Abstract
Because of the large variation in the response to psychoactive medication, many studies have attempted to uncover genetic factors that determine response. While considerable knowledge exists on the large effects of genetic polymorphisms on pharmacokinetics and plasma concentrations of drugs, effects of the concentration at the target site and pharmacodynamic effects on brain functions in disease are much less known. This article reviews the role of magnetic resonance imaging (MRI) to visualize response to medication in brain behaviour circuits in vivo in humans and assess the influence of pharmacogenetic factors. Two types of studies have been used to characterize effects of medication and genetic variation. In task-related activation studies the focus is on changes in the activity of a neural circuit associated with a specific psychological process. The second type of study investigates resting state perfusion. These studies provide an assessment of vascular changes associated with bioavailability of drugs in the brain, but may also assess changes in neural activity after binding of centrally active agents. Task-related pharmacogenetic studies of cognitive function have characterized the effects in the prefrontal cortex of genetic polymorphisms of dopamine receptors (DRD2), metabolic enzymes (COMT) and in the post-synaptic signalling cascade under the administration of dopamine agonists and antagonists. In contrast, pharmacogenetic imaging with resting state perfusion is still in its infancy. However, the quantitative nature of perfusion imaging, its non-invasive character and its repeatability might be crucial assets in visualizing the effects of medication in vivo in man during therapy.
Keywords: neuroleptics, perfusion imaging, pharmacogenetic imaging
Introduction
Notwithstanding the large effects of genetic polymorphisms on the pharmacokinetics of drugs 1, individual response is still poorly characterized 2. For this reason, there is a need for tools that visualize response in brain behaviour circuits in vivo in humans. Over the last 15 years, magnetic resonance imaging methods (MRI) have been used to assess the effects of centrally acting pharmacological agents on brain networks in vivo, giving rise to the kind of studies collectively known as pharmacological MRI (phMRI, 3,4). In parallel with the development of phMRI, imaging methods have also provided biomarkers of genetic variability of relevance for psychiatric disorders 5–7. In this article, we will focus on the potential importance for the field of pharmacogenomics of the simultaneous investigation of pharmacological and genetic variability with phMRI, and its potential contribution in understanding how the genetic makeup of patients may affect their response to pharmacological therapy. Even if they may never rival positron emission tomography (PET, 8) in molecular specificity, MRI techniques offer superior temporal and spatial resolution. Furthermore, they are cost-effective, radiation-free and widely available. These properties are essential for the collection of large samples (as required by clinical research or genetic imaging), and for the applicability of these methods in both preclinical models and in patients. For this reason, they may be of use not only in research on pharmacological effects, but also in future applications in the clinic.
A unifying concept underlying both pharmacological and genetic imaging research is that of ‘intermediate phenotype’ 6,9, corresponding to the notion of ‘endophenotype’ of psychiatric geneticists 10,11. Endophenotypes are state-independent heritable traits, not visible to the unaided eye but measurable with the appropriate instrumentation, that co-segregate with disorders within families 11. In contrast, the term intermediate phenotype is typically used in the context of neuroimaging research, where the role of intermediate phenotypes as biomarkers is not restricted to an association with genotype. Intermediate phenotypes are functional and quantifiable biomarkers of brain activity obtained in vivo with neuroimaging methods that may provide an assessment of brain function that is closer to the biological substrate affected by brain-related disorders, their treatment or genetic variation (Figure 1). The importance of this notion lies in the relative lack of specificity of symptomatic indices of improvement or remission from psychiatric disorders. Furthermore, by mapping these markers to what is known about brain circuits active during healthy functioning, intermediate phenotypes may provide proof-of-concept models of processes affected by therapy or individual differences. A related notion is that of Research Domain Criteria (RdoC). These are well-defined tasks recruiting fundamental processes, whose systematic investigation has been advocated as key to establish valid diagnostic criteria 12.
Figure 1.
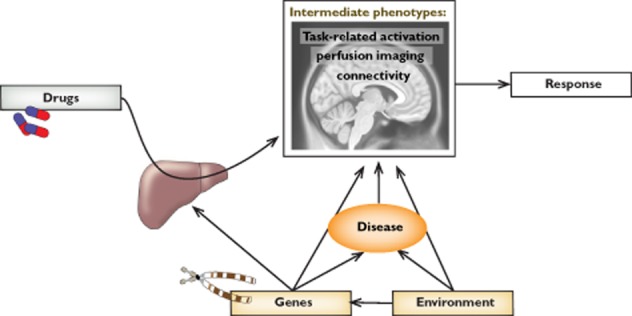
Intermediate phenotypes provided by imaging techniques are biological markers of brain function that may clarify the association between therapy, individual factors, including the genetic makeup, and response. In this article we review mainly the role of task-activated and resting perfusion phenotypes. A third emerging phenotype, connectivity, has just begun to be applied to pharmacological imaging studies in man
A typology of imaging studies of pharmacological and genetic effects
Task-related activation studies
Pharmacological and genetic imaging studies belong into two broad categories, assessing intermediate phenotypes with different properties. In the first type of study, participants are asked to perform a task while in the scanner (task-related activation studies). A control condition allows the identification of the brain structures associated with the recruitment of processes required by the task 13. Participants or scan sessions are further randomized to treatment with the active agent or placebo to assess the effect of the drug on the neural correlates of the process elicited by the task 4. For example, this approach successfully demonstrated modulation of the brain response to a painful stimulus under increasing analgesic concentrations 14. An advantage of this approach is the specificity of the brain function circuits assessed by the task, although the real degree of specificity depends on a careful choice of the task and control conditions (for a critical discussion, see 15). Because many such circuits may exist, studies of this type are usually guided by previous knowledge on the process that may be affected by the drug, or on the function of the polymorphic gene. Note, however, that the existence of learning or habituation effects in most tasks 16–18 complicates the longitudinal monitoring of the effects of medication.
The physiology and methodological underpinning of the signal in task-related activation studies has been extensively investigated 19. This signal is based on the blood oxygenation level dependent (BOLD) haemodynamic response 20,21 and on the tight coupling between brain metabolism and perfusion 22 (here we use the term ‘coupling’ to refer specifically to the mechanism through which a change in brain function is translated into an MRI signal). The increased glucose consumption accompanying neural activity brings about a vascular adjustment response, which increases blood oxygenation in the involved areas with a latency of 2–3 s. The MRI signal is generated by the different magnetic properties of haemoglobin at different oxidation levels 23. Task-related differences in metabolism or perfusion may also be detected with PET techniques or arterial spin labelling (ASL, 24) that measure the changes of these physiological parameters directly.
