Abstract
BACKGROUND & AIMS
Interleukin (IL)-28B (interferon-λ 3) genotype is the strongest predictor of response of patients with hepatitis C virus (HCV) infection to antiviral therapy. However, patients with HCV infection often have physical or mental comorbidities that contraindicate or complicate treatment, regardless of their genotype. The potential role of IL28B genotype within the context of patients’ clinical and social environment is therefore unclear.
METHODS
We characterized the IL28B genotype (for rs12980275 and rs8099917) in 308 patients (mean age, 56 y; 25% African American; 38% with advanced-stage fibrosis) with genotype 1 HCV infection seen at the Michael E. DeBakey Veterans Administration Medical Center in Houston, Texas, from May 1, 2009, through April 1, 2012. We evaluated their eligibility for antiviral treatment based on clinical and social factors such as physical or mental health comorbidity, ongoing alcohol or drug use, and noncompliance with treatment evaluation.
RESULTS
Of the 308 subjects, 40% were homozygous for rs12980275 (associated with response to therapy), 46% were heterozygous, and 15% were homozygous for alleles associated with reduced response to therapy. Overall, 36% of patients were considered to be ineligible for treatment; of these, 40% had the rs12980275 genotype. More than half of the patients with rs12980275 who were ineligible for treatment were excluded because of mental health comorbidities; one-third of these patients had advanced fibrosis. The reason(s) for treatment exclusion resolved in only 8% of patients during a mean 1.5 years of follow-up evaluation.
CONCLUSIONS
In a well-characterized cohort of patients with HCV, a large proportion (40%) with IL28B polymorphisms associated with response to therapy is ineligible for treatment because of contraindications. One potential role of IL28B genotype analysis could be to identify patients who, although not currently eligible for antiviral treatment, could become so by modifying fixable exclusions to treatment.
Keywords: Interferon Lambda 3, Genetics, Liver Disease, SNP
Chronic infection with hepatitis C virus (HCV) is a common, progressive, yet treatable condition.1,2 Recent data have shown that host genetic factors may influence response to HCV therapy. Four genome-wide association studies have identified genetic variation in the interleukin-28B (IL28B) gene coding for interferon-λ3 on chromosome 19 as the strongest predictor of treatment-induced sustained virologic response (SVR) among patients with HCV genotype 1.3–6
The use of IL28B testing in clinical practice is unclear. Although the predictive value of IL28B genotype for SVR in persons treated with direct acting antiviral (DAA) agents7,8 is lower than that for pegylated interferon and ribavirin alone,9 recent data have shown that the IL28B genotype remains one of the most important predictors of SVR with sofosbuvir-based triple therapy for HCV genotype 1 patients.10 IL28B also may remain relevant to some interferon-free DAA regimens in patients with HCV genotype 1 infection.11 Thus, IL28B genotyping might be used to identify candidates for shorter treatment durations with the current DAAs or perhaps those who will continue to need interferon as part of their treatment regimen with in-the-pipeline DAAs.9 These recommendations on using IL28B testing pertain to patients for whom the decision to start antiviral treatment already has been made.
The true promise of IL28B genotyping in the DAAs era indeed may lie in its application at a more proximal yet essential step—that is, at the time when the decision to start antiviral treatment is being weighed. The decision to start antiviral treatment often is difficult for both patients and their clinicians.12 Antiviral treatment is lengthy, expensive, and has significant side effects, with only a relatively modest chance of success in several patient groups.13 The effectiveness of antiviral treatment is compromised further because many patients have one or more conditions that contraindicate treatment in the first place.14–16 Co-existing mental and physical comorbidities are the leading barriers to starting and maintaining antiviral treatment in the United States.14–16 Although some of these contraindications may be modifiable or potentially reversible, most of these patients are excluded from treatment in routine practice.17 The issues limiting the use of HCV treatment have not changed in the DAA era—physical and mental comorbidity continues to contraindicate or complicate use of DAAs.18
Understanding the potential role an IL28B genetic test plays in clinical practice within the context of patients’ clinical and social environment is important in estimating the translational impact of this exciting genomic discovery. Most of the studies describing the association between IL28B and treatment outcomes were clinical trials or included cohorts outside the United States with limited applicability to diverse populations of HCV-infected patients in clinical practices in the United States.3–6 Previous studies also were limited solely to patients who started, and thus were eligible for, treatment, adding further uncertainty to the test’s clinical utility.
