Abstract
Dual antiplatelet therapy with aspirin and a P2Y12 receptor antagonist is the standard of care in patients undergoing percutaneous coronary intervention (PCI) and in patients with acute coronary syndromes (ACS) because this regimen has markedly decreased the rate of cardiovascular events. The substantial variability in pharmacodynamic response as well as the moderate antiplatelet efficacy of clopidogrel has raised major concerns, since high on-clopidogrel platelet reactivity has consistently been associated with increased risk for ischaemic events in PCI patients. Baseline demographic and clinical variables contributing to the observed variability have been identified. Besides this, research within the past decade has focused on the impact of genetic polymorphisms encoding transport systems or enzymes involved in the absorption and metabolism of these drugs. Loss-of-function polymorphisms in CYP2C19 are the strongest individual variables affecting pharmacokinetics and antiplatelet response to clopidogrel, but explain no more than 5 to 12% of the variability in adenosine diphosphate-induced platelet aggregation on clopidogrel. No genetic variables contributing to clinical outcomes of patients treated with the newer P2Y12 receptor antagonists, prasugrel or ticagrelor, have been identified so far. This review aims to provide an update on the current status of genotype-based personalized therapy with clopidogrel.
Keywords: clopidogrel, P2Y12 antagonist, platelet inhibitor, prasugrel, single nucleotide polymorphism, ticagrelor
Introduction
Platelet activation and aggregation play an important role in the development of ischaemic events during and after acute coronary syndromes (ACS) and percutaneous coronary interventions (PCI) 1. Acetylsalicylic acid (aspirin) was the first antiplatelet drug with proven benefit in ACS 2–4. Studies demonstrating significant platelet activation in ACS and during PCI despite treatment with aspirin and intense anticoagulant regimens stimulated clinical studies investigating novel antithrombotic regimens using two antiplatelet drugs namely aspirin and the thienopyridine derivative ticlopidine. Ticlopidine is an irreversible inhibitor of the platelet P2Y12 adenosine diphosphate (ADP) receptor 5,6. Compared with aspirin plus anticoagulation (heparin followed by vitamin K antagonists), dual antiplatelet therapy with aspirin and ticlopidine reduced not only the incidence of cardiac and vascular complications after the placement of coronary artery stents but also substantially the incidence of bleeding complications during follow-up 7. Dual antiplatelet therapy with aspirin and a P2Y12 receptor antagonist became therefore the standard of care for prevention of ischaemic complications in ACS patients and in patients undergoing PCI.
The clinical use of ticlopidine is hampered by serious haematological side effects. In clinical routine, ticlopidine has been widely replaced by clopidogrel which was approved in 1996/1997. Clopidogrel has demonstrated similar antiplatelet efficacy and an improved safety profile when compared with ticlopidine 8. Two additional P2Y12 receptor antagonists were approved within recent years by the regulatory agencies for treatment of patients with ACS, the thienopyridine derivative prasugrel (approval in 2009) and the cyclopentyltriazolopyrimidine ticagrelor (approved in 2010/2011).
A careful investigation of 700 aspirin-treated patients demonstrated that ‘non-response’ and/or a clinical meaningful variability in antiplatelet response is not an issue regarding aspirin 9. Clinically meaningful residual arachidonic acid-induced platelet activation was found in approximately 2% of patients and was most likely caused by under-dosing and/or non-compliance. In contrast, a substantial variability of antiplatelet effect was observed in clopidogrel-treated patients and high on clopidogrel platelet reactivity was associated with increased risk of ischaemic events in patients after PCI with stent placement 10–13.
Low systemic availability of the active metabolite of clopidogrel has been associated with blunted antiplatelet effect and adverse ischaemic events 14,15. Single nucleotide polymorphisms (SNP) in genes encoding for drug transporters and drug metabolizing enzymes have been linked to antiplatelet response of clopidogrel. Therefore, current evidence regarding the genetics of antiplatelet drugs with focus on clopidogrel is the topic of this review.
Therapeutic limitations of clopidogrel
Despite the widespread clinical use of clopidogrel for more than 15 years, large scale clinical studies assessing the antiplatelet efficacy of the drug were performed within the last decade. Pharmacodynamic studies demonstrated only a moderate inhibition of ADP-induced platelet aggregation and a substantial variability of effect in clopidogrel-treated patients (Figure 1) 16–18. High on-treatment platelet reactivity (HTPR) was associated with an increased incidence of adverse ischaemic/thrombotic events in patients after PCI 10,12. A white paper summarized the platelet function data from 28 studies comprising 11 477 patients on clopidogrel 19. HTPR was confirmed as an important risk factor for ischaemic events post-PCI irrespective of the clinical presentation of the patient (elective PCI, PCI in ACS w/o ST-segment elevation myocardial infarction). In contrast, risk of bleeding might be increased in patients with an exaggerated response to clopidogrel 20.
Figure 1.
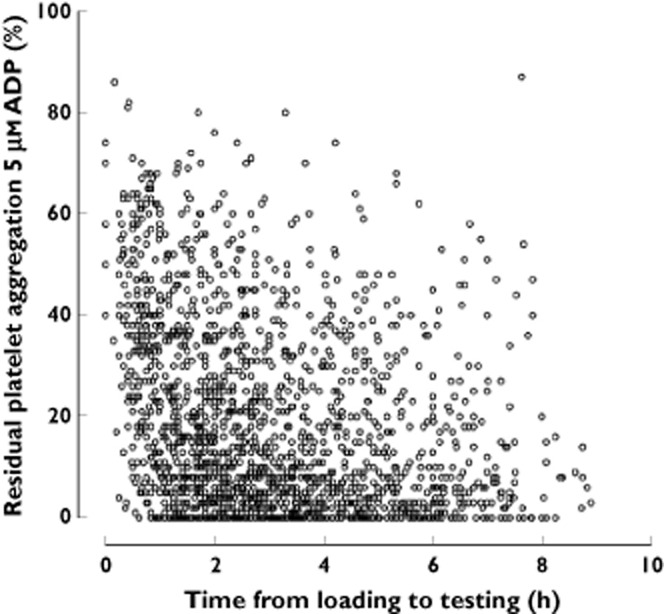
Residual platelet aggregation induced by 5 μm ADP in an unselected cohort of 2040 patients at elective cardiac catheterization dependent on time since administration of a loading dose of clopidogrel 600 mg 88. ADP, adenosine diphosphate
Various factors have been identified that contribute to this variability. Clinical and demographic variables such as older age (>65 years), increased body mass index, diabetes mellitus, reduced left ventricular function, renal failure (serum creatinine >1.5 mg dl−1) and presentation with ACS as well as drug–drug interactions were identified as predictors of HTPR 21,22. Clopidogrel is an inactive pro-drug requiring in vivo metabolism for formation of the active metabolite. Since the antiplatelet effect of clopidogrel is related to the amount of metabolite formed, recent research has focused on genetic factors with an impact on the activity of clopidogrel metabolizing enzymes and on bioavailability.
Metabolism of P2Y12 receptor antagonists
All thienopyridines (ticlopidine, clopidogrel and prasugrel) are inactive pro-drugs requiring metabolic activation in vivo. The active metabolites formed bind irreversibly to the P2Y12 receptor and therefore inhibit ADP-induced platelet activation for the life span of the platelet.
