning of the factors is designed to facilitate understanding by eliminating or minimizing the complexity of the relationship arrows (eg, avoiding overlaps so far as possible). SAPS = Simplified Acute Physiology Score; SSRI = selective serotonin reuptake inhibitor.
Results
Validation of Inclusion Criteria
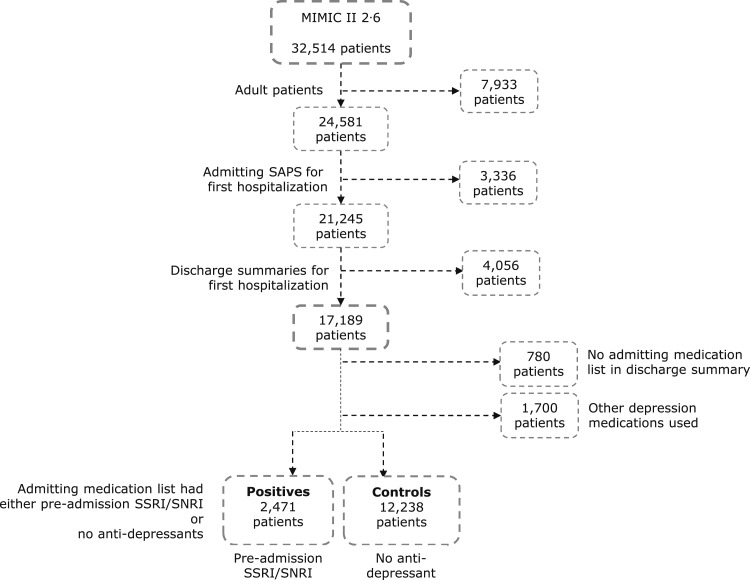
Of the eligible adult patients with an admitting SAPS (17,189), 2,480 were excluded for having no admitting medication section or for use of other types of antidepressants, leaving 14,709 patients for the study (12,238 unexposed, 2,471 SSRI/SNRI) (Fig 2). Patients with preadmission SSRI/SNRI use differed from the control group in that they were more often women, had lower Elixhauser scores, were slightly more likely to have COPD and diabetes mellitus, and were less likely to require mechanical ventilation or the use of vasopressors (Table 1).20 There were no significant clinical differences in the initial (first 24 h) laboratory results and vital signs between the groups (Table 2).
Figure 2.
Cohort selection process. MIMIC = Multiparameter Intelligent Monitoring in Intensive Care; SNRI = serotonin norepinephrine reuptake inhibitor. See Figure 1 legend for expansion of other abbreviations.
Table 1.
—Population Information on Groups Included in Study
| Included in Study | |||
| Variable | Control (12,238) | SSRI/SNRI Admission (2,471) | Rank Sum P Value |
| Demographics | |||
| Sex, % male | 61 | 44 | < .05 |
| Age, y | 66, IQR 26 | 64, IQR 25 | .4 |
| SAPS | 13, IQR 7 | 13, IQR 8 | < .05 |
| ICU type, % | |||
| CCU | 23 | 21 | .05 |
| CSRU | 38 | 33 | < .05 |
| MICU | 33 | 40 | < .05 |
| SICU | 6 | 5 | .2 |
| Selected acute diagnoses, % | |||
| Acute coronary syndrome (serum troponin > 0.5 ng/mL) | 8 | 7 | .2 |
| Sepsis (Martin criteriaa) | 3 | 4 | .7 |
| Comorbidities by ICD-9, % | |||
| COPD | 7 | 11 | < .05 |
| Diabetes | 17 | 21 | < .05 |
| Cardiovascular disease | 70 | 68 | .06 |
| Treatments, % | |||
| Ventilator use | 55 | 49 | < .05 |
| Vasopressor use | 39 | 31 | < .05 |
| Blood products transfused, mL | |||
| Total RBC volume | 750, IQR 1,125 | 750, IQR 1,125 | .2 |
| Total FFP volume | 608, IQR 866 | 614, IQR 895 | .6 |
| Total platelet volume | 325, IQR 487 | 27,5 IQR 380 | .07 |
Binary variables reported as prevalence percentages, continuous variables reported as data median with IQR, lengths of stay reported as data mean SD. CCU = coronary care unit; CSRU = cardiac surgery recovery unit; FFP = fresh frozen plasma; ICD-9 = International Classification of Diseases, Ninth Revision; IQR = interquartile range; MICU = medical ICU; SAPS = Simplified Acute Physiology Score; SICU = surgical ICU; SNRI = serotonin norepinephrine reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor.