In this study, we examined the prevalence of IL28 alleles in patients with HCV, determined how the IL28B allele frequency (genotype) overlapped with other patient characteristics (phenotypes) that determine treatment eligibility, and estimated the relative size of these genotype–phenotype subgroups.
Materials and Methods
Study Population
We prospectively recruited consecutive veterans with a confirmed chronic hepatitis C (HCV) diagnosis seen at the Michael E. DeBakey VA Medical Center in Houston, Texas, HCV clinics between May 1, 2009, and April 1, 2012. Veterans were eligible for inclusion in our current study if they met the following criteria: (1) they were between ages 18 and 70 years at recruitment; (2) had HCV viremia and were negative for both human immunodeficiency virus and hepatitis B virus surface antigen; (3) were not currently receiving antiviral therapy; and (4) had IL28B genotype data (see later). The study population and procedures have been described in detail previously.19
Data Collection and Study Measures
Interleukin 28B polymorphisms
For this study, we extracted genomic DNA from whole blood using the Puregene DNA kit (Qiagen, Alameda, CA). We genotyped 48 samples using the Illumina HumanOmni 1.0 DNA analysis BeadChip kit and 296 samples using the Illumina HumanOmni2.5-8v1 DNA Analysis BeadChip kit (Illumina, San Diego, CA). Genotypes were autocalled using the Illumina Genome Studio software. The following single-nucleotide polymorphisms (SNPs) around the IL28B gene were genotyped on these chips: rs12980275, rs8099917, rs12972991, rs8109886, and rs4803223. Although we did not genotype rs12979860 (which is 1 of the 2 SNPs strongly associated with SVR in the genome-wide association study and in other studies),3–6 data show that rs12979860 is in very strong linkage disequilibrium (R2 > 0.8) with the SNP rs12980275.3 We selected rs12980275 (as a surrogate for rs12979860) and rs8099917 for this study. Genotypes were defined as AA, AG, and GG for rs12980275, where A is the major and favorable allele; and TT, GT, and GG for rs8099917, where T is the major and favorable allele.
Serologic measures
Fasting blood was analyzed by the Michael E. DeBakey VA Medical Center’s Central Laboratory Service for HCV antibodies using immunometric immunoassay (Ortho Clinical Diagnostics, Raritan, NJ), HCV genotype using the InnoLiPA HCV II (Innogenetics, Ghent, Belgium), and quantitative viral load using the COBAS TaqMan HCV Test (Roche, Basel, Switzerland).
FibroSURE-ActiTest
We estimated the degree of hepatic fibrosis and inflammation using the FibroSURE-ActiTest (LabCorp, Burlington, NC). The FibroSURE-ActiTest has been validated against hepatic biopsy in multiple study populations including in HCV-positive populations.20,21 It provides METAVIR biopsy-based equivalent hepatic fibrosis (F0, no fibrosis present; to F4, cirrhosis) and inflammatory activity (A0, no inflammatory activity; to A3, severe inflammatory activity).
Survey
Trained research assistants administered a computerized sociodemographic survey that interrogated self-reported race/ethnicity.
Electronic medical record review
HCV clinicians used a structured template in the electronic medical records to systematically record physical (coronary artery disease, congestive heart failure, end-stage renal disease, chronic obstructive pulmonary disease, malignancy, poorly controlled diabetes, decompensated cirrhosis, other uncontrolled medical problems) and mental comorbidities (current street drug or alcohol use, depression, other psychiatric diagnoses), as well as other barriers (such as noncompliance with treatment evaluation, homelessness, patient refusal) to antiviral treatment. To ensure complete capture of comorbidity data for our study, a trained abstractor (D.L.) re-reviewed all records between patients’ date of enrollment and their last clinical follow-up evaluation or June 2012, whichever came first. For patients rendered treatment-ineligible (for the earlier-mentioned reasons), we also determined if these conditions improved during the follow-up evaluation. Last, we recorded the receipt and date of antiviral treatment for all study subjects.