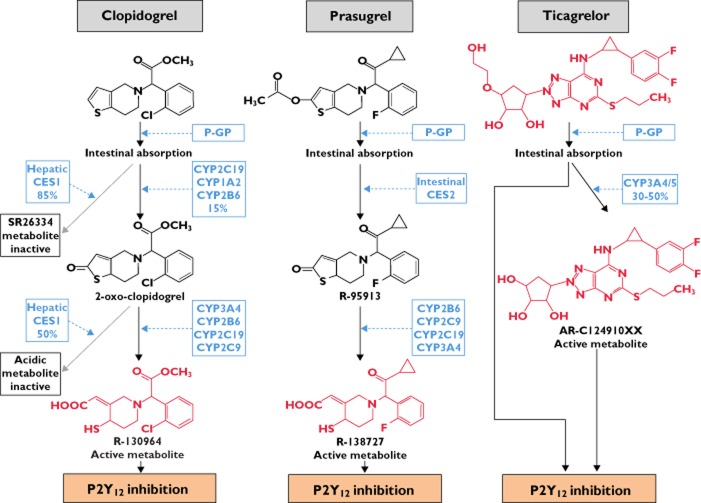
Clopidogrel is absorbed rapidly after oral ingestion. The majority of clopidogrel absorbed (∼85%) is hydrolyzed by human carboxylesterase 1 (CES1) which is primarily located in the liver to an inactive acid metabolite [S26334] (Figure 2) 23,24. Formation of the active metabolite is catalyzed by various cytochrome P450 (CYP) enzymes with formation of 2-oxo-clopidogrel as an intermediate metabolite. While CYP1A2, CYP2B6, CYP2C19 contribute to formation of 2-oxo-clopidogrel, CYP2B6, CYP2C9, CYP2C19 and CYP3A4/5 are involved in the subsequent formation of the active metabolite 24–26. In vitro experiments indicate that overall CYP2C19 is the main CYP contributing to the formation of the active metabolite of clopidogrel 26. Furthermore, approximately 50% of 2-oxo-clopidogrel formed is metabolized by CES1 to an inactive compound thus limiting the amount of active metabolite available for an antiplatelet effect.
Figure 2.
Metabolism of clopidogrel, prasugrel and ticagrelor 23,24,26,27,30. Compounds with P2Y12-receptor inhibiting properties are in red. CES, human carboxylesterase; CYP, cytochrome P450; P-GP, P-glycoprotein
Prasugrel is the most recently approved member of the thienopyridine-class of platelet inhibitors. It is rapidly absorbed and similar to clopidogrel is a substrate of intestinal P-glycoprotein. After absorption, prasugrel is extensively metabolized (Figure 2), and parent drug is not detected in human or animal plasma. The intermediate thiolactone metabolite of prasugrel is formed via hydrolysis predominantly by intestinal human carboxylesterase 2 (CES2) while CYP2B6, CYP2C9, CYP2C19 and CYP3A4 catalyze the second reaction in the formation process of the active metabolite. In contrast to clopidogrel, CYP3A4 and CYP2B6 are the main contributors to the formation of the active metabolite of prasugrel with smaller contributions by CYP2C9 and CYP2C19 27. It is estimated that at least 50 to 70% of the dose administered are converted into the active metabolite. In vitro experiments with washed human platelets yield similar EC50 values of the active metabolites of prasugrel and clopidogrel for inhibition of ADP-induced aggregation and provide evidence that the more efficient generation of the active metabolite is responsible for the superior antiplatelet efficacy of prasugrel in vivo 28.
Ticagrelor is a member of the new cyclopentyl-triazolopyrimidine class of agents. In contrast to the thienopyridines, ticagrelor is a direct-acting drug which interacts with the P2Y12 receptor via reversible, non-competitive binding to a ligand-site distinct from the binding site of ADP 29. Ticagrelor is also a substrate of P-glycoprotein. Approximately 30 to 50% of ticagrelor are eliminated via CYP3A4/5 metabolism (Figure 2) and the metabolite formed exerts antiplatelet effects, too 30. Due to its reversible binding characteristics, restoration of platelet function after cessation of ticagrelor is dependent on the rate of elimination of the parent compound and respective active metabolite.
Genetic polymorphisms and clopidogrel
CYP2C19 loss of function alleles
The pro-drug characteristics of clopidogrel, the dependence of the antiplatelet activity of clopidogrel on hepatic metabolism and the contribution of cytochrome P450 enzymes to this metabolic activation has already been determined in early pre-clinical studies 31,32. The two separate oxidative steps required for formation of the active metabolite are dependent on various CYP enzymes (CYP1A2, CYP2B6, CYP2C9, CYP2C19 and CYP3A4/5) and the genes encoding these enzymes are polymorphic. Evidence for the dominant contribution of CYP2C19 was derived in a pharmacodynamic study 33 and was confirmed in preclinical studies later 24–26. A genome-wide association study (GWAS) identified only single nucleotide polymorphisms on chromosome 10q24 within the CYP2C18–CYP2C19–CYP2C9–CYP2C8 cluster as being associated with clopidogrel response 34. At least 25 SNPs of the gene encoding the CYP2C19 isoenzyme have been described (http://www.cypallelees.ki.se/cyp2c19.htm). The fully functional and most prevalent version of the gene is CYP2C19*1. Variant alleles for which reduced or loss of function (LoF) have been shown comprise CYP2C19*2 to *8. The *2 allele is the most frequent variant allele (15%) in the Caucasian and the African population while *3 to *8 have only very minor clinical impact due to their low frequency (<1%). However, frequency of the *2 and the *3 alleles is much higher in the Asian population (*2: 27%, *3: 9%). The LoF SNP in the CYP2C19*2 gene variant is a G681A mutation (rs4244285) in exon 5 which encodes for a cryptic splice variant resulting in no enzyme activity in vivo, while a G636A mutation in exon 4 results in a premature stop codon in CYP2C19*3 35–37.
The clinical impact was initially observed in pharmacodynamic studies which showed that carriage of CYP2C19 LoF alleles is associated with a decreased antiplatelet activity of clopidogrel 11,33. Subsequently, pharmacokinetic/pharmacodynamic studies linked the attenuated efficacy of clopidogrel to a decreased systemic availability of the active metabolite 38. Clinical studies showed that patients undergoing PCI carrying CYP2C19 LoF alleles are at increased risk for adverse cardiovascular events (Figure 3) 38–41. A meta-analysis comprising 9685 cardiac patients with the vast majority (91%) enrolled after PCI confirmed this association and provided some evidence for a gene–dose effect 42. The risk of major adverse cardiovascular events and, in particular, the risk of stent thrombosis was increased in carriers of one or two reduced-function CYP2C19 alleles (Table 1).
Figure 3.
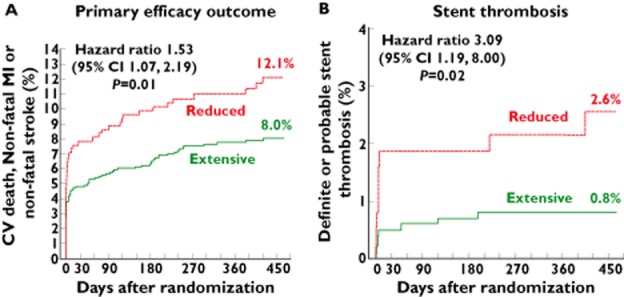
Risk for efficacy outcomes in non-carriers (CYP2C19*1/*1, *1/*17 and *17/*17) vs. carriers of CYP2C19 reduced function alleles (carriage of either *2, *3, *4,*5 or *8 allele) treated in TRITON-TIMI 38 with clopidogrel 38. A) Risk for the primary efficacy outcome of death from cardiovascular causes, myocardial infarction or stroke (n = 1459; n = 395 carriers of at least one CYP2C19 reduced-function allele and n = 1064 non-carriers). B) Risk of stent thrombosis (n = 1389; n = 375 carriers of at least one CYP2C19 reduced-function allele and n = 1014 non-carriers)
Table 1.