Resting state studies
A second approach historically preceded task-related activation studies and was common at the time when PET and related techniques were the only available neuroimaging probe. In this approach, an index of brain function such as metabolism or blood perfusion at rest provides the intermediate phenotype to associate with the effects of the pharmacological agent or to predict response 25. A possible disadvantage here is the lack of specificity of brain metabolism or perfusion at rest, compared with the former approach. In the absence of compelling hypotheses on the function affected by the drug or the genetic polymorphism, however, this may be an asset. In contrast, task-related activation studies are restricted to testing the modulation of the drug on one neural substrate among many, and one that was specified a priori. Furthermore, the signal from metabolism or perfusion at rest is stable over time, making it suitable to longitudinal investigations 26. More recently, the MRI technique of ASL 24 has been used as an alternative to PET for the assessment of brain perfusion at rest (Figure 2). While as yet less used than task-related phMRI, the coming of age of ASL may greatly expand the type of information obtainable with neuroimaging in pharmacogenetic research 27,28.
Figure 2.
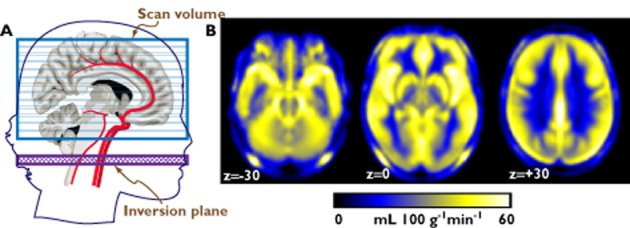
(A) Schematic illustration of the ASL technique. An inversion pulse inverts the spins of the water molecules in the blood in the neck, disrupting the signal from these molecules when they are sampled after reaching the brain. The cerebral blood flow (CBF) is estimated from the difference between two images, one taken with and one taken without the inversion pulse. (B) Regional CBF maps created from the ASL signal, averaged from about 300 hundred individuals. The maps represent transversal slices taken at the level of the midbrain, the thalamus and basal ganglia, and the cerebral hemispheres immediately above the corpus callosum (the coordinates are in Montreal Neurological Institute space). The figure shows that cortical and subcortical grey matter and regions rich in vessels are brighter than white matter and ventricular spaces, reflecting higher regional perfusion values
Studies of brain metabolism or perfusion at rest have shown that psychoactive substances affect the brain according to different regional patterns. Neuroleptics, for example, bring about an increase of metabolism and perfusion in the basal ganglia, and a variable degree of decrease in the cortex, especially in the frontal lobes (see 29,30 for a review of early work, and 31–33 for more recent ASL studies). These differences may be due to the very different receptor profiles of neuroleptics accompanying dopaminergic antagonism 33,34. Likewise, comparative studies of brain perfusion at rest of antidepressants with different profiles show different perfusion patterns 35.
While all task activation studies are based on the well-understood BOLD response neurovascular coupling, several mechanisms may be responsible for the rest perfusion changes associated with the administration of an active agent (Table 1). An unfavourable setting is when the mechanism is a direct action on vascular regulation. For example, a serotonomimetic compound may bind to vascular receptors and alter perfusion directly, masquerading as, or obscuring, a neural effect. However, the potential relevance of the effect on vascular tone and its informative value is dramatically different if it is serotonin itself which binds to vascular receptors. Then the study may assess the extracellular availability of the neurotransmitter, for example after transporter blockade 36, or the activation of intrinsic neurovascular regulatory circuits 37, thus providing an indirect assessment of drug activity in the brain. Most neurotransmitters have an effect on the regulation of cerebrovascular tone 37, so that the haemodynamic effect of the drug on vascular regulation through changes in the bioavailability of neurotransmitters 38 replaces the BOLD haemodynamic response as the coupling between stimulation (by the drug) and the signal (perfusion changes) 39. Validation of this concept comes from studies in laboratory animals showing the dependency of rest perfusion haemodynamic response on lesion of key receptor systems 36,39–41. In a third possibility, centrally acting compounds may alter neural activity, thus leading to changes detectable through the BOLD haemodynamic response coupling. For example, in an ASL rest perfusion study, changes in the brainstem under paroxetine and bupropion were observed that were consistent with neurophysiological changes in the firing rates observed in laboratory animals 35. A final interpretive strategy takes into account the broad range of receptor affinities of many drugs in clinical use. Because the effects on these receptors might involve any of the previous couplings, sometimes simultaneously, one may view changes in rest perfusion as multi-determined signatures or fingerprints of the receptor profiles of these drugs. An example of the issues tackled by this interpretive model arises in rest perfusion studies of neuroleptics, where the comparison of cortical decrements involves the discussion of notions of atypicality and the associated receptor profiles 32,33,42,43.
Table 1.
Neurovascular couplings leading to signal changes in rest perfusion MRI
| Mechanism | Interpretive model | Representative reference |
|---|---|---|
| Direct binding of drug to vascular receptor | Confound, since rarely of interest | Caffeine 60,105 |
| Direct binding of transmitter to vascular receptor | Assessment of central transmitter availability or vascular effector activation; assessment of central drug activity | Dopaminergic agents and vascular modulation 36,40; changes in dopamine function after nicotine withdrawal 106 |
| Changes in activity of target cell detected through hemodynamic response | Functional changes across networks | Brainstem nuclei after transporter blockade 35 |
| Essentially unknown, or a mixture of the above | Detection of signatures or fingerprints of receptor profiles | Cortical modulation of metabolism/perfusion by neuroleptics 42 |
Connectivity studies
Recently, a third type of study has emerged based on the estimation of correlations between seed voxels and the rest of the brain (‘intrinsic connectivity’, 44), which may be due to vasomotor activity reflecting structural or functional associations between brain areas 45. These correlation maps are obtained by regressing the signal in each voxel separately on the signal of a predefined voxel (‘seed’) in data collected with standard BOLD-sensitive techniques. These analyses show that variations of the BOLD signal, not due to changes in activity elicited by experimental tasks, tend to correlate with variations in other specific areas of the brain. These correlation patterns are stable, being qualitatively unchanged at rest and during the execution of a task 46. Several studies have shown modulation of strength of connectivity by pharmacological agents 47–54 and by genetic variation 55,56. However, little is known about the nature of the physiological coupling between pharmacological or genetic variability on the one hand and signal changes in intrinsic connectivity on the other. Because they are usually collected at rest, intrinsic connectivity studies are sometimes assimilated to rest studies. However, no association has been found between mean levels of perfusion at rest and connectivity patterns elicited by common seeds 57. It therefore appears that intrinsic connectivity and rest activity levels provide information about distinct properties of the brain. This result, however, like others in this field, may be dependent on the seeds chosen to elicit the connectivity maps. Key studies in laboratory animals are starting to investigate the physiological bases of connectivity changes caused by pharmacological agents 58, showing that connectivity analyses may profit from the use of specific seeds located in the subcortical centres most directly affected by the drug.