Statistical Analyses
We restricted all analyses to patients with HCV genotype 1 infection because the relevance of IL28B genotype in patients with non-1 HCV genotype infection is unclear. We determined the proportions of patients who were treatment eligible, ineligible because of physical health comorbidity, ineligible because of mental health comorbidity, and ineligible for other reasons. We then determined the frequency of rs12980275 and rs8099917 polymorphisms in the entire patient cohort and separately for both African American and Caucasian patients. For each SNP, we classified patients according to the observed IL28B genotype (favorable homozygous [AA and TT], heterozygous [AG and TG], and unfavorable homozygous [GG and GG] genotype for rs12980275 and rs8099917, respectively). Genetic analyses were performed using SNP and Variation Suite 7.0 (Golden Helix Inc, Bozeman, Montana), including quality assurance and the Hardy–Weinberg equilibrium test for each SNP.
We determined the proportions of patients who were treatment eligible, ineligible because of physical health comorbidity, ineligible because of mental health comorbidity, and ineligible for other reasons, stratified by their IL28B genotype in the entire cohort of patients and separately for both African American and Caucasian patients. All analyses were conducted using SPSS version 18 (SPSS Inc, Chicago, IL).
Results
Patient Characteristics
We determined the genotype distribution of rs12980275 and rs8099917 in 396 patients with HCV. Of these, 308 patients had HCV genotype-1 infection and constituted the cohort for this study. The average age of these patients was 56.1 years (standard deviation, 5.5 y) and all were male and of either African American (25%) or non-Hispanic white (75%) race/ethnicity (Table 1). Approximately 70% had HCV genotype 1a and 38% had advanced fibrosis (F3/F4) or cirrhosis.
Table 1.
Baseline Characteristics of 308 Patients With Chronic HCV Genotype 1 Infection
| Characteristic | Distribution |
|---|---|
| Demographics | |
| Mean age, y (SD) | 56.1 (5.5) |
| Race, % | |
| Caucasian | 75.0 |
| African American | 25.0 |
| Clinical characteristics, % | |
| FibroSURE-ActiTest | |
| F0–F3 | 61.6 |
| F3/F4–F4 | 38.4 |
| Necroinflammatory | |
| A0–A2 | 67.4 |
| A2/3–A3 | 32.6 |
| HCV genotype | |
| 1 (unspecified) | 3.6 |
| 1a | 70.1 |
| 1b | 26.3 |
| Overall treatment ineligible, % | 36.0 |
| Reasons for treatment ineligibility, % | |
| Physical comorbidity | 19.5 |
| Coronary artery disease | 2.6 |
| Other cardiac conditions | 0.3 |
| COPD | 3.2 |
| ESRD | 0.0 |
| Cancer | 4.5 |
| Decompensated cirrhosis | 4.5 |
| Othera | 9.7 |
| Mental comorbidity | 19.2 |
| Current drug/alcohol use | 13.0 |
| Depression | 10.1 |
| Other psychiatric diagnosesb | 0.6 |
| Other barriers | 8.4 |
| Noncompliance with evaluation | 1.9 |
| Homelessness | 0.3 |
| Noninterest in treatment | 3.6 |
| Failed previous treatment | 2.6 |
| Eventually treated, % | 11.0 |
COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease.
Included were anemia, awaiting hip surgery, cerebrovascular disease, colitis, diabetes, glaucoma, hyperthyroidism, hypothyroidism, neutropenia, obesity, pancytopenia, peripheral vascular disease, retinal vein occlusion, seizures, suspicious mass, suspicious lesions, thrombosis, and thrombocytopenia.
Included memory issues.
Approximately 36% of patients were deemed ineligible for antiviral treatment by their HCV clinicians. The reasons for treatment ineligibility were split almost equally between physical comorbidity and mental health comorbidity; 19% of patients had 1 or more physical comorbidities documented in their chart as a reason for treatment exclusion and a similar proportion had significant mental comorbidity. Table 1 lists the explicit reasons listed in the electronic medical records for ineligibility. We also found a variety of other conditions documented in patient charts as reasons for treatment exclusions. These included noncompliance with treatment (1.9%), noninterest in treatment (3.6%), and previous nonresponse to pegylated interferon and ribavirin treatment (2.6%). Approximately 11% of patients eventually were treated after a mean of 1.5 years of follow-up evaluation.
Genotype Distribution of IL28B Polymorphism
Table 2 displays the distribution of IL28B polymorphisms in our study cohort overall and by race. For rs12980275, 40.4%, 46.0%, and 13.6% of patients had the AA, AG, and GG genotype, respectively, where A represents the favorable allele. The race-specific distribution of rs12980275 was similar to that reported previously.3,22 For the rs8099917, 62.7%, 32.1%, and 5.2% of patients had TT, TG, and TT genotype, respectively, where T is the favorable allele. Although the frequency of favorable, intermediate, and unfavorable alleles in Caucasians was similar to that in the overall sample and previous report,22 a significant proportion (80.6%) of African Americans in our sample had the TT genotype (favorable), with no patients harboring the unfavorable GG alleles.
