Abstract
Tourette syndrome (TS) has a multifactorial etiology, in which genetic, environmental, immunological and hormonal factors interact to establish vulnerability. This review: (i) summarizes research exploring the exposure of TS patients to immune-activating environmental factors, and (ii) focuses on recent findings supporting a role of the innate and adaptive immune systems in the pathogenesis of TS and related disorders. A higher exposure prior to disease onset to group A β-haemolytic streptococcal (GABHS) infections in children with tics and obsessive-compulsive (OC) symptoms has been documented, although their influence upon the course of disease remains uncertain. Increased activation of immune responses in TS is suggested by changes in gene expression profiles of peripheral immune cells, relative frequency of lymphocyte subpopulations, and synthesis of immune effector molecules. Increased activity of cell-mediated mechanisms is suggested by the increased expression of genes controlling natural killer and cytotoxic T cells, increased plasma levels of some pro-inflammatory cytokines which correlate with disease severity, and increased synthesis of antineuronal antibodies. Important methodological differences might account for some inconsistency among results of studies addressing autoantibodies in TS. Finally, a general predisposition to autoimmune responses in TS patients is indicated by the reduced frequency of regulatory T cells, which induce tolerance towards self-antigens. Although the pathogenic role of immune activation in TS has not been definitively proven, a pathophysiological model is proposed to explain the possible effect of immunity upon dopamine transmission regulation and the generation of tics.
Keywords: group A beta haemolytic Streptococcus, tic, tourette syndrome, autoimmunity, antineuronal antibodies
INTRODUCTION
Tourette’s syndrome (TS) is a neurodevelopmental disorder with childhood onset, characterized by the presence of multiple motor and phonic tics lasting more than 1 year, with a tic-free period not longer than 3 months.1 The natural history and high heritability without a unique causative gene of TS and related psychiatric comorbid disorders (mainly, attention deficit hyperactivity disorder [ADHD] and obsessive-compulsive disorder [OCD]) supports a multifactorial etiology. During the course of development, it is likely that genetic, immunological, hormonal and environmental factors interact to establish a neurobiological vulnerability for TS and related disorders.2
Environmental factors resulting in immune activation have been proposed to play a role in a subgroup of patients with TS and pediatric-onset OCD. The possibility of such a link is based in part on the similarity of TS to Sydenham’s Chorea (SC), which occurs after group A β-hemolytic streptococcal (GABHS) infections.3,4 Patients with SC frequently display the sudden onset of anxiety, inattentiveness and obsessive-compulsive (OC) symptoms, and have increased risk of long-term psychiatric problems.5,6 The hypothesis that SC is a pathophysiological model for at least some TS or OCD cases gained favor after reports in the late 1980’s of a resurgence of SC and of sudden outbreaks of tics and OC symptoms temporally linked to GABHS infections.3,4 The prepubertal onset of OCD, TS, or tic disorder with abrupt symptom exacerbation after streptococcal infection has been termed PANDAS (pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection).4 The proposed working definition criteria for PANDAS are: (1) prepubertal onset; (2) Chronic Tic Disorder or OCD; (3) sudden periods of severe exacerbations (“saw tooth”)—relapsing-remitting course; (4) temporal association of onset and one or more exacerbations with clinical evidence of a GABHS infection; and (5) the presence of subtle neurological findings such as reduced fine motor coordination or increased motor hyperactivity (but not overt chorea).4 It is important in reviewing epidemiological studies and case reports to note that these stringent criteria are not always used. In particular, criterion number five can be problematic because subtle choreiform movements are quite common in children aged 3–12 years.7 On the other hand, SC patients may also develop tics occasionally.6 Furthermore, the distinction between chorea and tics may sometimes be difficult, posing a differential diagnostic issue between SC and PANDAS. Both chorea and tics consist in rapid, non-rhythmic, involuntary movements, but while choreic movements are nonstereotyped, irregular in distribution and almost constantly escape volitional control, tics are stereotyped, often preceded by premonitory sensations and partially under volitional control. Regarding criterion 2, many other neurological and psychiatric symptoms have been suggested to be variants of PANDAS. Finally, criterion number 4 is problematic, as numerous case reports of PANDAS base the diagnosis on a single event, not multiple exacerbations. It is worth noting however that SC is confidently diagnosed based on the dramatic emergence of an initial episode of chorea. In contrast, most PANDAS research has not focused on the initial clinical presentation.
Although it is still controversial whether stringently defined PANDAS criteria can be used to designate a unique clinical entity,8,9 we believe that the large number of clinical, epidemiological, and basic science observations supports further research into the potential role of the innate and adaptive immune systems in the pathogenesis of tics and OCD. This review summarizes recent findings related to this topic and integrates available data into a common pathophysiological model.
EXPOSURE TO ENVIRONMENTAL FACTORS TRIGGERING IMMUNE RESPONSES
Group A Streptococcal Infections and Psychosocial Stress
The link between GABHS infections and the onset or worsening of pediatric OCD, TS, and tic disorders has been supported by cross-sectional10,11 and longitudinal12–14 reports. These studies documented higher antistreptococcal antibody titers and a positive correlation between antibody titers and clinical severity in children with OCD or tic disorder. While this methodology provides the opportunity for rigorous clinical diagnosis, the small sample sizes suggest caution in interpreting results, particularly in cross-sectional studies.
