Abstract
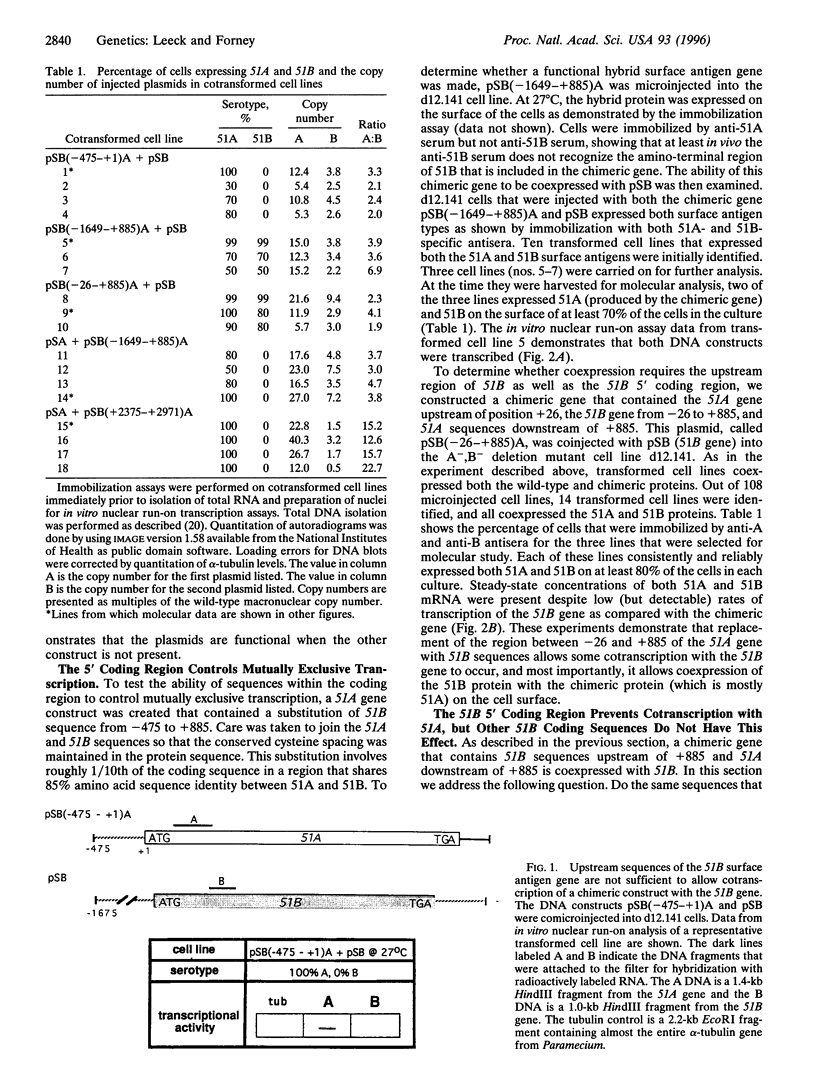
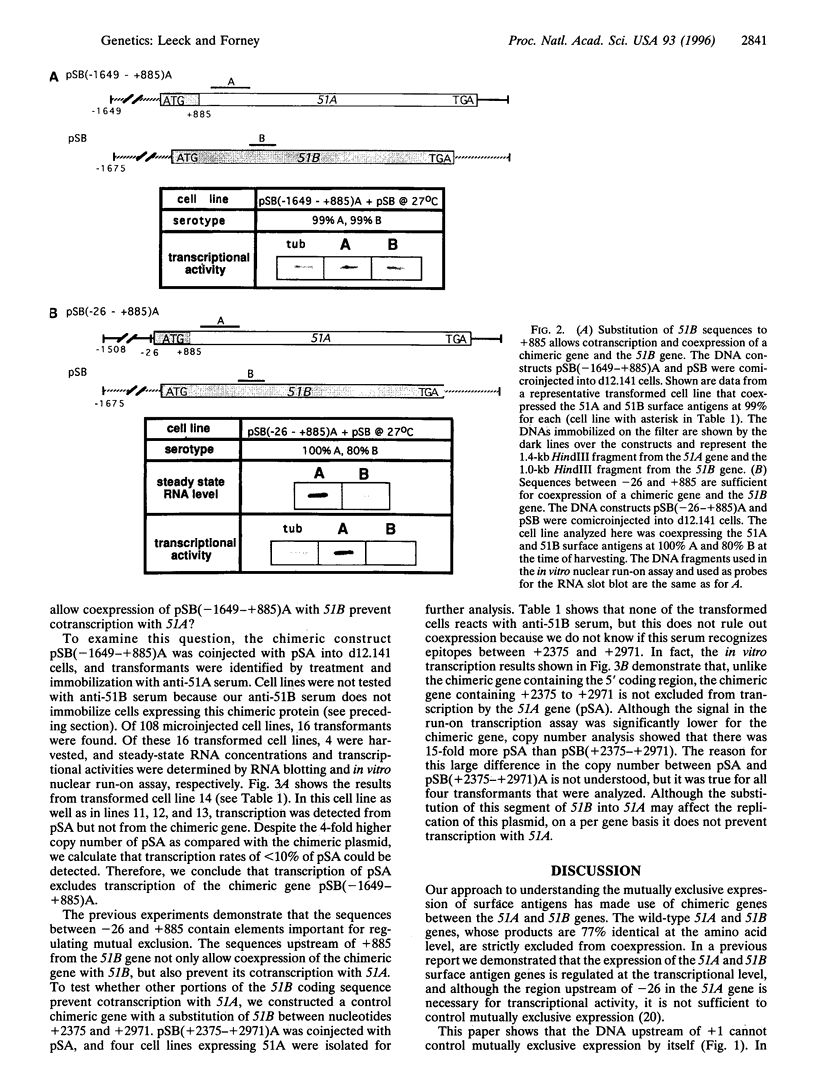
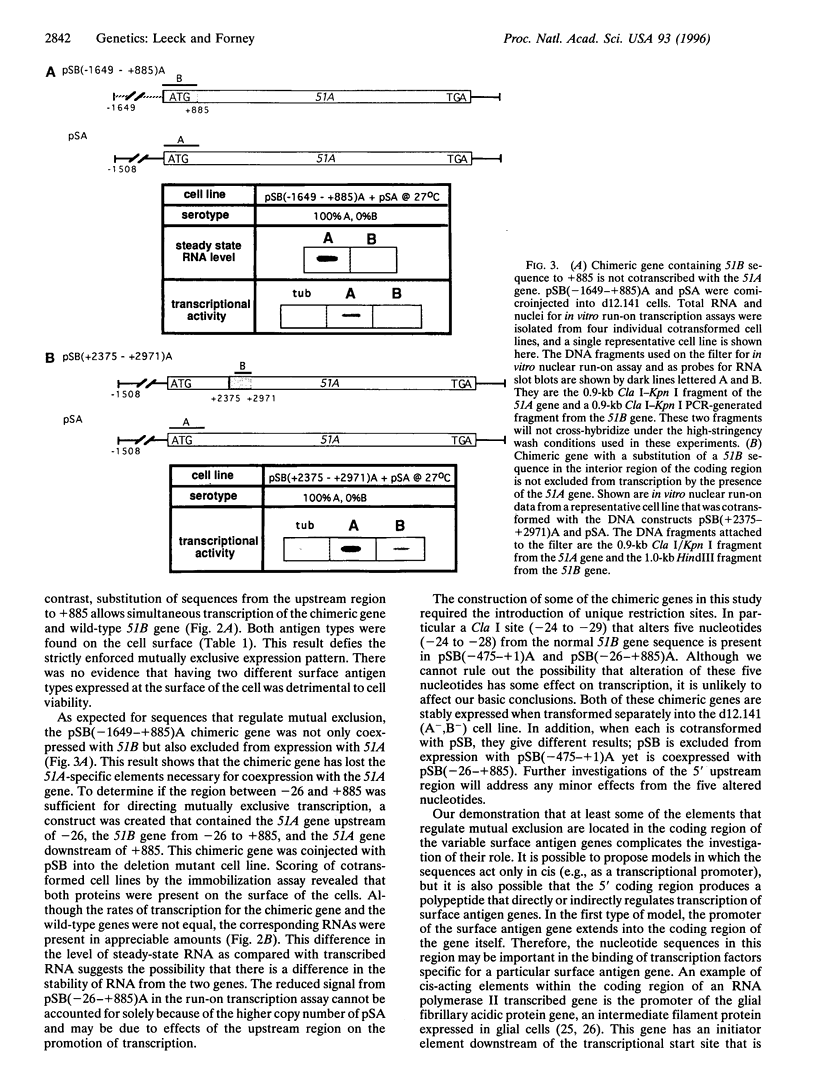
Paramecium tetraurelia stock 51 can express at least 11 different types of surface antigens, yet only a single type is expressed on the surface of an individual cell at any one time. The differential expression of stock 51 type A and B surface antigen genes (51A and 51B) is regulated at the level of transcription. Previously, we reported that nucleotide sequences upstream of position -26 (relative to the start of translation) in the 51A and 51B surface antigen genes are necessary for transcriptional activity but are not sufficient to direct differential transcriptional control. In this report we demonstrate that at least some of the critical elements necessary for differential transcription of the 51A and 51B genes lie within the 5' coding region. A hybrid gene that contains 51B upstream sequences (-475 to +1) attached to the ATG start codon of 51A is not cotranscribed with the 51B gene. In contrast, further substitution with 51B sequences (-1647 to +885) allows the chimeric gene to be coexpressed with 51B. A different hybrid gene containing a substitution of 51B sequence from -26 to +885 in the 51A gene is also coexpressed with 51B, revealing that the critical elements within the coding region of 51B do not require 51B upstream sequences for their effect. Coinjection of the 51A gene with the chimeric gene that contains 51B up to +885 showed that the same sequences that allow coexpression with 51B prevent cotranscription with 51A. Together, these results demonstrate that a region downstream of the transcriptional start site between nucleotide positions +1 and +885 (relative to translational start) is necessary to control differential transcriptional activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroin A., Prat A., Caron F. Telomeric site position heterogeneity in macronuclear DNA of Paramecium primaurelia. Nucleic Acids Res. 1987 Feb 25;15(4):1717–1728. doi: 10.1093/nar/15.4.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdeville Y., Cardoso de Almeida M. L., Deregnaucourt C. The membrane-anchor of Paramecium temperature-specific surface antigens is a glycosylinositol phospholipid. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1219–1225. doi: 10.1016/s0006-291x(87)80200-0. [DOI] [PubMed] [Google Scholar]