Table 2.
Genotype Distribution of IL28B Polymorphism in the Study Population of Patients With HCV Genotype 1 Infection
| All patients | Caucasians | African Americans | |
|---|---|---|---|
| Genotype, % | (n = 308) | (n = 231) | (n = 77) |
| rs12980275 | |||
| AA | 40.4 | 42.4 | 28.6 |
| AG | 46.0 | 44.2 | 51.9 |
| GG | 13.6 | 13.4 | 19.5 |
| rs8099917 | |||
| TT | 62.7 | 56.7 | 80.5 |
| TG | 32.1 | 36.4 | 19.5 |
| GG | 5.2 | 6.9 | 0 |
Genotype Distribution of IL28B by Treatment Eligibility
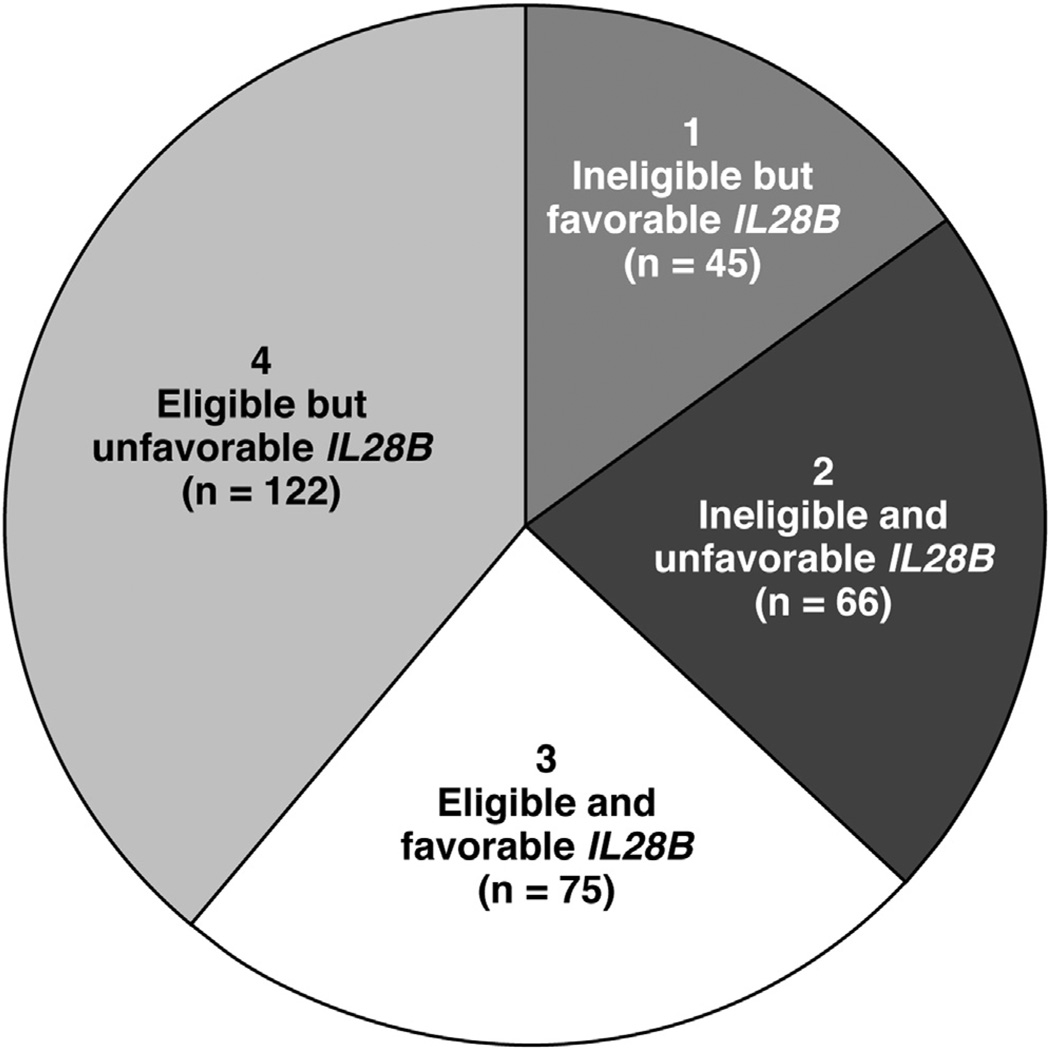
Of 308 patients, 111 patients were considered ineligible for antiviral treatment. Of these 111 treatment-ineligible patients, 45 (40.5%) had the favorable rs12980275 genotype (Figure 1). Of these 45 patients, 30 (66%) were deemed ineligible on the basis of mental health comorbidity (either alone [n = 18] or in combination with physical health [n = 9] or other issues [n = 3]), 12 (26.6%) were considered ineligible on the basis of physical health comorbidity, and 3 (6.6%) had other barriers to treatment. Approximately 45% of these patients had advanced hepatic fibrosis. The reasons that rendered patients ineligible resolved in 8% (n = 9) of patients by the end of the follow-up period (June 2012). These results did not change much when we used rs8099917 to define patients’ genotype.