Incidence of cardiovascular death, myocardial infarction or ischaemic stroke and stent thrombosis by CYP2C19 genotype comprising 9685 patients
| Hazard ratio | 95% confidence interval | P value | |
|---|---|---|---|
| Cardiovascular death, myocardial infarction or ischaemic stroke | |||
| Carriers of one or two CYP2C19 alleles with reduced function vs. non-carriers | 1.57 | 1.13, 2.16 | 0.006 |
| Carriers of one CYP2C19 allele with reduced function vs. non-carriers | 1.55 | 1.11, 2.17 | 0.01 |
| Carriers of two CYP2C19 alleles with reduced function vs. non-carriers | 1.76 | 1.24, 2.50 | 0.002 |
| Stent thrombosis | |||
| Carriers of one or two CYP2C19 alleles with reduced function vs. non-carriers | 2.81 | 1.81, 4.37 | <0.00001 |
| Carriers of one CYP2C19 allele with reduced function vs. non-carriers | 2.67 | 1.69, 4.22 | <0.0001 |
| Carriers of two CYP2C19 alleles with reduced function vs. non-carriers | 3.97 | 1.75, 9.02 | 0.001 |
Determined CYP2C19 alleles with reduced function: CYP2C19*2, CYP2C19*3, CYP2C19*4, CYP2C19*5 and CYP2C19*8. Data adopted from Mega et al., 2010 42.
The PREDICT (Residual Platelet Aggregation after Deployment of Intracoronary Stent) score provides an estimate for prediction of antiplatelet response after administration of a loading dose of clopidogrel 600 mg. The score comprises the weighted contribution of non-genetic variables and carriage of CYP2C19*2 43. The predictive value of CYP2C19 LoF alleles as well as baseline and demographic variables for an insufficient antiplatelet response were analyzed to determine the contribution of these factors to variability in antiplatelet response. Carriage of a CYP2C19*2 allele was the strongest individual factor contributing to variability in the ex vivo determined platelet-inhibitory effect of clopidogrel, but this polymorphism accounts only for 5.2 to 12% of the variation of ADP-induced aggregation 34,43,44. Overall, CYP2C19*2 carrier status together with all demographic and clinical predictors for high on-clopidogrel could only explain no more than 12–22% of platelet reactivity on treatment with clopidogrel 34,44.
CYP2C19 gain of function alleles
Polymorphisms within the CYP2C19 system comprise not only LoF alleles but also a gain-of-function (GoF) mutation (CYP2C19*17). This allelic variant, a C806T mutation in exon 5, is responsible for an increased catalytic activity most likely due to a higher transcription rate of the gene 45. Carriage of CYP2C19*17 allele(s) should result in an increased antiplatelet effect of clopidogrel due to an enhanced formation of the active metabolite which in turn may potentially expose the patients to an increased risk of bleeding. However, data on the impact of CYP2C19 GoF SNPs are conflicting. While three independent studies showed an association of CYP2C19*17 allele carriage with lower on-clopidogrel platelet reactivity 46–48, CYP2C19*17 was not associated with ADP-stimulated platelet aggregation in healthy Amish subjects treated with clopidogrel 34. However, an independent association of CYP2C19*17 allele carriage with risk of bleeding was observed in a large registry cohort as well as in the genetic substudy of the PLATO trial in patients randomized to clopidogrel 49,50. Two recently published meta-analysis confirmed that carriers of the CYP2C19*17 allele had a lower risk of cardiovascular events but an increased risk of bleeding 51,52.
ABCB1
Clopidogrel is a substrate of the intestinal ATP-dependent efflux transporter P-glycoprotein (MDR-1) and an increased activity of this transport system decreases bioavailability of clopidogrel and other substrates 53,54. P-glycoprotein is encoded by the ABCB1 gene which is expressed highly polymorphic with more than 50 variants residing in the coding region 55. Various studies investigated the impact of the C3435T SNP on pharmacokinetics and/or antiplatelet effect of clopidogrel as well as the impact on clinical outcome in patients on clopidogrel.
Taubert and co-workers investigated the antiplatelet effects and pharmacokinetics of clopidogrel after 300, 600 and 900 mg bolus doses of clopidogrel and their association with ABCB1 C3435T genotype 53. Cmax and AUC values of clopidogrel and its active metabolite in the 300 mg and 600 mg groups (but not in the 900 mg group) were lower in subjects homozygous for the ABCB1 C3435T variant alleles compared with subjects with the 3435C/T and 3435C/C genotypes. However, subsequently performed clinical studies yielded conflicting results. Carriers of one or two ABCB1 C3435T variant alleles in the FAST-MI registry had an increased risk of the combined endpoint of cardiovascular death, myocardial infarction, or stroke 40 while an increase in risk in the genetic substudy of the TRITON–TIMI 38 study was evident only in TT homozygotes treated with clopidogrel 56. ABCB1 C3435T genotype was not associated in two studies with ADP-stimulated platelet aggregation and patients with the C/C genotype on treatment with clopidogrel in the PLATO genetic substudy had the highest risk for the combined ischaemic endpoint 34,50,57. A recently performed meta-analysis also failed to show an association between ABCB1 C3435T genotype and risk of overall recurrent ischaemic events, stent thrombosis or bleeding in clopidogrel treated patients 58.
Only one study analyzed the association between G2677T SNP and platelet reactivity in a small cohort of patients after elective PCI. This study could not show any difference in platelet reactivity between the genotypes 59.
Carboxylesterase 1 G143E
The vast majority of absorbed clopidogrel is shunted by carboxylesterase 1 (CES1) to inactive metabolites. Thus, inhibition of CES1 in vitro increased formation of the active metabolite of clopidogrel 60. A missense single nucleotide polymorphism (SNP) in exon 4 of CES1 (rs71647871) results in a catalytic site glycine (G)-to-glutamic acid (E) amino acid change at position 143 (G143E). The G143E mutation severely decreased CES1 catalytic function 61. A recently published study showed that systemic availability of the active metabolite of clopidogrel was increased and ADP-induced platelet aggregation was amplified in carriers of the CES1 143E-allele 62. Due to lack of adequately powered studies it is currently unknown whether CES1 G143E mutations impact on clinical outcome of clopidogrel treated patients.