As described in Martin et al.20
Table 2.
—Comparison of Initial Laboratory Values and First 24-h Vital Signs in Groups Included in Study
| Variable | Control (12,238) | SSRI/SNRI Admission (2,471) |
| Selected initial laboratory values | ||
| Initial creatinine | 1, IQR 0 | 1, IQR 1 |
| Initial hematocrit | 35, IQR 9 | 35, IQR 8 |
| Initial platelet count | 222, IQR 123 | 239, IQR 129 |
| Selected vital signs | ||
| Total min with abnormal HRa | 125, IQR 1,020 | 155, IQR 1,020 |
| Total min with abnormal BPb | 60, IQR 315 | 75, IQR 360 |
Variables were reported as data median with IQR; lengths of stay were reported as data mean SD. HR = heart rate. See Table 1 legend for expansion of other abbreviations.
Heart rate > 100 beats/min.
Mean arterial pressure < 60 mm Hg.
Study Outcome Analysis
Our outcome analysis tested the relationship between preadmission SSRI/SNRI use and in-hospital mortality. After adjusting for differences in age, sex, SAPS, Elixhauser score, ventilator use, and vasopressor administration, multivariate logistic regression found a significant increase in the odds of death for those with preadmission SSRI/SNRI use (OR, 1.19; 95% CI, 1.02-1.40; P = 0.026).
Subgroup Examination of Study Outcome
Patients were divided into several subcategories to determine if the relationship between preadmission SSRI/SNRI use and in-hospital mortality varied across patient subsets. Separate logistic regression models were built for each subgroup using the same confounding variables (age, sex, SAPS, Elixhauser score, ventilator usage, and vasopressor usage), shown in Table 3. Using Holm’s stepdown procedure to determine significance, we found that SSRI/SNRI use was associated with higher mortality in patients with cardiovascular disease (OR, 1.24; P = .025), those admitted to the cardiac surgery recovery unit (OR, 1.45; P = .016), and those with acute coronary syndrome (OR, 1.95; P = .006).
Table 3.
—OR of SSRI/SNRI vs Control for Multivariate Logistic Regression for Hospital Mortality
| Patient Subgroup | Population Total (Control/Positive) | OR | 95% CI | P Value |
| Entire cohorta | 14,709 (12,238/2,471) | 1.19 | (1.02-1.40) | .026 |
| Comorbidities by ICD-9 | ||||
| Cardiovascular diseasea | 10,195 (8,521/1,674) | 1.24 | (1.03-1.49) | .025 |
| Diabetes mellitus | 2,571 (2,063/508) | 1.08 | (0.75-1.58) | .7 |
| Selected ICU types | ||||
| CSRUa | 5,463 (4,647/816) | 1.45 | (1.07-1.95) | .016 |
| MICU | 5,000 (4,006/994) | 1.05 | (0.83-1.34) | .7 |
| Selected acute diagnoses | ||||
| Acute coronary syndromea (serum troponin > 0.5 ng/mL) | 1,171 (990/181) | 1.95 | (1.21-3.13) | .006 |
| Acute renal failure by ICD-9 | 2,071 (1,670/401) | 1.22 | (0.90-1.66) | .2 |
| Acute respiratory failure by ICD-9 | 1,847 (1,448/399) | 1.05 | (0.79-1.38) | .7 |
| Sepsis (Martin criteria) | 509 (420/89) | 1.31 | (0.67-2.54) | .4 |
Each row was obtained from the regression model for the specific patient subset. See Table 1 legend for expansion of abbreviations.
These results reject the null hypothesis using Holm’s stepdown procedure on the families previously defined, with an initial P value of .05 for the entire cohort of < .05.