Figure 1.
Distribution of IL28B genotype (rs12980275) by treatment eligibility. Segments 1 and 2 represent subgroups of patients considered ineligible for antiviral treatment. Of these, approximately 40% had a favorable IL28B genotype (segment 1). One fourth of the patients had a favorable genotype and also was eligible for treatment (segment 3). However, a significant proportion of patients without any major treatment exclusion had an unfavorable IL28B genotype (segment 4).
Approximately 21.4% of patients were treatment-ineligible and had an unfavorable IL28B genotype. In contrast, 24.0% of patients (with genotype 1 HCV) were eligible for treatment and also had a favorable IL28B genotype (Figure 1); 40% of these patients had advanced hepatic fibrosis and 82% had viral load greater than 600,000 IU/mL. However, a significant proportion of patients (39.6%) who were deemed eligible for antiviral treatment had an unfavorable IL28B genotype.
The proportion of patients considered treatment-ineligible on the basis of mental comorbidity was higher in patients with favorable rs12980275 genotype than those with less favorable genotypes (Table 3). There was no difference in the proportion of patients considered treatment-ineligible on the basis of physical comorbidities or other barrier to treatment in patients with favorable, intermediate, and unfavorable rs12980275 genotype.
Table 3.
Reasons for Treatment Ineligibility and Treatment Rates in Patients With HCV Genotype 1 Stratified by rs12980275 Genotype Status
| Distribution of genotype | ||||
|---|---|---|---|---|
| Characteristic | Favorable AA | Intermediate AG | Unfavorable GG | P value |
| Reasons for treatment ineligibility, % | ||||
| Physical comorbidity | ||||
| Coronary artery disease | 3.3 | 1.4 | 4.3 | .448 |
| Other cardiac conditions | 0.0 | 0.0 | 2.2 | .057 |
| COPD | 3.3 | 2.8 | 4.3 | .876 |
| ESRD | 0.0 | 0.0 | 0.0 | NA |
| Cancer | 3.3 | 5.6 | 4.3 | .671 |
| Decompensated cirrhosis | 5.0 | 3.5 | 6.5 | .665 |
| Othera | 11.7 | 7.7 | 10.9 | .545 |
| Mental comorbidity | ||||
| Current drug/alcohol use | 16.7 | 12.7 | 4.3 | .106 |
| Depression | 15.0 | 9.2 | 0.0 | .014 |
| Other psychiatric diagnosesb | 1.7 | 0.0 | 0.0 | .207 |
| Other barriers | 7.5 | 9.2 | 8.7 | .889 |
| Noncompliance with evaluation | 2.5 | 1.4 | 2.2 | .811 |
| Homelessness | 0.8 | 0.0 | 0.0 | .456 |
| Noninterest in treatment | 1.7 | 4.2 | 6.5 | .272 |
| Failed previous treatment | 2.5 | 3.5 | 0.0 | .425 |
| Overall treatment ineligible, % | 37.5 | 35.9 | 32.6 | .841 |
| Antiviral treatment, % | 10.0 | 12.0 | 10.9 | .878 |
COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease.
Included were anemia, awaiting hip surgery, cerebrovascular disease, colitis, diabetes, glaucoma, hyperthyroidism, hypothyroidism, neutropenia, obesity, pancytopenia, peripheral vascular disease, retinal vein occlusion, seizures, suspicious mass, suspicious lesions, thrombosis, and thrombocytopenia.