- Capdeville Y. Regulation of surface antigen expression in Paramecium primaurelia. II. Role of the surface antigen itself. J Cell Physiol. 1979 Jun;99(3):383–393. doi: 10.1002/jcp.1040990313. [DOI] [PubMed] [Google Scholar]
- Caron F., Meyer E. Molecular basis of surface antigen variation in paramecia. Annu Rev Microbiol. 1989;43:23–42. doi: 10.1146/annurev.mi.43.100189.000323. [DOI] [PubMed] [Google Scholar]
- Forney J. D., Blackburn E. H. Developmentally controlled telomere addition in wild-type and mutant paramecia. Mol Cell Biol. 1988 Jan;8(1):251–258. doi: 10.1128/mcb.8.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney J. D., Epstein L. M., Preer L. B., Rudman B. M., Widmayer D. J., Klein W. H., Preer J. R., Jr Structure and expression of genes for surface proteins in Paramecium. Mol Cell Biol. 1983 Mar;3(3):466–474. doi: 10.1128/mcb.3.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley D., Rudman B. M., Preer J. R., Jr, Polisky B. Multilevel regulation of surface antigen gene expression in Paramecium tetraurelia. Mol Cell Biol. 1990 Apr;10(4):1538–1544. doi: 10.1128/mcb.10.4.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godiska R., Aufderheide K. J., Gilley D., Hendrie P., Fitzwater T., Preer L. B., Polisky B., Preer J. R., Jr Transformation of Paramecium by microinjection of a cloned serotype gene. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7590–7594. doi: 10.1073/pnas.84.21.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godiska R. Structure and sequence of the H surface protein gene of Paramecium and comparison with related genes. Mol Gen Genet. 1987 Jul;208(3):529–536. doi: 10.1007/BF00328151. [DOI] [PubMed] [Google Scholar]