PON1
Paraoxonase-1 (PON1) is an esterase synthesized in the liver and associated with HDL-cholesterol in blood 63. The catalytic activity of PON1 is determined by a common SNP in PON1 c.575A>G resulting in an amino acid substitution Gln>Arg at position 192 (Q192R). A case-cohort study in survivors of a stent thrombosis on clopidogrel and matched controls linked PON1 Q192R polymorphism with the antiplatelet activity of clopidogrel and the risk of stent thrombosis 64. The link between PON1 Q192R polymorphism and the risk of cardiovascular events on treatment with clopidogrel was supported by a replication study using a prospective cohort of 1982 individuals with ACS. Furthermore, investigation of the pharmacokinetics of clopidogrel, ex vivo determination of ADP-induced platelet aggregation and a series of in vitro experiments using microsomal expression system of metabolizing enzymes corroborated the hypothesis. The authors suggested that PON1 and not CYP2C19 seems to be the key enzyme involved in metabolism of 2-oxo-clopidogrel to the active metabolite. They estimated that more than 70% of variability in platelet aggregation could be attributed to PON1 Q192R polymorphism which thus seems to be the primary predictor of clopidogrel response 64. A series of other studies and a meta-analysis did not find an association between PON1 Q192R polymorphism either with the antiplatelet effect of clopidogrel regardless of the test used or with risk of cardiovascular events in patients treated with clopidogrel 59,65–70. The reason for the discrepancy between the original work and the subsequently performed studies is unclear up to now. It was hypothesized that differences in the study populations might be an issue because the distribution of Q192R genotypes in the study of Bouman et al. 64 differed from the subsequently performed studies 59,65–70. The specificity of the analytical methods used by Bouman et al. 64 in their in vitro studies was questioned by Dansette and co-workers 71. These authors investigated the impact of CYP enzymes and PON1 on the complex stereochemistry of the metabolism of 2-oxo-clopiodgrel to the active thiol metabolite. They showed that formation of the most active cis-diastereoisomers of the thiol metabolite of clopidogrel is catalyzed by CYP enzymes. In contrast, contribution of esterases such as PON1, results in formation of a trans-diastereoisomer metabolite which exhibits only minor antiplatelet properties 71.
Pharmacogenetics of prasugrel and ticagrelor
The impact of the ABCB1 C3435T polymorphism and SNPs in genes encoding for cytochrome P450 enzymes was analyzed in various studies within the clinical study programme of the new P2Y12 receptor antagonists, prasugrel and ticagrelor. Neither the pharmacodynamics nor the pharmacokinetics of prasugrel or ticagrelor were altered in patients with this genetic background 72–74. A recent study showed that the antiplatelet effect of prasugrel might also be modulated by CYP2C19*2 and *17 if platelet reactivity index vasodilator-stimulated phosphoprotein (PRI VASP) is used for assessment 75. The latter observation requires confirmation since the same authors reported in a preceding paper that this association holds true only for PRI VASP assay while no significant influence of CYP2C19 genotype on platelet reactivity assessed by ADP-induced platelet aggregation was observed 76.
At present, there are no data supporting any impact of genetic polymorphisms in pharmacokinetics of prasugrel or ticagrelor on clinical outcomes of patients. Large subgroups of patients enrolled in the large scale phase III clinical outcomes studies TRITON-TIMI 38 (prasugrel) and PLATO (ticagrelor) consented for additional genetic analysis. Prasugrel as well as ticagrelor provided overall a significant beneficial effect by reducing the incidence of the combined endpoint (cardiovascular death, myocardial infarction, stroke) compared with clopidogrel 77,78. The observed net clinical benefit of prasugrel/ticagrelor over clopidogrel was not affected by ABCB1 C3435T polymorphism or carriage of CYP2C19 LoF allele(s) 50,73
Current status of therapeutic strategies based on genotype
Triggered by the compelling data on the association between CYP2C19 LoF polymorphism and increased risk for ischaemic events in clopidogrel-treated patients after PCI the FDA announced a ‘Boxed warning’ on March 12, 2010, informing physicians and patients about the reduced effectiveness of clopidogrel in patients with an impaired ability to convert the drug into its active form (FDA 03-12-2010). This warning was based upon the CYP2C19 LoF genetic background of attenuated antiplatelet efficacy of clopidogrel and triggered several actions:
US [American College of Cardiology Foundation/American Heart Association (ACCF/AHA)] and European Society of Cardiology (ESC) implemented recommendations for genotyping in their respective guidelines 79,80. However, one has to admit that classes of recommendation and levels of evidence attributed to genotyping as part of antiplatelet therapy are weak (ACCF/AHA: class IIb, level of evidence C; ESC: class IIb, level of evidence B).
Bedside tests for the rapid assessment of CYP2C19*2 genotype were developed.
Clinical studies were initiated to investigate if HTPR in carriers of CYP2C19 LoF allele(s) can be overcome by high dose regimens of clopidogrel or treatment with alternate P2Y12 receptor antagonists.
Clinical implementation of CYP2C19 genotyping
A prerequisite for implementation of genotype based antiplatelet therapy especially in patients presenting with ACS is the option for a simple bedside assay which can be performed in the coronary angiography laboratory with a rapid turnover of the test and, of course, reliability of test results.
At present, at least two point-of-care assays are available for CYP2C19*2 genotyping: the Verigene CYP2C19 XP system (Nanosphere, Northbrook, IL, USA) and the Spartan RX CYP2C19 system (Spartan Bioscience Inc, Ottawa, Ontario, Canada). The CE-certified SPARTAN RX assay uses a buccal swap and results are available within 60 min. CYP2C19*2 carrier status is provided as homozygous for the wild-type allele (*1/*1), heterozygous (*1/*2) or homozygous for the *2 allele (*2/*2). Assessment of the technical performance of the SPARTAN RX assay provided a sensitivity of 100% (95% CI 92.3, 100) and a specificity of 99.3% (95% CI 96.3, 100) using direct DNA sequencing as reference. A proof of concept study (RAPID GENE) was published recently 81. Patients undergoing PCI for ACS or stable angina were randomly assigned to rapid CYP2C19*2 allele screening at randomization or conventional treatment with subsequent CYP2C19*2 genotyping. Patients randomized to rapid screening and carrying CYP2C19*2 alleles were treated with prasugrel 10 mg daily, while non-carriers and patients in the conventional treatment arm received clopidogrel 75 mg daily. The proportion of patients with HTPR (PRU >234 by VerifyNow P212™ test) was analyzed after 1 week of treatment. None of the CYP2C19*2 carriers in the rapid genotyping group treated with prasugrel had HTPR, while 30% of CYP2C19*2 patients in the conventional group treated with clopidogrel had HTPR. However, 9.9% and 9.4% of non-carriers of CYP2C19*2 alleles in both groups had HTPR after 1 week of clopidogrel treatment.
The Verigene CYP2C19 XP assay is performed in a blood sample from the patient and provides data on the presence of either CYP2C19*2 or *3 allele(s). Turnaround time is about 3 h.
The key question is what will be the role for genotyping in personalized antiplatelet therapy given the background of the new P2Y12 receptor antagonists, prasugrel and ticagrelor? The more potent P2Y12 receptor antagonists prasugrel or ticagrelor decreased the incidence of ischaemic endpoints but at the cost of a slightly increased rate of non-CABG related major bleedings 77,78. These benefit–risk considerations and the availability of substantially less expensive generic clopidogrel in most countries provide an attractive stimulus for optimizing the therapeutic benefit of clopidogrel not only from a pharmaco-economic point of view.