Included memory issues.
There was also no difference in the rate of antiviral treatment across patient genotypes in the overall sample and in Caucasians (Table 3 and Supplementary Table 1). However, none of the 21 African American patients with a favorable genotype received treatment during the observation period (Supplementary Table 1).
Discussion
We characterized the candidacy and suitability for antiviral treatment in a large cohort of patients with HCV seen in general HCV clinics on the basis of IL28B genotype and other nongenetic factors. We found that, similar to previous studies, approximately 40% of Caucasian patients seen in the general HCV clinics were homozygous for the favorable allele for rs12980275 SNP—and thus had a reasonable to high chance of response to antiviral treatment—approximately 44% were heterozygous, and 13% were homozygous for the unfavorable alleles. In contrast, the frequency of unfavorable alleles was higher in African Americans, with approximately 20% being homozygous for unfavorable alleles. The frequency of rs8099917 SNP in our Caucasian patients was fairly similar to a previous report.22 However, we found that most of our African American patients had the favorable TT genotype for rs8099917, with only 20% being heterozygous. The frequency of rs8099917 polymorphisms has not been studied thoroughly in the general population of African American patients with HCV in the United States.
We found that 40% of HCV patients who were thought to be ineligible for treatment indeed had a fair chance of responding to such treatment based on their IL28B genotype. This finding is particularly important because more than half of these patients were excluded based on conditions that were potentially modifiable. These included, for example, depression, drug and alcohol use, and noninterest in treatment. Approximately a third of patients with favorable IL28B genotype and treatment exclusions also had evidence of advanced fibrosis and/or cirrhosis—a group in whom risks of delaying therapy may far outweigh any potential benefits associated with such delay. Genotype testing therefore can identify the subset who, although they may not be currently treatment-eligible, might become so by modifying fixable exclusions to treatment—data that may have major implications for quality improvement in HCV. Indeed, it is plausible that knowledge of favorable genotype might in itself have a motivating effect on providers and patients to correct potentially modifiable contraindications.
Second, a possible implication of our finding relates to the potential role of pegylated interferon and ribavirin alone in treating patients with HCV. Data show that among patients who are homozygous for the favorable IL28B alleles, the rates of SVR are high (~ 80%) regardless of whether they receive a DAA as part of their treatment regimen. Our data show that this therapeutic equipoise may occur in at least approximately 40% of patients with HCV. The choice of treatment for this group of patients ideally should be guided by factors over and beyond the chance of SVR. One consideration may be a shorter course of treatment with DAA-based therapy (than with pegylated interferon and ribavirin alone) in this group with a genotypic advantage. However, this potential benefit likely will be offset by new challenges of DAAs, such as greater risk of viral resistance and requirement of strict adherence to the treatment regimen. Comorbidity may continue to contraindicate the use of the new agents in some patients. The new treatment also will be substantially more expensive with the estimates for drug-related annual costs starting in the hundreds of millions of dollars to treat all HCV-infected patients. As the menu of treatment options for HCV grows over the next few years, IL28B (and other genomic markers) likely will play a key role in personalizing our approach to treatment for patients with HCV.23 Knowledge of patients’ favorable IL28B genotype can guide both clinicians and their patients to mutually discuss and elicit outcome expectancies, risks, and benefits associated with the available options, and subsequent preferences for treatment. This shared decision-making approach may be a way to help stem the increasing costs associated with new DAAs.
Third, our data may be used to foretell potential community effectiveness of the available DAAs. A significant proportion (40%) is unlikely to receive these treatments on the basis of physical and mental comorbidity. Of the remaining patients, more than half (39.6% of the total) have the unfavorable IL28B allele, further attenuating DAA effectiveness (ie, up to 75% of patients were either ineligible or had an unfavorable genotype). Patients’ genotype is a fixed, uncorrectable variable. Dedicated efforts to improve modifiable comorbidity may be a high-yield target to modify in order to fully benefit from these highly efficacious treatments. For example, we found that co-existing depression with or without substance use disorders was the leading patient-related barrier to antiviral treatment. Depression and substance use are potentially modifiable barriers to antiviral treatment and may continue to play an important role in the era of new (and potentially interferon-sparing) antiviral treatments. Interferon serves as the backbone for several DAAs in the pipeline and still may be necessary for the more common and hard-to-cure HCV genotype 1a (~ 70% of the patients in our cohort had HCV genotype 1a infection). Co-existing mental health conditions will continue to be a barrier to starting antiviral treatment with these interferon-based treatments. Mental health illness is also a known and modifiable risk factor for poor compliance and medication nonadherence.24,25 Thus, even in patients who are eligible for interferon-sparing treatments, mental health illness—through its effect on retention in care and adherence to treatment—may remain an important predictor of treatment receipt in HCV. Given these considerations, the benefits of any efforts targeted at depression and co-existing mental health comorbidities may extend beyond better mental health to include improved HCV care. By expanding the pool of eligible patients, these programs may lead to more HCV patients receiving and responding to the highly potent antiviral agents.