Methodological issues in interpreting imaging studies of drug effects and genetic polymorphisms
Several issues may compromise the valid interpretation of results in genetic or pharmacological MRI studies, but affect task-related and baseline perfusion studies differently. For the inference in task-related activation studies to be valid, the medication or the genetic polymorphism should not alter the BOLD haemodynamic response mechanism. Furthermore, effects detected in task-related phMRI studies may be caused by the effects of drugs on the vascular tree or its regulation, rather than by changes in the functional activity of neurons 59. Two well-known cases are caffeine 60–62 and indomethacin 63. These effects are readily detectable with rest perfusion studies 27, where, as noted above, they constitute a powerful instrument to assess drug activity at target sites 39.
Several observations suggest that results of task-related phMRI are not all due to confounding effects of this nature. In a study measuring both electrophysiological and functional imaging effects, these two measures provided comparable assessments of the cortical response to stimulation 64. Medication-induced changes were present in both measurements, indicating that changes in the functional imaging signal reflected changes in the response of cortical neurons, not in the BOLD haemodynamic response mechanism through which neural activity is detected. Another indirect line of evidence for the validity of task-related phMRI is given by the sparseness of task-related activation effects, in contrast to vascular effects that may be expected to be distributed across large regions. The same argument may be applied to regionally identifiable effects in rest perfusion phMRI. For example, rest perfusion under serotonin transporter inhibitors is characterized not only by diffuse cortical decrements that are consistent with the vascular activity of serotonin 65, but also by regionally specific effects 35,66.
A limitation of the BOLD approach in task-related activation studies lies in the non-quantitative character of detected signal changes. Hence, it is impossible to tell if changes in signal amplitudes associated with the task are due to changes in the activation or in baseline levels. Several laboratories have demonstrated that reduction in baseline perfusion levels is associated with higher activation responses 67–69. This difficulty is overcome by quantitative approaches such as ASL that can provide baseline measurements in task-related studies. However, ASL currently suffers from lower signal : noise ratio and slow acquisition times, which makes it unsuitable for the detection of fast activity changes of some task-related studies 70.
Pharmacogenetic MRI studies
Because of differences in the intermediate phenotype they expose through different couplings, task-related activation phMRI and rest perfusion phMRI have different applicability. The former can identify a brain behavioural circuit that may be affected by neuro-psychiatric disorders, and therefore provide an index of change related to a cognitive or emotional process affected by pathology or therapy. The latter is closer to the physiological substrate of centrally active agents, and may be more appropriately used to assess their biological activity. This broadly suggests the suitability of task-related activation phMRI and rest perfusion phMRI for the investigation of individual variation in pharmacodynamic and pharmacokinetic factors, respectively.
Pharmacogenetic MRI studies with focus on pharmacodynamics
Reflecting the state of research in phMRI, most pharmacogenetic studies conducted to date have adopted a task-related approach, and targeted the neural substrates of specific processes affected by medication and pharmacogenetic polymorphisms to identify the pharmacodynamic mechanisms underlying individual variation in response (Table 2). Most efforts have been directed to study the effects of dopaminergic agonists and antagonists on intermediate phenotypes exposed by cognitive function. In contrast, pharmacogenetic imaging studies of affect and antidepressants are in the early stages 71.
Table 2.
Task-related phMRI studies with pharmacogenetic implications
| Intermediate phenotype/gene | Drug | Main findings | Ref. |
|---|---|---|---|
| Working memory/COMT | Amphetamine | Performance improvement and modulation of working memory network (PFC) in carriers of the high activity allele (see text) | 72 |
| Working memory + declarative memory/COMT | Tolcapone | Performance improvement and modulation of working memory network (PFC) in carriers of the high activity allele | 107 |
| Working memory/DRD2(C957T) | Nicotine | Polymorphism modulates changes in ventral associative areas | 108 |
| Working memory/COMT | Nicotine abstinence | Smokers with the high activity allele were more sensitive to withdrawal and had larger effects in working memory network (PFC) | 109 |
| Reward/DRD2(TaqIA) | Bromocriptine | Increased ventral striatum reward prediction signal in carriers of the low density D2 receptor allele | 110 |
| Reversal learning/DRD2(TaqIA) | Cabergoline | Increased striatal signal in carriers of the low density D2 receptor allele | 111 |
| Working memory/COMT | Olanzapine | Response in carriers of the high activity allele, and modulation of attentional networks (see text) | 78 |
| Working memory/DRD2(rs1076560), AKT1, GSK-3β | Olanzapine | Epistatic interactions associated with response and attentional networks (see text) | 84 |
| Working memory connectivity/AKT1, COMT, DRD2(rs1076560) | Neuroleptics | Epistatic interactions associated with response and cortical-subcortical connectivity (see text) | 85 |
| Declarative memory/AKT1, COMT, BDNF | Lithium/valproate addition to neuroleptics | Epistatic interaction associated with response and structural brain changes | 104 |
| Perception emotional stimuli/CNR1 | Antidepressants | Association with response paralleled by subcortical signal changes | 112 |
D2, dopamine D2 receptor; PFC, prefrontal cortex.