Medication-Specific Primary Outcome Analysis
We examined the role that the degree of serotonin reuptake inhibition might play, given the variation in this factor among the SSRIs (Table 4).21 As SSRI and SNRI medications operate via different mechanisms, we included only SSRI agents in this segment of the analysis. Here, we compared the five main SSRI types found: citalopram (622 patients), escitalopram (203 patients), fluoxetine (388 patients), paroxetine (406 patients), and sertraline (555 patients). We excluded fluvoxamine, as the number of patients taking this medication was small (15 patients).
Table 4.
—Proportion of In-Hospital Death for Specific Drugs as Compared With Those in the Control Population
| Drug | Population on Medication | Hospital Mortality Rate in Positives, % | Dissociation Constant for Serotonin Transportera |
| Citalopram | 622 | 9 | 1.16 |
| Escitalopram | 203 | 5 | Unknownb |
| Fluoxetine | 388 | 10 | 0.81 |
| Sertraline | 555 | 14 | 0.29 |
| Paroxetine | 406 | 12 | 0.13 |
The hospital mortality rate in the control group is 10%.
Lower dissociation constant denotes a higher degree of serotonin reuptake inhibition. From Tatsumi et al.14
Escitalopram is the active s-enantiomer of citalopram and is, thus, presumed to have a similar dissociation constant.
We first tested for the statistical existence of a trend in the baseline mortality rate. This rate tends to increase as the dissociation constant decreases, so that those medications with a higher degree of serotonin reuptake inhibition (eg, sertraline and paroxetine) are associated with higher mortality rates. Using the reuptake inhibition coefficient as the ordinal population value and the in-hospital mortality as the outcome, the cumulative Mann-Kendall trend test rejected the null hypothesis that there was no trend (P < .001).
To determine if some SSRIs are more strongly associated with mortality than others, we performed a likelihood ratio test between a logistic regression model that dummy codes each SSRI used and a null model with a single indicator variable for SSRI use. Both models adjusted for age, sex, SAPS, and combined Elixhauser Score. The SSRI-specific model had a better log-likelihood than the single indicator variable model, with P = .02. This demonstrates that an in-hospital mortality model that includes SSRI type is significantly better than the one that does not.
Results of the SSRI-specific model are shown in Table 5. We found that paroxetine and sertraline use (which possess the two strongest degrees of serotonin reuptake) were more strongly correlated with in-hospital mortality (OR, 1.52; P = .015; OR, 1.47 P = .007; respectively) than the other SSRI drugs.
Table 5.
—ORs Associated With Specific Drugs and Relevant Risk Factors in the Control and SSRI Population
| Covariate | OR (95% CI) | P Value |
| Sex | 0.87 (0.77-0.98) | .2 |
| Age | 1.01 (1.01-1.02) | < .001 |
| SAPS | 1.13 (1.12-1.15) | < .001 |
| Ventilator use | 1.67 (1.42-1.98) | < .001 |
| Vasopressor use | 1.52 (1.33-1.74) | < .001 |
| Combined Elixhauser | 1.23 (1.21-1.25) | < .001 |
| Citalopram | 1.06 (0.78-1.43) | .7 |
| Escitalopram | 0.54 (0.28-1.04) | .07 |
| Fluoxetine | 1.11 (0.76-1.62) | .6 |
| Sertraline | 1.47 (1.11-1.94) | .007 |
| Paroxetine | 1.52 (1.08-2.12) | .015 |
See Table 1 legend for expansion of abbreviations.
Falsification Hypothesis Analysis
We defined two falsification hypotheses: Hospital mortality is not associated with the use of either stool softeners or calcium supplements. We evaluated the relationship between hospital mortality and use of stool softeners (senna, Dulcolax, docusate, milk of magnesia, Colace, MiraLAX, and bisacodyl) in both the entire cohort (1,449 positive, 13,260 control subjects) and in those ≥ 75 years old (450 positive, 4,047 control subjects). We also examined the relationship between hospital mortality and use of calcium supplements in both the entire cohort (647 positive, 14,062 control subjects) and in female patients ≥ 50 years old without end-stage renal disease (ICD-9. 585.6) (225 positive, 4,4323 control subjects). Using logistic regression adjusted for age, sex, SAPS, vasopressor use, ventilator use, and combined Elixhauser score, we found that medication use in all scenarios was not associated with in-hospital mortality (P > .1 in all cases).