Our study had several limitations. First, the high-throughput gene chip that we used for our study did not identify the most commonly reported SNP: rs12979860. However, this SNP has been shown to be in high linkage disequilibrium (r2 > 0.8) with the primary SNP rs12980275 identified on our study chip with almost identical favorable and unfavorable allelic frequencies.3,22 Indeed, the first US-based genome-wide association study by Ge et al3 ranked rs12980275 as second on the list of top SNPs associated with SVR (after rs12979860 and higher than rs8099917). A recent meta-analysis of published data also showed that the pooled odds ratio for SVR in patients with favorable (AA) vs unfavorable genotypes at rs12980275 was 3.95 (95% confidence interval, 2.39–6.53)26; this was not much different than the pooled odds ratio across studies that reported on rs12979860 (odds ratio, 3.77; 95% confidence interval, 3.25–4.37)—showing that both SNPs have a similar association with SVR. Second, we focused on conditions that were documented explicitly as reasons that may contraindicate or complicate antiviral treatment. We did not abstract information regarding the full range of patients’ comorbidities for this study. Third, our study may be underpowered to make definitive inferences regarding the prevalence of SNP alleles in African Americans. Nonetheless, this study reports the frequency of IL28B SNPs in general patients with HCV seen within the VA, stratified by race. Given that our data were obtained from a predominantly male veteran population in one VA hospital, some of our findings may not be generalized to women and nonveterans in other health care systems. However, the fact that the frequency of the 2 SNPs that we studied was remarkably similar to those reported from other patient populations renders several findings extendable to general patients with HCV seen in other health care settings.
In conclusion, in this well-characterized HCV cohort, approximately 40% of patients who were ineligible for treatment had a favorable IL28B genotype. Most of these patients were rendered ineligible related to potentially fixable treatment exclusions. Future studies will examine whether knowledge of favorable genotype results in better motivation for providers and patients to correct modifiable contraindications.
Supplementary Material
Acknowledgments
Funding
This material is based on work supported in part by the Texas Medical Center Digestive Disease Core Grant (P30 Center Grant DK56338), a VA Clinical Research and Development Merit Review Award (H-22934, H.E.-S.), and the Houston VA Health Services Research and Development Center of Excellence (HFP90-020).
Abbreviations used in this paper
- DAA
direct acting antiviral
- HCV
hepatitis C virus
- SNP
single-nucleotide polymorphism
- SVR
sustained virologic response
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2013.08.034.
Conflicts of interest
The authors disclose no conflicts.
The opinions and assertions contained herein are the sole views of the authors and are not to be construed as official or as reflecting the views of the Department of Veterans Affairs.