- Gottschling D. E., Aparicio O. M., Billington B. L., Zakian V. A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990 Nov 16;63(4):751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- Laurenson P., Rine J. Silencers, silencing, and heritable transcriptional states. Microbiol Rev. 1992 Dec;56(4):543–560. doi: 10.1128/mr.56.4.543-560.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeck C. L., Forney J. D. The upstream region is required but not sufficient to control mutually exclusive expression of Paramecium surface antigen genes. J Biol Chem. 1994 Dec 9;269(49):31283–31288. [PubMed] [Google Scholar]
- Love H. D., Jr, Allen-Nash A., Zhao Q. A., Bannon G. A. mRNA stability plays a major role in regulating the temperature-specific expression of a Tetrahymena thermophila surface protein. Mol Cell Biol. 1988 Jan;8(1):427–432. doi: 10.1128/mcb.8.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin P. An Exception to Mutual Exclusion of the Ciliary Antigens in Paramecium Aurelia. Genetics. 1956 Sep;41(5):685–699. doi: 10.1093/genetics/41.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L. D., Pollack S., Preer J. R., Jr, Polisky B. DNA sequence requirements for the regulation of immobilization antigen A expression in Paramecium tetraurelia. Dev Genet. 1994;15(5):443–451. doi: 10.1002/dvg.1020150507. [DOI] [PubMed] [Google Scholar]
- Nakatani Y., Brenner M., Freese E. An RNA polymerase II promoter containing sequences upstream and downstream from the RNA startpoint that direct initiation of transcription from the same site. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4289–4293. doi: 10.1073/pnas.87.11.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani Y., Horikoshi M., Brenner M., Yamamoto T., Besnard F., Roeder R. G., Freese E. A downstream initiation element required for efficient TATA box binding and in vitro function of TFIID. Nature. 1990 Nov 1;348(6296):86–88. doi: 10.1038/348086a0. [DOI] [PubMed] [Google Scholar]
- Nielsen E., You Y., Forney J. Cysteine residue periodicity is a conserved structural feature of variable surface proteins from Paramecium tetraurelia. J Mol Biol. 1991 Dec 20;222(4):835–841. doi: 10.1016/0022-2836(91)90573-o. [DOI] [PubMed] [Google Scholar]