So far, only pharmacodynamic studies investigating treatment algorithms based on genotype are available. Three non-randomized open label studies determined antiplatelet response to increased loading doses of clopidogrel 82–84. The frequency of patients with high on clopidogrel platelet reactivity can be reduced in CYP2C19*2 carriers by increasing the loading dose of clopidogrel. Bonello and co-workers used a target approach: They administered additional day by day 600 mg loading doses of clopidogrel up to a total dose of 2400 mg until the pre-defined antiplatelet effect assessed by vasodilator-stimulated phosphoprotein (VASP) assay was obtained. This attempt was successful in 88% of patients carrying at least one CYP2C19*2 allele and in five out of six CYP2C19*2 homozygous patients 83.
ELEVATE-TIMI used a randomized, double-blind study design to investigate if increasing the maintenance doses of clopidogrel up to 300 mg daily can improve platelet reactivity in CYP2C19*2 carriers with stable cardiovascular disease 85. A maintenance dose of 225 mg clopidogrel daily provided in CYP2C19*2 heterozygotes levels of platelet reactivity assessed with the VerifyNow P212™ assay which were similar to that determined on the standard 75 mg dose in non-carriers. It seems that CYP2C19*2 homozygous patients did not achieve degrees of platelet reactivity comparable with non-carriers despite treatment with clopidogrel doses up to 300 mg daily. However, the number of patients with this genotype was insufficient to draw any firm conclusions.
There are no studies available that investigated the time course of platelet reactivity in patients with CYP2C19 LoF genotype in the first days after switching from clopidogrel to prasugrel or ticagrelor. The RAPID GENE study showed that a superior antiplatelet effect is achieved at day 7 after switching from clopidogrel to prasugrel 10 mg daily in CYP2C19*2 patients 81. There is no dedicated switching study from clopidogrel to ticagrelor in CYP2C19*2 patients available. A pharmacodynamic study confirmed that the antiplatelet response to ticagrelor is not influenced by CYP2C19 genotype 74.
Summary and conclusions
High on-treatment platelet reactivity is associated with increased risk for ischaemic events in patients undergoing PCI with stent placement. Data supporting the impact of ABCB1 genotype on antiplatelet effect and clinical outcome of patients are controversial while the majority of studies obviate any impact of the PON1 Q192R polymorphism. So far, no clinical outcome studies investigating the contribution of carboxylesterase 1 G143E genotype are available.
Carriage of CYP2C19 LoF allele(s) is the strongest individual variable associated with HTPR on clopidogrel but CYP2C19*2 carrier status accounted for only 5 to 12% of the variability in on-clopidogrel platelet reactivity. Easy to use validated bedside tests for rapid assessment of this genetic background are available. Prospective double-blind randomized clinical outcome studies should be the next step for assessment of the clinical utility of personalized CYP2C19 genotype-guided antiplatelet therapy and patients presenting with ACS should be the target patient population. Currently, a combination of genotyping and phenotyping (i.e determination of platelet reactivity) seems to be the most promising approach to personalize antiplatelet therapy and improve efficacy and safety. This approach should be compared with the one size fits all strategy by using the new P2Y12 platelet antagonists prasugrel or ticagrelor in ACS patients. At present, the latter strategy is favoured above clopidogrel in the current guidelines of ACCF/AHA and ESC for ACS 79,86,87. In order to progress pharmacogenetics in antiplatelet therapy, utilization of next generation sequencing technologies seems promising after clear phenotype definitions balancing risk of thrombosis vs. bleeding risk have been determined.
Competing Interests
Both authors of the paper have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted manuscript. DT reported receiving consulting fees or paid advisory board fees and lecture fees from Eli Lilly, Daiichi Sankyo, AstraZeneca, Bayer Vital, and lecture fees from Boehringer Ingelheim KG, Bristol Myers Squibb, and Merck Sharp & Dohme in the previous 3 years. WH reported serving on a consultant/advisory board for Sanofi-Aventis in the previous 3 years.
References
- 1.Lange RA, Hillis LD. Antiplatelet therapy for ischemic heart disease. N Engl J Med. 2004;350:277–280. doi: 10.1056/NEJMe038191. [DOI] [PubMed] [Google Scholar]
- 2.Lewis HD, Jr, Davis JW, Archibald DG, Steinke WE, Smitherman TC, Doherty JE, 3rd, Schnaper HW, LeWinter MM, Linares E, Pouget JM, Sabharwal SC, Chesler E, DeMots H. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1983;309:396–403. doi: 10.1056/NEJM198308183090703. [DOI] [PubMed] [Google Scholar]
- 3.Cairns JA, Gent M, Singer J, Finnie KJ, Froggatt GM, Holder DA, Jablonsky G, Kostuk WJ, Melendez LJ, Myers MG, Sackett DL, Sealey BJ, Tanser PH. Aspirin, sulfinpyrazone, or both in unstable angina. Results of a Canadian multicenter trial. N Engl J Med. 1985;313:1369–1375. doi: 10.1056/NEJM198511283132201. [DOI] [PubMed] [Google Scholar]
- 4.Theroux P, Ouimet H, McCans J, Latour J-G, Joly P, Lévy G, Pelletier E, Juneau M, Stasiak J, deGuise P, Pelletier GB, Rinzler D, Waters DD. Aspirin, heparin, or both to treat acute unstable angina. N Engl J Med. 1988;319:1105–1111. doi: 10.1056/NEJM198810273191701. [DOI] [PubMed] [Google Scholar]
- 5.Neumann FJ, Gawaz M, Ott I, May A, Mössmer G, Schömig A. Prospective evaluation of hemostatic predictors of subacute stent thrombosis after coronary Palmaz-Schatz stenting. J Am Coll Cardiol. 1996;27:15–21. doi: 10.1016/0735-1097(95)00433-5. [DOI] [PubMed] [Google Scholar]
- 6.Gawaz M, Neumann FJ, Ott I, Schiessler A, Schömig A. Platelet function in acute myocardial infarction treated with direct angioplasty. Circulation. 1996;93:229–237. doi: 10.1161/01.cir.93.2.229. [DOI] [PubMed] [Google Scholar]
- 7.Schömig A, Neumann FJ, Kastrati A, Schühlen H, Blasini R, Hadamitzky M, Walter H, Zitzmann Roth EM, Richardt G, Alt E, Schmitt C, Ulm K. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084–1089. doi: 10.1056/NEJM199604253341702. [DOI] [PubMed] [Google Scholar]
- 8.Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the Clopidogrel Aspirin Stent International Cooperative Study (CLASSICS) Circulation. 2000;102:624–629. doi: 10.1161/01.cir.102.6.624. [DOI] [PubMed] [Google Scholar]
- 9.Frelinger AL, 3rd, Furman MI, Linden MD, Li Y, Fox ML, Barnard MR, Michelson AD. Residual arachidonic acid-induced platelet activation via an adenosine diphosphate-dependent but cyclooxygenase-1-and cyclooxygenase-2-independent pathway: a 700-patient study of aspirin resistance. Circulation. 2006;113:2888–2896. doi: 10.1161/CIRCULATIONAHA.105.596627. [DOI] [PubMed] [Google Scholar]