Discussion
In our study, we found that after adjustment for confounding variables, patients being treated with an SSRI or SNRI prior to admission to the ICU had significantly higher hospital mortalities than patients not receiving one of these medications. This article explores whether there is an association between SSRI use and ICU outcomes; our results do not reflect risk of harm from SSRI use among those who do not develop critical illness.
One difficult question to answer in any study investigating possible adverse effects of antidepressants is whether the observed effect is related to the drug or to the disease. To complicate matters, the population receiving antidepressants may include a substantial number of patients who are not depressed by standard criteria. Depression has been shown to be associated with worse outcomes in multiple medical conditions, and we were unable to control for or measure the degree of depression in this study. In an effort to answer this question, we analyzed patients based on which specific SSRI they were taking prior to admission.21‐23 SSRIs have varying degrees of serotonin reuptake inhibition (see Table 4), as has been reported previously,14 but the literature suggests that the choice of SSRI is based primarily on provider preference rather than the degree of depression or other patient factors.9 We, therefore, posited that if the effect on mortality is related to the medication, then mortality would be expected to vary by the potency of the specific SSRI rather than by the severity of depression. Our analysis revealed that mortality in patients taking citalopram and escitalopram was not significantly higher than in control subjects. Citalopram is known to be a lower-affinity SSRI,21 and as escitalopram is the active enantiomer of citalopram, it is presumed to be similar in affinity. In contrast, patients taking paroxetine and sertraline, both higher-affinity SSRIs,14 demonstrated significantly higher mortalities.
Long-term SSRI use has previously been linked to increased mortality, perhaps most notably in the Women’s Health Initiative study. That data revealed that SSRI use was associated with a hazard ratio for death of 1.32 and a hazard ratio of 1.45 for stroke, compared with patients not taking an antidepressant.6
Another study found that continuation of SSRIs during the perioperative period is associated with a higher risk of adverse events, including hospital mortality and readmission at 30 days.24 These authors state that determining whether patient factors or SSRIs themselves are responsible for elevated risks requires prospective study. Unfortunately, it would not be possible to prospectively examine the interaction between long-term SSRI use and critical illness.
We were unable to find any evidence in our database that would indicate that any of the known adverse effects of SSRIs were a potential cause for the increased mortality in these patients. Specifically, there was no significant difference in hematocrit level, initial BP, or heart rate between patients taking SSRIs and control subjects. Patients taking SSRIs did not require more blood transfusions.
Of the different patient subsets, we found the most significant correlation between SSRI/SNRI use and hospital mortality among cardiac surgery patients and patients who developed acute coronary syndrome with significant troponin elevation (troponin-T level > 0.5 ng/mL). A trend was also noted among patients with chronic cardiovascular disease based on ICD-9 diagnoses, suggesting that the association is secondary to the interaction between SSRI/SNRI and cardiovascular disease, or between SSRI/SNRI and a cardiovascular medication. In view of the very strong effect noted in acute coronary syndrome, one speculative possibility is that the increased mortality seen might be a withdrawal effect on the control of coronary vascular tone.
Our study has several limitations. First, our data are retrospective, which prevents us from evaluating causality. We were also unable to control for smoking, which is a potentially significant confounder for mortality, as the prevalence of smoking is significantly higher in patients with depression than in the general population. Neither the dose nor the duration of the SSRI administration was available, so we were unable to assess any potential effects of these factors.
Although our report examines the impact of specific drugs on outcomes, the potential for the approach described is not so limited. In fact, there are far-reaching implications for this mode of data examination that can include and analyze any of the elements captured in the available database. Although the current MIMIC database is limited to one academic hospital in the United States, plans are already in motion to extend the data to other hospitals, including institutions outside the United States. As the database expands quantitatively and qualitatively across diverse care environments, the power and significance of any individual analysis will only increase over time. Furthermore, such analyses can be easily repeated, modified, and strategically improved based on iterative interpretation of prior findings.