- Pays E., Vanhamme L., Berberof M. Genetic controls for the expression of surface antigens in African trypanosomes. Annu Rev Microbiol. 1994;48:25–52. doi: 10.1146/annurev.mi.48.100194.000325. [DOI] [PubMed] [Google Scholar]
- Pays E., Vanhamme L., Berberof M. Genetic controls for the expression of surface antigens in African trypanosomes. Annu Rev Microbiol. 1994;48:25–52. doi: 10.1146/annurev.mi.48.100194.000325. [DOI] [PubMed] [Google Scholar]
- Prat A. Conserved sequences flank variable tandem repeats in two alleles of the G surface protein of Paramecium primaurelia. J Mol Biol. 1990 Feb 5;211(3):521–535. doi: 10.1016/0022-2836(90)90263-L. [DOI] [PubMed] [Google Scholar]
- Prat A., Katinka M., Caron F., Meyer E. Nucleotide sequence of the Paramecium primaurelia G surface protein. A huge protein with a highly periodic structure. J Mol Biol. 1986 May 5;189(1):47–60. doi: 10.1016/0022-2836(86)90380-3. [DOI] [PubMed] [Google Scholar]
- Preer J. R., Preer L. B., Rudman B. M. mRNAs for the immobilization antigens of Paramecium. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6776–6778. doi: 10.1073/pnas.78.11.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudman B., Preer L. B., Polisky B., Preer J. R., Jr Mutants affecting processing of DNA in macronuclear development in paramecium. Genetics. 1991 Sep;129(1):47–56. doi: 10.1093/genetics/129.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J., Leeck C., Forney J. Molecular and genetic analyses of the B type surface protein gene from Paramecium tetraurelia. Genetics. 1993 May;134(1):189–198. doi: 10.1093/genetics/134.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Karpen G. H. Sixty years of mystery. Genetics. 1990 Dec;126(4):779–784. doi: 10.1093/genetics/126.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y., Scott J., Forney J. The role of macronuclear DNA sequences in the permanent rescue of a non-mendelian mutation in Paramecium tetraurelia. Genetics. 1994 Apr;136(4):1319–1324. doi: 10.1093/genetics/136.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]