References
- 1.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Ghany MG, Strader DB, Thomas DL, et al. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ge D, Fellay J, Thompson A, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 4.Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon- alpha and ribavirin therapy. Nat Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 6.Rauch A, Kutalik Z, Descombes P, et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338–1345. 1345e1–1345e7. doi: 10.1053/j.gastro.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 7.Chayama K, Hayes CN, Abe H, et al. IL28B but not ITPA polymorphism is predictive of response to pegylated interferon, ribavirin, and telaprevir triple therapy in patients with genotype 1 hepatitis C. J Infect Dis. 2011;204:84–93. doi: 10.1093/infdis/jir210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poordad F, Bronowicki JP, Gordon SC, et al. SPRINT-2 and RESPOND-2 Investigators. Factors that predict response of patients with hepatitis C virus infection to boceprevir. Gastroenterology. 2012;143:608–618. doi: 10.1053/j.gastro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Thompson AJ, McHutchison JG. Will IL28B polymorphism remain relevant in the era of direct-acting antiviral agents for hepatitis C virus? Hepatology. 2012;56:373–381. doi: 10.1002/hep.25792. [DOI] [PubMed] [Google Scholar]
- 10.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 11.Zeuzem S, Soriano V, Asselah T, et al. SVR4 and SVR12 with an interferon-free regimen of BI201335 and BI207127, +/− ribavirin, in treatment-naïve patients with chronic genotype-1 HCV infection: interim results of SOUND-C2. J Hepatology. 2012;56(Suppl 2):S45. [Google Scholar]
- 12.Fraenkel L, McGraw S, Wongcharatrawee S, et al. What do patients consider when making decisions about treatment for hepatitis C? Am J Med. 2005;118:1387–1391. doi: 10.1016/j.amjmed.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 13.Bruno S, Vierling JM, Esteban R, et al. Efficacy and safety of boceprevir plus peginterferon-ribavirin in patients with HCV G1 infection and advanced fibrosis/cirrhosis. J Hepatol. 2013;58:479–487. doi: 10.1016/j.jhep.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Bini EJ, Brau N, Currie S, et al. Prospective multicenter study of eligibility for antiviral therapy among 4084 U.S. veterans with chronic hepatitis C virus infection. Am J Gastroenterol. 2005;100:1772–1779. doi: 10.1111/j.1572-0241.2005.41860.x. [DOI] [PubMed] [Google Scholar]
- 15.Cawthorne CH, Rudat KR, Burton MS, et al. Limited success of HCV antiviral therapy in United States veterans. Am J Gastroenterol. 2002;97:149–155. doi: 10.1111/j.1572-0241.2002.05439.x. [DOI] [PubMed] [Google Scholar]
- 16.Kramer JR, Kanwal F, Richardson P, et al. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol. 2012;56:320–325. doi: 10.1016/j.jhep.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Kanwal F, Hoang T, Kramer J, et al. The performance of process measures in hepatitis C. Am J Gastroenterol. 2012;107:1512–1521. doi: 10.1038/ajg.2012.201. [DOI] [PubMed] [Google Scholar]
- 18.Chen EY, Sinclair SN, Czul F, et al. A small percentage of patients with hepatitis C receive triple therapy with boceprevir or telaprevir. Clin Gastroenterol Hepatol. 2013;11:1014–1020. doi: 10.1016/j.cgh.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 19.White DL, Tavakoli-Tabasi S, Kuzniarek J, et al. Higher serum testosterone is associated with increased risk of advanced hepatitis C-related liver disease in males. Hepatology. 2012;55:759–768. doi: 10.1002/hep.24618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poynard T, Imbert-Bismut F, Munteanu M, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol. 2004;3:8. doi: 10.1186/1476-5926-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halfon P, Munteanu M, Poynard T. FibroTest-ActiTest as a non-invasive marker of liver fibrosis. Gastroenterol Clin Biol. 2008;32(Suppl 1):22–39. doi: 10.1016/S0399-8320(08)73991-5. [DOI] [PubMed] [Google Scholar]
- 22.Fischer J, Böhm S, Scholz M, et al. Combined effects of different interleukin-28B gene variants on the outcome of dual combination therapy in chronic hepatitis C virus type 1 infection. Hepatology. 2012;55:1700–1710. doi: 10.1002/hep.25582. [DOI] [PubMed] [Google Scholar]
- 23.Holmes JA, Desmond PV, Thompson AJ. Does IL28B genotyping still have a role in the era of direct-acting antiviral therapy for chronic hepatitis C infection? J Viral Hepat. 2012;19:677–684. doi: 10.1111/jvh.12003. [DOI] [PubMed] [Google Scholar]
- 24.Singh N, Squier C, Sivek C, et al. Determinants of compliance with antiretroviral therapy in patients with human immunodeficiency virus: prospective assessment with implications for enhancing compliance. AIDS Care. 1996;8:261–269. doi: 10.1080/09540129650125696. [DOI] [PubMed] [Google Scholar]
- 25.Gordillo V, del Amo J, Soriano V, et al. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13:1763–1769. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- 26.Jiménez-Sousa MA, Fernández-Rodríguez A, Guzmán-Fulgencio M, et al. Meta-analysis: implications of interleukin-28B polymorphisms in spontaneous and treatment-related clearance for patients with hepatitis C. BMC Med. 2013;11:6. doi: 10.1186/1741-7015-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.