- 10.Hochholzer W, Trenk D, Bestehorn HP, Fischer B, Valina CM, Ferenc M, Gick M, Caputo A, Büttner HJ, Neumann FJ. Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. J Am Coll Cardiol. 2006;48:1742–1750. doi: 10.1016/j.jacc.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 11.Trenk D, Hochholzer W, Fromm MF, Chialda LE, Pahl A, Valina CM, Stratz C, Schmiebusch P, Bestehorn HP, Büttner HJ, Neumann FJ. Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J Am Coll Cardiol. 2008;51:1925–1934. doi: 10.1016/j.jacc.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 12.Sibbing D, Braun S, Morath T, Mehilli J, Vogt W, Schömig A, Kastrati A, von Beckerath N. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J Am Coll Cardiol. 2009;53:849–856. doi: 10.1016/j.jacc.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Geisler T, Langer H, Wydymus M, Göhring K, Zürn C, Bigalke B, Stellos K, May AE, Gawaz M. Low response to clopidogrel is associated with cardiovascular outcome after coronary stent implantation. Eur Heart J. 2006;27:2420–2425. doi: 10.1093/eurheartj/ehl275. [DOI] [PubMed] [Google Scholar]
- 14.von Beckerath N, Taubert D, Pogatsa-Murray G, Wieczorek A, Schömig E, Schömig A, Kastrati A. A patient with stent thrombosis, clopidogrel-resistance and failure to metabolize clopidogrel to its active metabolite. Thromb Haemost. 2005;93:789–791. [PubMed] [Google Scholar]
- 15.Heestermans AA, van Werkum JW, Taubert D, Seesing TH, von Beckerath N, Hackeng CM, Schömig E, Verheugt FW, ten Berg JM. Impaired bioavailability of clopidogrel in patients with a ST-segment elevation myocardial infarction. Thromb Res. 2008;122:776–781. doi: 10.1016/j.thromres.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45:246–251. doi: 10.1016/j.jacc.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 17.Gurbel PA, Bliden KP, Hiatt BL, O'Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 18.Hochholzer W, Trenk D, Frundi D, Blanke P, Fischer B, Andris K, Bestehorn HP, Büttner HJ, Neumann FJ. Time dependence of platelet inhibition after a 600 mg loading dose of clopidogrel in a large, unselected cohort of candidates for percutaneous coronary intervention. Circulation. 2005;111:2560–2564. doi: 10.1161/01.CIR.0000160869.75810.98. [DOI] [PubMed] [Google Scholar]
- 19.Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, Bhatt DL, Cattaneo M, Collet JP, Cuisset T, Gachet C, Montalescot G, Jennings LK, Kereiakes D, Sibbing D, Trenk D, Van Werkum JW, Paganelli F, Price MJ, Waksman R, Gurbel PA. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–933. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 20.Sibbing D, Koch W, Gebhard D, Schuster T, Braun S, Stegherr J, Morath T, Schömig A, von Beckerath N, Kastrati A. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. 2010;121:512–518. doi: 10.1161/CIRCULATIONAHA.109.885194. [DOI] [PubMed] [Google Scholar]
- 21.Geisler T, Grass D, Bigalke B, Stellos K, Drosch T, Dietz K, Herdeg C, Gawaz M. The Residual Platelet Aggregation after Deployment of Intracoronary Stent (PREDICT) score. J Thromb Haemost. 2008;6:54–61. doi: 10.1111/j.1538-7836.2007.02812.x. [DOI] [PubMed] [Google Scholar]
- 22.Bates ER, Lau WC, Angiolillo DJ. Clopidogrel–drug interactions. J Am Coll Cardiol. 2011;57:1251–1263. doi: 10.1016/j.jacc.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, Pascal M, Herbert JM. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost. 2000;84:891–896. [PubMed] [Google Scholar]
- 24.Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel and prasugrel in humans. J Clin Pharmacol. 2010;50:126–142. doi: 10.1177/0091270009343005. [DOI] [PubMed] [Google Scholar]
- 25.Hagihara K, Kazui M, Kurihara A, Yoshiike M, Honda K, Okazaki O, Farid NA, Ikeda T. A possible mechanism for the differences in efficiency and variability of active metabolite formation from thienopyridine antiplatelet agents, prasugrel and clopidogrel. Drug Metab Dispos. 2009;37:2145–2152. doi: 10.1124/dmd.109.028498. [DOI] [PubMed] [Google Scholar]
- 26.Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, Ikeda T, Kurihara A. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92–99. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 27.Rehmel JL, Eckstein JA, Farid NA, Heim JB, Kasper SC, Kurihara A, Wrighton SA, Ring BJ. Interactions of two major metabolites of prasugrel, a thienopyridine antiplatelet agent, with the cytochromes P450. Drug Metab Dispos. 2006;34:600–607. doi: 10.1124/dmd.105.007989. [DOI] [PubMed] [Google Scholar]
- 28.Sugidachi A, Ogawa T, Kurihara A, Hagihara K, Jakubowski JA, Hashimoto M, Niitsu Y, Asai F. The greater in vivo antiplatelet effects of prasugrel as compared to clopidogrel reflect more efficient generation of its active metabolite with similar antiplatelet activity to that of clopidogrel's active metabolite. J Thromb Haemost. 2007;5:1545–1551. doi: 10.1111/j.1538-7836.2007.02598.x. [DOI] [PubMed] [Google Scholar]
- 29.Van Giezen JJJ, Nilsson L, Berntsson P, Wissing B-M, Giordanetto F, Tomlinson W, Greasley PJ. Ticagrelor binds to human P2Y12 independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. J Thromb Haemost. 2009;7:1556–1565. doi: 10.1111/j.1538-7836.2009.03527.x. [DOI] [PubMed] [Google Scholar]
- 30.Teng R, Oliver S, Hayes MA, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010;38:1514–1521. doi: 10.1124/dmd.110.032250. [DOI] [PubMed] [Google Scholar]
- 31.Savi P, Herbert JM, Pflieger AM, Dol F, Delebassee D, Combalbert J, Defreyn G, Maffrand JP. Importance of hepatic metabolism in the antiaggregating activity of the thienopyridine clopidogrel. Biochem Pharmacol. 1992;44:527–532. doi: 10.1016/0006-2952(92)90445-o. [DOI] [PubMed] [Google Scholar]
- 32.Savi P, Combalbert J, Gaich C, Rouchon MC, Maffrand JP, Berger Y, Herbert JM. The antiaggregating activity of clopidogrel is due to a metabolic activation by the hepatic cytochrome P450-1A. Thromb Haemost. 1994;72:313–317. [PubMed] [Google Scholar]
- 33.Hulot JS, Bura A, Villard E, Azizi M, Remones V, Goyenvalle C, Aiach M, Lechat P, Gaussem P. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244–2247. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- 34.Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem. 1994;269:15419–15422. [PubMed] [Google Scholar]
- 36.Goldstein JA. Clinical relevance of genetic polymorphisms in the human CYP2C subfamily. Br J Clin Pharmacol. 2001;52:349–355. doi: 10.1046/j.0306-5251.2001.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wedlund PJ. The CYP2C19 enzyme polymorphism. Pharmacology. 2000;61:174–183. doi: 10.1159/000028398. [DOI] [PubMed] [Google Scholar]