The study by Mattay et al. 72 was among the first to demonstrate the genetic modulation of the response to a centrally active drug on a task-related intermediate phenotype with phMRI. The cortical activation was assessed in participants carrying out a working memory task and receiving amphetamine, a dopamine agonist that increases alertness and modulates attention, and is member of a class of drugs used in the clinic to treat attention deficit hyperactivity disorder 73. Mattay and colleagues hypothesized that individual differences in the response to amphetamine reflected differences in baseline dopamine tone 74,75, associated with prefrontal cortex function 76. They therefore tested the modulation of the effect of amphetamine by the val158-met COMT polymorphism, the drug metabolizing enzyme (DME) that removes endogenous dopamine, on the prefrontal brain circuit known to be associated with working memory function 77. Homozygous carriers of the high activity COMT allele were characterized by improved reaction times and a decrease of the prefrontal BOLD signal in the working memory task under amphetamine, consistently with a compensation of a lower basal dopamine tone. In contrast, these improvements were not observed in homozygous carriers of the low activity COMT allele, whose performance degraded at high levels of task difficulty. The authors concluded that these findings accounted for an increased risk of adverse response to amphetamine in the low activity COMT allele carriers 72.
Following this seminal work, several innovative studies have explored the genetic modulation of the dopamine system under neuroleptic medication. Bertolino et al. assessed the working memory intermediate phenotype to locate the mechanism through which the same COMT polymorphism may affect response to olanzapine in a sample of schizophrenic patients 78. They reported that response to treatment was limited to patient carriers of the high activity allele, and a corresponding modulation of the signal elicited by the working memory task in the prefrontal and parietal attentional network. The authors concluded that the effects of olanzapine on working memory capacity interact with its response profile. The same group extended these results with an in-depth investigation of post-synaptic dopamine D2 receptor transmission, involving polymorphisms of the dopamine D2 receptor (DRD2, 79), and of AKT1, a kinase in the signalling pathways of post-synaptic D2 80 and in the growth factor-induced cell survival in the developing nervous system 81. Polymorphism in these genes was previously associated with differences in cognitive performance 82,83. They found that interactions between polymorphisms in these two genes were associated with response to olanzapine treatment in schizophrenic patients. In both polymorphisms as in the previous COMT study, the alleles associated with response were those leading to reduced dopaminergic function. They also reported a modulation of the signal in the medial prefrontal cortex by the interaction of these two polymorphisms in an attentional task 84. These results were confirmed by subsequent work by Tan et al. 85, where a sophisticated connectivity analysis traced the effect of AKT1 polymorphism on dopaminergic function in the efficiency of the interactions between prefrontal cortex and striatum in a working memory task. In schizophrenic patients receiving neuroleptic treatment, they replicated the association of D2 receptor and AKT1 polymorphism with response in the form of a dose-response effect on cognitive change.
Imaging studies with focus on pharmacokinetics
In contrast with pharmacogenetic studies targeting pharmacodynamic mechanisms, applications of MRI to study genetic variation in pharmacokinetics belong to the future. An issue affecting response is the concentration of a drug at the target site, which may be only loosely related to plasma concentrations due to the existence of anatomical and functional structures such as the blood–brain barrier. Transporters at the blood brain barrier such as the organic cation transporters (OCTs, 86) display functional polymorphisms that affect the distribution of the drug at the target site and therapy response 87,88. Imaging methods are valuable here because they provide a means to quantify changes in function in vivo. One approach exploits the vasoactive coupling of rest perfusion phMRI to assess drug activity indirectly, provided that the active compound does not itself alter vascular tone 40. An alternative, a few phMRI studies have been conducted to estimate the pharmacokinetic curve of the drug in the brain 14,89–91. Beyond MRI approaches, PET may be used to assess receptor occupancy 92 or drug distribution at the target tissue, provided that radiolabelling does not alter its pharmacokinetic properties 93. This listing shows that there are several potential approaches that may be exploited to target the possible effects of genetic polymorphisms on the pharmacokinetics of drugs at their target.
Another important issue for response concerns the activity of DMEs in the brain, a consequence of the common existence of endogenous substrates of DMEs 94. Psychotropic DMEs, especially those in the cytochrome P450 group (CYP), are expressed not only in the liver, but also in the brain 95,96, where they may contribute to the local drug metabolism and the local biochemical homeostasis. Many endogenous neurotransmitters and neurohormones are metabolized by CYPs 97, possibly explaining their reported roles in neurodevelopment 98, neuroprotection 99 and behavioural affective traits 100. In man, variation in brain levels of CYPs among individuals, either through genetics 101 or regulation 97, may contribute not only to differences in drug response 96,101, but may also explain the reported associations between genetic polymorphisms of CYPs and cognitive function, personality and vulnerability to mental disorders 96. The importance of these associations lies not only in their intrinsic value in explaining individual variability in vulnerability to affective disorders and risk for drug-induced neurotoxicity, but also to the fact that many drugs commonly used in the treatment of mental disorders are either metabolized by these enzymes and/or actively inhibit these enzymes in the brain. A comprehensive account of the effects of DME polymorphism on drug effects may therefore be more complex than the one provided by the observation window on plasma concentrations, crossing the distinction between pharmacokinetics and pharmacodynamics.
Two imaging studies have provided evidence on the modulation of brain function by CYP2D6 polymorphism. The first study provided evidence of differences in rest perfusion levels in the brain, primarily affecting the thalamus and the posterior cortical regions 102. The effect of CYP2D6 polymorphism in the same region was found also in a second study, which used task-related activation in a cognitive and emotional processing task 103. As would be expected from the influence of baseline levels on task-related activation, task-related activation was larger in the individuals whose genetic make-up was associated in the previous study with lower baseline perfusion.
Conclusion
The joint investigation of genetic and pharmacological variates in a MRI design is still rare, requiring large samples for genetic analysis and sophisticated experimental manipulations for pharmacological treatment. As the recent pharmacogenetic studies on the effects of neuroleptics demonstrate 84,85,104, extremely valuable information may be obtained by combining analysis of samples of patients obtained in a relatively naturalistic design with larger samples of healthy participants, genetic databases 85 and biobanks for the validation of genetic polymorphisms and investigation of genetic expression 84.