Although this study specifically reports the evaluation of outcomes in critically ill patients who have received SSRI/SSRN agents prior to admission, there is a more general and ultimately more important implication of this work: the use of an open clinical database for targeted analyses with documented methodologies and subsequent expert interpretation of the findings.25 We advocate that these analyses can be (1) repeated by other investigators using the same, updated, or another ICU database; (2) selectively modified to include and/or exclude covariates as more clinicians weigh in on the theoretical causal pathway between the exposure, the confounders, and the outcome of interest; and (3) repeated using other (including nonparametric) algorithms that might be better suited to represent the complexities of the relationship between the covariates, either real or perceived, during clinical decision-making. This kind of powerful, generalizable approach, requiring transparency in both data and methods, is necessary to realize and optimize the potentially achievable benefit of the secondary use of health data in the oncoming era of evidence-generating medicine.
Conclusions
In conclusion, we found in-hospital mortality to be increased in patients admitted to an ICU who were receiving SSRI or SNRI agents prior to admission. This relationship persisted after adjustment for several confounders, and risk appeared to be higher with increasing potency of the SSRIs. This information raises important questions about the long-term safety of these commonly prescribed medications, which require further investigation.
Currently, no recommendations can be made on the basis of this study regarding the continued use of these agents in patients who are in or discharged from an ICU. The next step is to validate our findings in another ICU database and design additional analyses that will address the limitations of this study, including taking into consideration the dose and duration of SSRI/SNRI use prior to critical illness. We envision using the same data-based approach in investigating an unlimited variety of clinical questions, such as potential interactions in diverse populations between drugs (or medical devices), and illuminating complex clinical scenarios, such as systemic inflammatory response syndrome, surgery, general anesthesia, and acute coronary syndrome, which, typically, are not captured during premarket testing.
As we learn more about maximizing the clinical information and guidance that can be gained from such an approach, we will begin to enter an era of transparency in data and methods based on what will be virtually continuous addition to and use of the accrued data. This lends to numerous benefits. First, the digitization of the medical record will then begin to truly pay off for clinicians burdened with what they may perceive as onerous data entry duties of dubious value. Second, this will begin to reduce the nearly universal loss of the precious information that is gained in every clinical encounter, enabling population-based clinical guidelines to be customized to individual patients across clinical contexts. Third, we will begin to create a beneficial and transparently cooperative clinical environment in which the care of each patient extends well beyond the individual to benefit larger patient populations, and the care of populations, in turn, powerfully benefits individuals. In addition, the increasing availability of all necessary data will enable users to perform such studies in a more complete and timely manner and, thus, complement and supplement traditional RCTs for the study of new (as well as accepted) therapies in diverse populations and settings. Finally, we will be able to rapidly identify even subtle adverse effects from drugs, devices, and practices and understand treatment effect heterogeneity that may be inadvertently glossed over in traditional RCTs.
Acknowledgments
Author contributions: Ms Ghassemi is guarantor of the integrity of the study.
Ms Ghassemi: contributed to the conception of the study, the study design, and the approach to data analysis; extracted the data from MIMIC-II; performed the statistical analysis; and participated in the interpretation of the findings and in the writing of the manuscript.
Dr Marshall: contributed to the conception of the study, the study design, and the approach to data analysis and participated in the interpretation of the findings and in the writing of the manuscript.
Mr Singh: contributed to the conception of the study, the study design, and the approach to data analysis; performed the statistical analysis; and participated in the interpretation of the findings and in the writing of the manuscript.
Dr Stone: contributed to the conception of the study, the study design, and the approach to data analysis and participated in the interpretation of the findings and in the writing of the manuscript.
Dr Celi: contributed to the conception of the study, the study design, and the approach to data analysis and participated in the interpretation of the findings and in the writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Abbreviations
- ICD-9
International Classification of Diseases, Ninth Revision
- MIMIC
Multiparameter Intelligent Monitoring in Intensive Care
- RCT
randomized controlled trial
- SAPS
Simplified Acute Physiology Score
- SNRI
serotonin norepinephrine reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
Footnotes
Drs Stone and Celi are co-senior authors of this manuscript.