- 38.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, Sabatine MS. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 39.Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, Payot L, Brugier D, Cayla G, Beygui F, Bensimon G, Funck-Brentano C, Montalescot G. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309–317. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 40.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Meneveau N, Steg PG, Ferrieres J, Danchin N, Becquemont L. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 41.Sibbing D, Stegherr J, Latz W, Koch W, Mehilli J, Dorrler K, Morath T, Schömig A, Kastrati A, von Beckerath N. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur Heart J. 2009;30:916–922. doi: 10.1093/eurheartj/ehp041. [DOI] [PubMed] [Google Scholar]
- 42.Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, Horne BD, Hulot JS, Kastrati A, Montalescot G, Neumann FJ, Shen L, Sibbing D, Steg PG, Trenk D, Wiviott SD, Sabatine MS. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geisler T, Schaeffeler E, Dippon J, Winter S, Buse V, Bischofs C, Zuern C, Moerike K, Gawaz M, Schwab M. CYP2C19 and nongenetic factors predict poor responsiveness to clopidogrel loading dose after coronary stent implantation. Pharmacogenomics. 2008;9:1251–1259. doi: 10.2217/14622416.9.9.1251. [DOI] [PubMed] [Google Scholar]
- 44.Hochholzer W, Trenk D, Fromm MF, Valina CM, Stratz C, Bestehorn HP, Büttner HJ, Neumann FJ. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J Am Coll Cardiol. 2010;55:2427–2434. doi: 10.1016/j.jacc.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 45.Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, Ingelman-Sundberg M. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther. 2006;79:103–113. doi: 10.1016/j.clpt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Sibbing D, Gebhard D, Koch W, Braun S, Stegherr J, Morath T, Von Beckerath N, Mehilli J, Schömig A, Schuster T, Kastrati A. Isolated and interactive impact of common CYP2C19 genetic variants on the antiplatelet effect of chronic clopidogrel therapy. J Thromb Haemost. 2010;8:1685–1693. doi: 10.1111/j.1538-7836.2010.03921.x. [DOI] [PubMed] [Google Scholar]
- 47.Frere C, Cuisset T, Gaborit B, Alessi MC, Hulot JS. The CYP2C19*17 allele is associated with better platelet response to clopidogrel in patients admitted for non-ST acute coronary syndrome. J Thromb Haemost. 2009;7:1409–1411. doi: 10.1111/j.1538-7836.2009.03500.x. [DOI] [PubMed] [Google Scholar]
- 48.Harmsze AM, van Werkum JW, Hackeng CM, Ruven HJ, Kelder JC, Bouman HJ, Breet NJ, Ten Berg JM, Klungel OH, de Boer A, Deneer VH. The influence of CYP2C19*2 and *17 on on-treatment platelet reactivity and bleeding events in patients undergoing elective coronary stenting. Pharmacogenet Genomics. 2012;22:169–175. doi: 10.1097/FPC.0b013e32834ff6e3. [DOI] [PubMed] [Google Scholar]
- 49.Sibbing D, Koch W, Gebhard D, Schuster T, Braun S, Stegherr J, Morath T, Schömig A, von Beckerath N, Kastrati A. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. 2010;121:512–518. doi: 10.1161/CIRCULATIONAHA.109.885194. [DOI] [PubMed] [Google Scholar]
- 50.Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, Husted S, Katus H, Steg PG, Shah SH, Becker RC. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–1328. doi: 10.1016/S0140-6736(10)61274-3. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Tang HL, Hu YF, Xie HG. The gain-of-function variant allele CYP2C19*17: a double-edged sword between thrombosis and bleeding in clopidogrel-treated patients. J Thromb Haemost. 2012;10:199–206. doi: 10.1111/j.1538-7836.2011.04570.x. [DOI] [PubMed] [Google Scholar]
- 52.Zabalza M, Subirana I, Sala J, Lluis-Ganella C, Lucas G, Tomás M, Masiá R, Marrugat J, Brugada R, Elosua R. Meta-analyses of the association between cytochrome CYP2C19 loss-and gain-of-function polymorphisms and cardiovascular outcomes in patients with coronary artery disease treated with clopidogrel. Heart. 2012;98:100–108. doi: 10.1136/hrt.2011.227652. [DOI] [PubMed] [Google Scholar]
- 53.Taubert D, von Beckerath N, Grimberg G, Lazar A, Jung N, Goeser T, Kastrati A, Schömig A, Schömig E. Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther. 2006;80:486–501. doi: 10.1016/j.clpt.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Greiner B, Eichelbaum M, Fritz P, Kreichgauer HP, von Richter O, Zundler J, Kroemer HK. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest. 1999;104:147–153. doi: 10.1172/JCI6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fung KL, Gottesman MM. A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim Biophys Acta. 2009;1794:860–871. doi: 10.1016/j.bbapap.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mega JL, Close SL, Wiviott SD, Shen L, Walker JR, Simon T, Antman EM, Braunwald E, Sabatine MS. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376:1312–1319. doi: 10.1016/S0140-6736(10)61273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trenk D, Hochholzer W, Fromm MF, Kleyer A, Pahl A, Neumann F-J. Polymorphism of MDR-1 and the organic anion-transporting polypeptide (OATP) 1B1 have no impact on antiplatelet effect of a 600-mg loading dose of clopidogrel in patients undergoing PCI. Eur Heart J. 2008;29:832. Abstract Suppl.: Abstract P4781. [Google Scholar]
- 58.Luo M, Li J, Xu X, Sun X, Sheng W. ABCB1 C3435T polymorphism and risk of adverse clinical events in clopidogrel treated patients: a meta-analysis. Thromb Res. 2012;129:754–759. doi: 10.1016/j.thromres.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Rideg O, Komócsi A, Magyarlaki T, Tőkés-Füzesi M, Miseta A, Kovács GL, Aradi D. Impact of genetic variants on post-clopidogrel platelet reactivity in patients after elective percutaneous coronary intervention. Pharmacogenomics. 2011;12:1269–1280. doi: 10.2217/pgs.11.73. [DOI] [PubMed] [Google Scholar]
- 60.Zhu HJ, Wang X, Gawronski BE, Brinda BJ, Angiolillo DJ, Markowitz JS. Carboxylesterase 1 as a determinant of clopidogrel metabolism and activation. J Pharmacol Exp Ther. 2013;344:665–672. doi: 10.1124/jpet.112.201640. [DOI] [PubMed] [Google Scholar]
- 61.Zhu HJ, Patrick KS, Yuan HJ, Wang JS, Donovan JL, DeVane CL, Malcolm R, Johnson JA, Youngblood GL, Sweet DH, Langaee TY, Markowitz JS. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82:1241–1248. doi: 10.1016/j.ajhg.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lewis JP, Horenstein RB, Ryan K, O'Connell JR, Gibson Q, Mitchell BD, Tanner K, Chai S, Bliden KP, Tantry US, Peer CJ, Figg WD, Spencer SD, Pacanowski MA, Gurbel PA, Shuldiner AR. The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response. Pharmacogenet Genomics. 2013;23:1–8. doi: 10.1097/FPC.0b013e32835aa8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blatter MC, James RW, Messmer S, Barja F, Pometta D. Identification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein-associated protein, K-45. Identity of K-45 with paraoxonase. Eur J Biochem. 1993;211:871–879. doi: 10.1111/j.1432-1033.1993.tb17620.x. [DOI] [PubMed] [Google Scholar]
- 64.Bouman HJ, Schömig E, van Werkum JW, Velder J, Hackeng CM, Hirschhauser C, Waldmann C, Schmalz HG, ten Berg JM, Taubert D. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med. 2011;17:110–116. doi: 10.1038/nm.2281. [DOI] [PubMed] [Google Scholar]