A conceptual attraction of pharmacogenetic MRI is the triangulation of outcome measures, genetics and intermediate phenotypes, which provides a link with the neurobiological mechanism mediating the effects of medication and genetic polymorphisms on response. Furthermore, since the methods would be available in the clinic, discoveries in research programmes of this kind may be translated to the clinic without the need of additional infrastructure.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work and no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years. RV received consultancy fees from the BfArM, Bonn. All other authors declare no other relationships or activities that could appear to have influenced the submitted work. This paper was prepared without recourse to extramural funding sources.
References
- 1.Kirchheiner J, Nickchen K, Bauer M, Wong M-L, Licinio J, Roots I, Brockmöller J. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9:442–473. doi: 10.1038/sj.mp.4001494. [DOI] [PubMed] [Google Scholar]
- 2.Kirchheiner J, Seeringer A, Viviani R. Pharmacogenetics in psychiatry. A useful clinical tool or wishful thinking for the future? Curr Pharm Des. 2010;16:136–144. doi: 10.2174/138161210790112728. [DOI] [PubMed] [Google Scholar]
- 3.Leslie RA, James MF. Pharmacological magnetic resonance imaging: a new application for functional MRI. Trends Pharmacol Sci. 2000;21:314–318. doi: 10.1016/s0165-6147(00)01507-8. [DOI] [PubMed] [Google Scholar]
- 4.Honey GD, Bullmore E. Human pharmacological MRI. Trends Pharmacol Sci. 2004;25:366–374. doi: 10.1016/j.tips.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin functional and corticolimbic affective processing. Biol Psychiatry. 2006;59:888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nature Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 7.Siebner HR, Callicott JH, Sommer T, Mattay VS. From the genome to the phenome and back: linking genes with human brain function and structure using genetically informed neuroimaging. Neurosci. 2009;164:1–6. doi: 10.1016/j.neuroscience.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sokoloff L, Reivich M, Kennedy C, Des Rosier MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure and normal values in the conscious and anaesthetised albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 9.Matthews PM, Honey GD, Bullmore ET. Applications of fMRI in translational medicine and clinical practice. Nature Rev Neurosci. 2006;7:732–744. doi: 10.1038/nrn1929. [DOI] [PubMed] [Google Scholar]
- 10.Glahn DC, Thompson PM, Blangero J. Neuroimaging endophenotypes: strategies for finding genes influencing brain structure and function. Hum Br Mapping. 2007;28:488–501. doi: 10.1002/hbm.20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottesmann II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 12.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 13.Posner MI, Raichle ME. Images of the Mind. New York: Freeman; 1994. [Google Scholar]
- 14.Wise RG, Rogers R, Painter D, Bantick S, Ploghaus A, Williams P, Rapeport G, Tracey I. Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentalin. NeuroImage. 2002;16:999–1014. doi: 10.1006/nimg.2002.1146. [DOI] [PubMed] [Google Scholar]
- 15.Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends Cogn Sci. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 17.Raichle ME, Fiez JA, Videen TO, MacLeod AMK, Pardo JV, Fox PT, Petersen SE. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 18.Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Logothetis MK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Phil Trans R Soc B. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng HM, Brady TJ, Rosen BR. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokoloff L. Local cerebral energy metabolism: its relationship to local functional activity and blood flow. 1978;56 Cerebral Vascular Smooth Muscle and its Control; vol., Ciba Foundation Symposium. [PubMed] [Google Scholar]
- 23.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin-inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89:212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JJ, Aguirre GK, Kimberg DY, Roc AC, Li L, Detre JA. Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med. 2003;49:796–802. doi: 10.1002/mrm.10437. [DOI] [PubMed] [Google Scholar]
- 27.Wang DJJ, Chen Y, Fernández-Sara MA, Detre JA. Potential and challenges for arterial spin labelling in pharmacological magnetic resonance imaging. J Pharmacol Exp Ther. 2011;337:359–366. doi: 10.1124/jpet.110.172577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wise RG, Preston C. What is the value of human FMRI in CNS drug development? Drug Discov Today. 2010;15:973–980. doi: 10.1016/j.drudis.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Holcomb HH, Cascella NG, Thaker GK, Medoff DR, Dannals RF, Tamminga CA. Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol. Am J Psychiatry. 1996;153:41–49. doi: 10.1176/ajp.153.1.41. [DOI] [PubMed] [Google Scholar]
- 30.Miller DD, Rezai K, Alliger R, Andreasen NC. The effect of antipsychotic medication on relative cerebral blood perfusion in schizophrenia: assessment with Technetium-99m Hexamethyl-Propyleneamine Oxime single photon emission computer tomography. Biol Psychiatry. 1997;41:550–559. doi: 10.1016/s0006-3223(96)00110-2. [DOI] [PubMed] [Google Scholar]
- 31.Fernández-Seara MA, Aznárez-Sanado M, Mengual E, Irigoyen J, Heukamp F, Pastor MA. Effects on resting state cerebral blood flow and functional connectivity induced by metoclopramide: a perfusion MRI study in healthy volunteers. Br J Pharmacol. 2011;163:1639–1652. doi: 10.1111/j.1476-5381.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handley R, Zelaya FO, Reinders AATS, Reis Marques T, Mehta MA, O'Gorman R, Alsop DC, Taylor H, Johnston A, Williams S, McGuire P, Pariante CM, Kapur S, Dazzan P. Acute effects of single-dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Hum Br Mapping. 2013;34:272–282. doi: 10.1002/hbm.21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viviani R, Graf H, Wiegers M, Abler B. Effects of amisulpride on human resting cerebral perfusion. Psychopharmacol (Berl) 2013 doi: 10.1007/s00213-013-3091-z. doi: 10.1007/s00213-013-3091-z. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 34.Miller DD, Andreasen NC, O'Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Comparison of the effects of risperidone and haloperidol on regional cerebral blood flow in schizophrenia. Biol Psychiatry. 2001;49:704–715. doi: 10.1016/s0006-3223(00)01001-5. [DOI] [PubMed] [Google Scholar]