Funding/Support: This research was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health [Grant R01 EB001659], the National Library of Medicine’s university-based Biomedical Informatics Research Training Program, and the Intel Science and Technology Center.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Moses C, Celi LA, Marshall J. Pharmacovigilance: an active surveillance system to proactively identify risks for adverse events. Popul Health Manag. 2013;16(3):147-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman CP, Wong AK, Blumenthal D. Achieving a nationwide learning health system. Sci Transl Med. 2010;2(57):57cm29. [DOI] [PubMed] [Google Scholar]
- 3.Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848-856 [DOI] [PubMed] [Google Scholar]
- 4.Pirraglia PA, Stafford RS, Singer DE. Trends in prescribing of selective serotonin reuptake inhibitors and other newer antidepressant agents in adult primary care. Prim Care Companion J Clin Psychiatry. 2003;5(4):153-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mojtabai R. Clinician-identified depression in community settings: concordance with structured-interview diagnoses. Psychother Psychosom. 2013;82(3):161-169 [DOI] [PubMed] [Google Scholar]
- 6.Smoller JW, Allison M, Cochrane BB, et al. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women’s Health Initiative study. Arch Intern Med. 2009;169(22):2128-2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan A, Okereke OI, Sun Q, et al. Depression and incident stroke in women. Stroke. 2011;42(10):2770-2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mojtabai R. Unmet need for treatment of major depression in the United States. Psychiatr Serv. 2009;60(3):297-305 [DOI] [PubMed] [Google Scholar]
- 9.Petersen T, Dording C, Neault NB, et al. A survey of prescribing practices in the treatment of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(1):177-187 [DOI] [PubMed] [Google Scholar]
- 10.MIMIC II database PhysioNet website. http://physionet.org/mimic2. Updated October 4, 2012. Accessed May 20, 2013
- 11.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65-70 [Google Scholar]
- 12.Kendall MG, Gibbons JD. Rank Correlation Methods. 5th ed. London, England: Oxford University Press; 1990.
- 13.Hollander M, Wolfe DA. Nonparametric Statistical Methods. 2nd ed New York, NY: Wiley; 1999 [Google Scholar]
- 14.Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340(2-3):249-258 [DOI] [PubMed] [Google Scholar]
- 15.Prasad V, Jena AB. Prespecified falsification end points: can they validate true observational associations? JAMA. 2013;309(3):241-242 [DOI] [PubMed] [Google Scholar]
- 16.Strand K, Flaatten H. Severity scoring in the ICU: a review. Acta Anaesthesiol Scand. 2008;52(4):467-478 [DOI] [PubMed] [Google Scholar]
- 17.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27 [DOI] [PubMed] [Google Scholar]
- 18.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633 [DOI] [PubMed] [Google Scholar]
- 19.Pearl J. The art and science of cause and effect. In: Causality: Models, Reasoning, and Inference. Cambridge, England: Cambridge University Press; 2000:331-358 [Google Scholar]
- 20.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546-1554 [DOI] [PubMed] [Google Scholar]
- 21.Damen NL, Pelle AJ, Boersma E, Serruys PW, van Domburg RT, Pedersen SS. Reduced positive affect (anhedonia) is independently associated with 7-year mortality in patients treated with percutaneous coronary intervention: results from the RESEARCH registry. Eur J Prev Cardiol. 2013;20(1):127-134 [DOI] [PubMed] [Google Scholar]
- 22.Tsai YC, Chiu YW, Hung CC, et al. Association of symptoms of depression with progression of CKD. Am J Kidney Dis. 2012;60(1):54-61 [DOI] [PubMed] [Google Scholar]
- 23.Ramasubbu R, Taylor VH, Samaan Z, et al. ; Canadian Network for Mood and Anxiety Treatments (CANMAT) Task Force The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and select comorbid medical conditions. Ann Clin Psychiatry. 2012;24(1):91-109 [PubMed] [Google Scholar]
- 24.Auerbach AD, Vittinghoff E, Maselli J, Pekow PS, Young JQ, Lindenauer PK. Perioperative use of selective serotonin reuptake inhibitors and risks for adverse outcomes of surgery. JAMA Intern Med. 2013;173(12):1075-1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stang PE, Ryan PB, Racoosin JA, et al. Advancing the science for active surveillance: rationale and design for the Observational Medical Outcomes Partnership. Ann Intern Med. 2010;153(9):600-606 [DOI] [PubMed] [Google Scholar]