- 65.Fontana P, James R, Barazer I, BerdaguÉ P, Schved JF, Rebsamen M, Vuilleumier N, Reny JL. Relationship between paraoxonase-1 activity, its Q192R genetic variant and clopidogrel responsiveness in the ADRIE study. J Thromb Haemost. 2011;9:1664–1666. doi: 10.1111/j.1538-7836.2011.04409.x. [DOI] [PubMed] [Google Scholar]
- 66.Lewis JP, Fisch AS, Ryan K, O'Connell JR, Gibson Q, Mitchell BD, Shen H, Tanner K, Horenstein RB, Pakzy R, Tantry US, Bliden KP, Gurbel PA, Shuldiner AR. Paraoxonase 1 (PON1) gene variants are not associated with clopidogrel response. Clin Pharmacol Ther. 2011;90:568–574. doi: 10.1038/clpt.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sibbing D, Koch W, Massberg S, Byrne RA, Mehilli J, Schulz S, Mayer K, Bernlochner I, Schömig A, Kastrati A. No association of paraoxonase-1 Q192R genotypes with platelet response to clopidogrel and risk of stent thrombosis after coronary stenting. Eur Heart J. 2011;32:1605–1613. doi: 10.1093/eurheartj/ehr155. [DOI] [PubMed] [Google Scholar]
- 68.Simon T, Steg PG, Becquemont L, Verstuyft C, Kotti S, Schiele F, Ferrari E, Drouet E, Grollier G, Danchin N. Effect of paraoxonase-1 polymorphism on clinical outcomes in patients treated with clopidogrel after an acute myocardial infarction. Clin Pharmacol Ther. 2011;90:561–567. doi: 10.1038/clpt.2011.193. [DOI] [PubMed] [Google Scholar]
- 69.Trenk D, Hochholzer W, Fromm MF, Zolk O, Valina CM, Stratz C, Neumann FJ. Paraoxonase-1 Q192R polymorphism and antiplatelet effects of clopidogrel in patients undergoing elective coronary stent placement. Circ Cardiovasc Genet. 2011;4:429–436. doi: 10.1161/CIRCGENETICS.111.960112. [DOI] [PubMed] [Google Scholar]
- 70.Reny J-L, Combescure C, Daali Y, Fontana P for the PON1 Meta-Analysis Group. Influence of the paraoxonase-1 Q192R genetic variant on clopidogrel responsiveness and recurrent cardiovascular events: a systematic review and meta-analysis. J Thromb Haemost. 2012;10:1242–1251. doi: 10.1111/j.1538-7836.2012.04756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dansette PM, Rosi J, Bertho G, Mansuy D. Paraoxonase-1 and clopidogrel efficacy. Nat Med. 2011;17:1040–1041. doi: 10.1038/nm.2436. [DOI] [PubMed] [Google Scholar]
- 72.Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS, 2nd, Lachno DR, Salazar D, Winters KJ. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5:2429–2436. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 73.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias WL, Braunwald E, Sabatine MS. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119:2553–2560. doi: 10.1161/CIRCULATIONAHA.109.851949. [DOI] [PubMed] [Google Scholar]
- 74.Tantry US, Bliden KP, Wei C, Storey RF, Armstrong M, Butler K, Gurbel PA. First analysis of the relation between CYP2C19 genotype and pharmacodynamics in patients treated with ticagrelor versus clopidogrel: the ONSET/OFFSET and RESPOND genotype studies. Circ Cardiovasc Genet. 2010;3:556–566. doi: 10.1161/CIRCGENETICS.110.958561. [DOI] [PubMed] [Google Scholar]
- 75.Grosdidier C, Quilici J, Loosveld M, Camoin L, Moro PJ, Saut N, Gaborit B, Pankert M, Cohen W, Lambert M, Beguin S, Morange PE, Bonnet JL, Alessi MC, Cuisset T. Effect of CYP2C19*2 and *17 genetic variants on platelet response to clopidogrel and prasugrel maintenance dose and relation to bleeding complications. Am J Cardiol. 2013;111:985–990. doi: 10.1016/j.amjcard.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 76.Cuisset T, Loosveld M, Morange PE, Quilici J, Moro PJ, Saut N, Gaborit B, Castelli C, Beguin S, Grosdidier C, Fourcade L, Bonnet JL, Alessi MC. CYP2C19*2 and *17 alleles have a significant impact on platelet response and bleeding risk in patients treated with prasugrel after acute coronary syndrome. JACC Cardiovasc Interv. 2012;5:1280–1287. doi: 10.1016/j.jcin.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 77.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 78.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA The PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 79.Hamm CW, Bassand J-P, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 80.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC., Jr 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e426–579. doi: 10.1161/CIR.0b013e318212bb8b. [DOI] [PubMed] [Google Scholar]
- 81.Roberts JD, Wells GA, Le May MR, Labinaz M, Glover C, Froeschl M, Dick A, Marquis JF, O'Brien E, Goncalves S, Druce I, Stewart A, Gollob MH, So DY. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet. 2012;379:1705–1711. doi: 10.1016/S0140-6736(12)60161-5. [DOI] [PubMed] [Google Scholar]
- 82.Gladding P, Webster M, Zeng I, Farrell H, Stewart J, Ruygrok P, Ormiston J, El-Jack S, Armstrong G, Kay P, Scott D, Gunes A, Dahl M-L. The pharmacogenetics and pharmacodynamics of clopidogrel response: an analysis from the PRINC (Plavix Response in Coronary Intervention) Trial. JACC Cardiovasc Interv. 2008;1:620–627. doi: 10.1016/j.jcin.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 83.Bonello L, Armero S, Ait Mokhtar O, Mancini J, Aldebert P, Saut N, Bonello N, Barragan P, Arques S, Giacomoni M-P, Bonello-Burignat C, Bartholomei M-N, Dignat-George F, Camoin-Jau L, Paganelli F. Clopidogrel loading dose adjustment according to platelet reactivity monitoring in patients carrying the 2C19*2 loss of function polymorphism. J Am Coll Cardiol. 2010;56:1630–1636. doi: 10.1016/j.jacc.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 84.Collet J-P, Hulot J-S, Anzaha G, Pena A, Chastre T, Caron C, Silvain J, Cayla G, Bellemain-Appaix A, Vignalou J-B, Galier S, Barthélémy O, Beygui F, Gallois V, Montalescot G. High doses of clopidogrel to overcome genetic resistance: the randomized crossover CLOVIS-2 (Clopidogrel and Response Variability Investigation Study 2) JACC Cardiovasc Interv. 2011;4:392–402. doi: 10.1016/j.jcin.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 85.Mega JL, Hochholzer W, Frelinger AL, Kluk MJ, Angiolillo DJ, Kereiakes DJ, Isserman S, Rogers WJ, Ruff CT, Contant C, Pencina MJ, Scirica BM, Longtine JA, Michelson AD, Sabatine MS. Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA. 2011;306:2221–2228. doi: 10.1001/jama.2011.1703. [DOI] [PubMed] [Google Scholar]
- 86.Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey JDE, Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non–ST-elevation myocardial infarction (Updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60:645–681. doi: 10.1016/j.jacc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 87.Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van't Hof A, Widimsky P, Zahger D. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 88.Trenk D, Zolk O, Fromm MF, Neumann FJ, Hochholzer W. Personalizing antiplatelet therapy with clopidogrel. Clin Pharmacol Ther. 2012;92:476–485. doi: 10.1038/clpt.2012.133. [DOI] [PubMed] [Google Scholar]