- 35.Viviani R, Abler B, Seeringer A, Stingl JC. Effect of paroxetine and bupropion on human resting brain perfusion: an arterial spin labelling study. NeuroImage. 2012;61:773–779. doi: 10.1016/j.neuroimage.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. NeuroImage. 2006;30:700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 37.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz AJ, Zocchi A, Reese T, Gozzi A, Garzotti M, Varnier G, Curcuruto O, Sartori I, Girlanda E, Biscaro B, Crestan V, Bertani S, Heidbreder C, Bifone A. Concurrent pharmacological MRI and in situ microdialysis of cocaine reveal a complex relationship between the central hemodynamic response and local dopamine concentration. NeuroImage. 2004;23:296–304. doi: 10.1016/j.neuroimage.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins BG. Pharmacologic magnetic resonance imaging (phMRI): imaging drug action in the brain. NeuroImage. 2012;62:1072–1085. doi: 10.1016/j.neuroimage.2012.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen YCI, Galpern WR, Brownell AL, Matthews RT, Bogdanov M, Isacson O, Keltner JR, Beal MF, Rosen BR, Jenkins BG. Detection of dopaminergic neurotransmitter activity using pharmacologic MRI: correlation with PET, microdialysis, and behavioral data. Magn Reson Med. 1997;38:389–398. doi: 10.1002/mrm.1910380306. [DOI] [PubMed] [Google Scholar]
- 41.Chen YC, Choi JK, Andersen SL, Rosen BR, Jenkins BG. Mapping dopamine D2/D3 receptor function using pharmacological magnetic resonance imaging. Psychopharmacol (Berl) 2005;180:705–715. doi: 10.1007/s00213-004-2034-0. [DOI] [PubMed] [Google Scholar]
- 42.Miller DD, Andreasen NC, O'Leary DS, Rezai K, Watkins L, Boles Ponto LL, Hichwa RD. Effect of antipsychotics on regional cerebral blood flow measured with positron emission tomography. Neuropsychopharmacology. 1997;17:230–240. doi: 10.1016/S0893-133X(97)00042-0. [DOI] [PubMed] [Google Scholar]
- 43.Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol Psychiatry. 2003;53:601–608. doi: 10.1016/s0006-3223(02)01602-5. [DOI] [PubMed] [Google Scholar]
- 44.Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SARB, Rypma B, Schlaggar BL, Schmidt S, Seidler RS, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biswal BB, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 46.Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 47.Kiviniemi V, Jauhiainen J, Tervonen O, Pääkkö E, Oikarinen J, Vainionpää V, Ranrala H, Biswal B. Slow vasomotor fluctuation in the fMRI of the anesthetized child brain. Magn Reson Med. 2000;44:378–383. doi: 10.1002/1522-2594(200009)44:3<373::aid-mrm5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 48.Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP, McMahon K. L-Dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci. 2009;29:7364–7378. doi: 10.1523/JNEUROSCI.0810-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li SJ, Biswal B, Li Z, Risinger R, Rainey C, Cho JK, Salmeron BJ, Stein EA. Cocaine adminstration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med. 2000;43:45–51. doi: 10.1002/(sici)1522-2594(200001)43:1<45::aid-mrm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 50.Greicius MD, Kiviniemi V, Tervonen O, Vainionpää V, Alahuta S, Reiss AL, Menon V. Persistent default-mode network connectivity during light sedation. Hum Br Mapping. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. NeuroImage. 2010;52:590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- 52.McCabe C, Mishor Z. Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. NeuroImage. 2011;57:1317–1323. doi: 10.1016/j.neuroimage.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stamatakis EA, Adapa RM, Absalom AR, Menon DK. Changes in resting neural connectivity during propofol sedation. PLoS ONE. 2011;5:e14224. doi: 10.1371/journal.pone.0014224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sambataro F, Blasi G, Fazio L, Caforio G, Taurisano P, Romano R, Di Giorgio A, Gelao B, Lo Bianco L, Papazacharias A, Popolizio T, Nardini M, Bertolino A. Treatment with olanzapine is associated with modulation of the default mode network in patients with schizophrenia. Neuropsychopharmacology. 2010;35:904–912. doi: 10.1038/npp.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu B, Song M, Li J, Liu Y, Li K, Yu C, Jiang T. Prefrontal-related functional connectivities with the default network are modulated by COMT val158met in healthy young adults. J Neurosci. 2010;30:64–69. doi: 10.1523/JNEUROSCI.3941-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tost H, Bilek E, Meyer-Lindenberg A. Brain connectivity in psychiatric imaging genetics. NeuroImage. 2012;62:2250–2260. doi: 10.1016/j.neuroimage.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 57.Viviani R, Messina I, Walter M. Resting state functional connectivity in perfusion imaging: correlation maps with BOLD connectivity and resting state perfusion. PLoS ONE. 2011;6:e27050. doi: 10.1371/journal.pone.0027050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bifone A, Gozzi A, Schwarz AJ. Functional connectivity in the rat brain: a complex network approach. Magn Res Imaging. 2010;28:1200–1209. doi: 10.1016/j.mri.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 59.Kuschinsky W, Suda S, Bünger R, Yaffe S, Sokoloff L. The effects of intravenous norepinephrine on the local coupling between glucose utilization and blood flow in the rat brain. Pflugers Arch. 1983;398:134–138. doi: 10.1007/BF00581061. [DOI] [PubMed] [Google Scholar]
- 60.Mulderinck TA, Gitelman DR, Marsel Mesulam M, Parrish TB. On the use of caffeine as a contrast booster for BOLD fMRI studies. NeuroImage. 2002;15:37–44. doi: 10.1006/nimg.2001.0973. [DOI] [PubMed] [Google Scholar]
- 61.Addicott MA, Yang LL, Peiffer AM, Burnett LR, Burdette JH, Chen MY, Hyasaka S, Kraft RA, Maldjian JA, Laurienti PI. The effect of daily caffeine use on cerebral blood flow: how much caffeine can we tolerate? Hum Brain Mapp. 2009;30:3102–3114. doi: 10.1002/hbm.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Y, Parrish TB. Caffeine dose effect on activation-induced BOLD and CBF responses. NeuroImage. 2009;46:577–583. doi: 10.1016/j.neuroimage.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawrence K, Ye FQ, Lewise BK, Frank JA, McLaughlin AC. Measuring the effects of indomethacin on changes in cerebral oxidative metabolism and cerebral blood flow during sensorimotor activation. Magn Reson Med. 2003;50:99–106. doi: 10.1002/mrm.10502. [DOI] [PubMed] [Google Scholar]
- 64.Arthurs PJ, Stephenson CME, Rice K, Lupson VC, Spiegelhalter DJ, Boniface SJ, Bullmore ET. Dopaminergic effects on electrophysiological and functional MRI measures of human cortical stimulus-response power laws. NeuroImage. 2004;21:540–546. doi: 10.1016/j.neuroimage.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 65.Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog Neurobiol. 1996;50:335–362. doi: 10.1016/s0301-0082(96)00033-0. [DOI] [PubMed] [Google Scholar]
- 66.Chen Y, Wan HI, O'Reardon JP, Wang DJJ, Wang Z, Korczykowski M, Detre JA. Quantification of cerebral blood flow as biomarker of drug effect: arterial spin labeling phMRI after a single dose of oral citalopram. Clin Pharmacol Ther. 2011;89:251–258. doi: 10.1038/clpt.2010.296. [DOI] [PubMed] [Google Scholar]
- 67.Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J Cereb Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Hyder F, Rothman DL, Shulman RG. Total neuroenergetics support localized brain activity: implications for the interpretation of fMRI. Proc Natl Acad Sci U S A. 2002;99:10771–10776. doi: 10.1073/pnas.132272299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li A, Gong L, Xu F. Brain-state-independent neural representation of peripheral stimulation in rat olfactory bulb. Proc Natl Acad Sci U S A. 2011;108:5087–5092. doi: 10.1073/pnas.1013814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aguirre GK, Detre JA, Zarahn E, Alsop DC. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. NeuroImage. 2002;15:488–500. doi: 10.1006/nimg.2001.0990. [DOI] [PubMed] [Google Scholar]
- 71.Rabl U, Scharinger C, Müller M, Pezawas L. Imaging genetics: implications for research on variable antidepressant drug response. Expert Rev Clin Pharmacol. 2010;3:471–489. doi: 10.1586/ecp.10.35. [DOI] [PubMed] [Google Scholar]
- 72.Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41(Suppl. 2):26S–49S. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- 74.Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mattay VS, Callicott JH, Bertolino A, Heaton I, Frank JA, Coppola R, Berman KF, Goldberg TE, Weinberger DR. Effects of dextroamphetamine on cognitive performance and cortical activation. NeuroImage. 2000;12:268–275. doi: 10.1006/nimg.2000.0610. [DOI] [PubMed] [Google Scholar]
- 76.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Owen AM, McMillan K, Laird AR, Bullmore E. N-Back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bertolino A, Caforio G, Blasi G, De Candia M, Latorre V, Petruzzella V, Altamura M, Nappi G, Papa S, Callicott JH, Mattay VS, Bellomo A, Scarabino T, Weinberger DR, Nardini M. Interaction of COMT val108/158 met genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004;161:1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Lee MLT, Xiao T, Papp A, Wang D, Sadée W. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci U S A. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry. 2010;167:388–396. doi: 10.1176/appi.ajp.2009.08121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 82.Bertolino A, Fazio L, Caforio G, Blasi G, Rampino A, Romano R, Di Giorgio A, Taurisano P, Papp A, Pinsonneault J, Wang D, Nardini M, Popolizio T, Sadée W. Functional variants of the dopamine receptor D2 gene modulate profronto-striatal phenotypes in schizophrenia. Brain. 2009;132:417–425. doi: 10.1093/brain/awn248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tan HY, Nicodemus KK, Chen Q, Li Z, Brooke JK, Honea R, Kolachana BS, Straub RE, Meyer-Lindenberg A, Sei Y, Mattay VS, Callicott JH, Weinberger DR. Genetic variation in AKT1 is linked to dopamine-associated prefrontal cortical structure and function in humans. J Clin Invest. 2008;118:2200–2208. doi: 10.1172/JCI34725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blasi G, Napolitano F, Ursini G, Tausrisano P, Romano R, Caforio G, Fazio L, Gelao B, Di Giorgio A, Iacovelli L, Sinibaldi L, Popolizio T, Usiello A, Bertolino A. DRD2/AKT1 interaction on D2 c-AMP independent signaling, attentional processing, and response to olanzapine treatment in schizophrenia. Proc Natl Acad Sci U S A. 2011;108:1158–1163. doi: 10.1073/pnas.1013535108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tan HY, Chen AG, Kolachana B, Apud JA, Mattay VS, Callicott JH, Chen Q, Weinberger DR. Effective connectivity of AKT1-mediated dopaminergic working memory networks and pharmacogenetics of anti-dopaminergic treatment. Brain. 2012;135:1436–1445. doi: 10.1093/brain/aws068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, functions, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 87.Haenisch B, Drescher E, Thiemer L, Xin H, Giros B, Gautron S, Bönisch H. Interaction of antidepressant and antipsychotic drugs with the human organic cation transporters hOCT1, hOCT2 and hOCT3. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:1017–1023. doi: 10.1007/s00210-012-0781-8. [DOI] [PubMed] [Google Scholar]
- 88.Bacq A, Balasse L, Biala G, Guiard B, Gardier AM, Schinkel A, Louis F, Vialou V, Martres MP, Chevarin C, Hamon M, Giros B, Gautron S. Organic cation transporter 2 controls brain norepinephrine and serotonin clearance and antidepressant response. Mol Psychiatry. 2012;17:926–939. doi: 10.1038/mp.2011.87. [DOI] [PubMed] [Google Scholar]
- 89.Bloom AS, Hoffmann RG, Fuller SA, Pankiewicz J, Harsch HH, Stein EA. Determination of drug-induced changes in functional MRI signal using a pharmacokinetic model. Hum Brain Mapp. 1999;8:235–244. doi: 10.1002/(SICI)1097-0193(1999)8:4<235::AID-HBM7>3.0.CO;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nordin LE, Li TQ, Brogren J, Johansson P, Sjögren N, Hannesdottir K, Björk C, Segerdahl M, Wang DJJ, Julin P. Cortical responses to amphetamine exposure by pCASL MRI and pharmacokinetic/pharmacodynamic dose modeling. NeuroImage. 2013;68:75–82. doi: 10.1016/j.neuroimage.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 91.Schouw MLJ, Kaag AM, Caan MWA, Hejtel DFR, Majoie CBLM, Nederveen AJ, Booij J, Reneman L. Mapping the hemodynamic response in human subjects to a dopaminergic challenge with dextroamphetamine using ASL-based pharmacological MRI. NeuroImage. 2013;72:1–9. doi: 10.1016/j.neuroimage.2012.12.056. [DOI] [PubMed] [Google Scholar]
- 92.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–520. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 93.Matthews PM, Rabiner I, Gunn R. Non-invasive imaging in experimental medicine for drug development. Curr Opin Pharmacol. 2011;11:5101–5107. doi: 10.1016/j.coph.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 94.Nebert DW. Polymorphisms in drug-metabolizing enzymes: what is their clinical relevance and why do they exist? Am J Med Hum Genet. 1997;60:265–271. [PMC free article] [PubMed] [Google Scholar]
- 95.Ferguson CS, Tyndale RF. Cytochrome P450 enzymes in the brain: emerging evidence for biological significance. Trends Pharmacol Sci. 2011;32:798–14. doi: 10.1016/j.tips.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stingl JC, Brockmöller J, Viviani R. Genetic variability of drug-metabolizing enzymes: the dual impact on psychiatric therapy and regulation of brain function. Mol Psychiatry. 2013;18:273–287. doi: 10.1038/mp.2012.42. [DOI] [PubMed] [Google Scholar]
- 97.Miksys S, Tyndale RF. Cytochrome P450-mediated drug metabolism in the brain. J Psychiatry Neurosci. 2012;37:120–133. doi: 10.1503/jpn.120133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Persson A, Sim SC, Virding S, Onishchenko N, Schulte G, Ingelman-Sundberg M. Decreased hippocampal volume and increased anxiety in a transgenic mouse model expressing the human CYP2C19 gene. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mann A, Tyndale RF. Cytochrome P450 2D6 enzyme neuroprotects against 1-methyl-4-phenylpyridinium toxicity in SH-SY5Y neuronal cells. Mol Dev Neurosci. 2010;31:1185–1193. doi: 10.1111/j.1460-9568.2010.07142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sim SC, Nordin L, Andersson TML, Virding S, Olsson M, Pedersen NL, Ingelman-Sundberg M. Association between CYP2C19 polymorphism and depressive symptoms. Am J Med Genet (Neuropsychiatr Genet) 2010;153B:1160–1166. doi: 10.1002/ajmg.b.31081. [DOI] [PubMed] [Google Scholar]
- 101.Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. Influence of cytochrome P450 polymorphism on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther. 2007;116:496–526. doi: 10.1016/j.pharmthera.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 102.Kirchheiner J, Seeringer A, Godoy AL, Maier C, Beschoner P, Sim EJ, Viviani R. CYP2D6 in the brain: genotype effects on resting brain perfusion. Mol Psychiatry. 2011;16:333–341. doi: 10.1038/mp.2010.42. [DOI] [PubMed] [Google Scholar]
- 103.Stingl JC, Esslinger C, Tost H, Bilek E, Kirsch P, Ohmle B, Viviani R, Walter H, Rietschel M, Meyer-Lindenberg A. Genetic variation in CYP2D6 impacts neural activation during cognitive tasks in humans. NeuroImage. 2011;59:2818–2823. doi: 10.1016/j.neuroimage.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 104.Tan HY, Chen AG, Chen Q, Browne LB, Verchinski B, Kolachana B, Zhang F, Apud J, Callicott JH, Mattay VS, Weinberger DR. Epistatic interactions of AKT1 on human medial temporal lobe biology and pharmacogenetic implications. Mol Psychiatry. 2012;17:1007–1016. doi: 10.1038/mp.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hahn T, Heinzel S, Plichta MM, Reif A, Lesch KP, Fallgatter AJ. Neurovascular coupling in the human visual cortex is modulated by cyclooxygenase-1 (COX-1) gene variant. Cereb Cortex. 2011;21:1659–1666. doi: 10.1093/cercor/bhq236. [DOI] [PubMed] [Google Scholar]
- 106.Wang Z, Ray R, Faith M, Tang K, Wileyto EP, Detre JA, Lerman C. Nicotine abstinence-induced cerebral blood flow changes by genotype. Neurosci Lett. 2008;438:272–280. doi: 10.1016/j.neulet.2008.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, Alce G, Iudicello JE, Akbar N, Egan MF, Goldberg TE, Weinberger DR. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology. 2007;32:1011–1020. doi: 10.1038/sj.npp.1301227. [DOI] [PubMed] [Google Scholar]
- 108.Jacobsen LK, Pugh KR, Mencl WE, Gelernter J. C957T polymorphism of the dopamine D2 receptor gene modulates the effect of nicotine on working memory performance and cortical processing efficiency. Psychopharmacol (Berl) 2006;188:530–540. doi: 10.1007/s00213-006-0469-1. [DOI] [PubMed] [Google Scholar]
- 109.Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, Ruparel K, Ray R, Gur RC, Lerman C. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Mol Psychiatry. 2009;14:820–826. doi: 10.1038/mp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kirsch P, Reuter M, Mier D, Lonsdorf T, Stark R, Gallhofer B, Vaitl D, Hennig J. Imaging gene-substance interactions: the effect of the DRD2 TaqlA polymorphism and the dopamine agonist bromocriptine on the brain activation during the anticipation of reward. Neurosci Lett. 2006;405:196–201. doi: 10.1016/j.neulet.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 111.Cohen MX, Krohn-Grimberghe A, Elger CE, Weber B. Dopamine gene predicts the brain's response to dopaminergic drug. Eur J Neurosci. 2007;26:3652–3660. doi: 10.1111/j.1460-9568.2007.05947.x. [DOI] [PubMed] [Google Scholar]
- 112.Domschke K, Dannlowski U, Ohrmann P, Lawford B, Bauer J, Kugel H, Heindel W, Young R, Morris P, Arolt V, Deckert J, Suslow T, Baune BT. Cannabinoid receptor 1 (CNR1) gene: impact on antidepressant treatment response and emotion processing in major depression. Eur Neuropsychopharmacol. 2008;18:751–759. doi: 10.1016/j.euroneuro.2008.05.003. [DOI] [PubMed] [Google Scholar]