Research using health services methodology in large datasets is also informative. Using ICD-9 codes in a large dataset from a health maintenance organization in the Seattle, Washington area, Mell et al.,15 identified 144 cases of new-onset OCD, TS or tic disorder along with 609 matched controls and found that patients with new-onset OCD, TS or tic disorder were significantly more likely to have prior streptococcal infections diagnosed in the year before illness onset. The most striking result was that when TS was considered separately having >1 GABHS infection in the 12 months before the onset date was 13-fold more common among cases with TS than controls (OR: 13.6; 95% CI: 1.93, 51.0). Similarly, Leslie et al.16 in a USA national health insurance sample of 479 cases of new-onset OCD, TS or tic disorder and 3,647 matched controls, found that children with newly diagnosed OCD, TS, or tic disorder were more likely to have had a diagnosis of streptococcal infection in the previous year. Of note, prior streptococcal infection was also modestly associated with incident diagnoses of ADHD (odds ratio = 1.20, 95% CI: 1.06–1.35) and major depressive disorder (MDD) (odds ratio = 1.63, 95% CI: 1.12–2.30). These findings provide epidemiologic evidence that some pediatric-onset neuropsychiatric disorders, including OCD, tic disorders, ADHD and MDD, may be temporally related to prior streptococcal infections. Several limitations of this methodology require mention. First, in contrast to the Mell et al’s study,15 the Leslie et al’s16 data does not include rigorous ascertainment of tic or OCD cases using consistent diagnostic criteria. Secondly, the date of the appearance of the neuropsychiatric diagnoses in the health claims data does not definitely coincide temporally with the onset of tic or OCD symptoms.
A few prospective studies have recently been published. In a prospective, multicenter study of children who met stringent criteria for PANDAS (n = 40) and matched children with OCD or Tic Disorders (n = 40), with monthly throat cultures, 3-month blood antibody tests, and monthly phone or in-clinic evaluations, Kurlan et al.17 found that the PANDAS group had a significantly higher rate of GABHS infections as well as a higher rate of clinical exacerbations. However, no more than 25% of the exacerbations in the PANDAS cases were temporally associated with a GABHS infection. In addition, there was no relationship between changes in the levels of antistreptococcal antibody titers and the occurrence of clinical exacerbations.18 Three possible limitations of the Kurlan et al’s study bear consideration. Firstly, this study informed primary health care providers of the results of throat cultures. Thus, primary clinicians were free, if they chose, to prescribe short term antibiotics for symptomatic or asymptomatic patients with positive cultures. This practice potentially could have limited the number of exacerbations observed.19 Secondly, both the total number of clinical exacerbations and the total number of GABHS infections were lower than had been estimated, raising the possibility that the study was under-powered, that it ascertained patients too late in their clinical course, or that study participation changed patient behaviors in some other way. Third, the study’s outcome variable was based on the PANDAS concept that a significant symptom exacerbation is a discrete event recognizable to clinicians. Therefore, the analysis tested for the observation of events at a rate that exceeded expectations. Although this study did not convincingly support PANDAS, it is still possible that GABHS infections alter tic or OCD severity. Whether infections influence tics and OCD as a result of a non-specific stress response or secondary to an activation of the immune system remains to be determined.
Clinical observations as well as studies of TS and early-onset OCD have consistently suggested that these disorders are sensitive to psychosocial stress.20–23 Psychosocial stress has also been shown to be an important factor in the onset and course of MDD24,25 and ADHD.26,27 In addition, a number of reports documented an abnormal response to stress in TS patients.21,28–31 Recently Lin et al.31 monitored 45 children with tic disorder and/or OCD and 41 matched healthy control subjects over a 2-year period for the level of psychosocial stress. Consecutive monthly ratings of tic, OC and depressive symptom severity were obtained. State-of-the-art structural equation modeling for unbalanced repeated measures was used to assess the temporal sequence of psychosocial stress measure changes with the severity of tic, OC and depressive symptoms. Psychosocial stress proved to be a powerful predictor of future tic, OC and depressive symptom severity.31
Alternative Precipitants in Addition to Psychosocial Stress and GABHS Infections
GABHS infection has been postulated as the main initial autoimmune response-inciting event contributing to the onset and clinical course of a subgroup of patients with TS and related disorders. However, it has been documented that tic exacerbations can be triggered also by other infectious agents (e.g. herpes simplex virus,32 varicella zoster virus,33 human immunodeficiency virus,34 Borrelia burgdorferi32), and a number of other precipitants have been identified including sinusitis,16 the common cold,35 and Mycoplasma pneumoniae.36–38 Future prospective longitudinal studies are needed to confirm these findings and to clarify whether there is a common underlying immunological response that triggers tic worsening.
ABNORMALITIES OF IMMUNE RESPONSE REGULATION
General Aspects of Immune Regulation
The immune system has two functional components, the innate and the adaptive components, which cooperate to protect the host against infections.39 The innate immune system is organized in functionally distinct ‘modules’, and recognizes a relatively small number of pathogen-associated molecular patterns through a likewise small number of pattern-recognition receptors. It represents the first line of inflammatory defence, but may also stimulate the adaptive immune response. Dendritic, phagocytes and natural killer (NK) cells are the principal ‘actors’ of innate immunity, and are specialized in killing a variety of intra- and extracellular pathogens. The adaptive immune system is capable of recognizing a much wider multitude of antigens, presented by specialized antigen-presenting cells, thereby building up an antigen-specific response in a more delayed fashion. T and B lymphocytes are the main effectors of adaptive immunity, supporting cell-mediated and antibody-mediated immune responses. Among T lymphocytes, T-helper lymphocytes (CD4+-lymphocytes) play the role of ‘orchestra conductor’, modulating both cell-mediated activity through macrophages and T-cytotoxic lymphocytes (CD8+-lymphocytes) and antibody production by plasma cells (which originate from B lymphocytes). The adaptive immune system also activates in turn innate effector mechanisms in an antigen-specific manner. In immune-mediated diseases the predominance of cell-mediated or humoral responses upon each other is relevant in both pathophysiological and therapeutic terms.
Gene Expression Profile Related to Immune Functioning
TS is genetically heterogeneous, but none of the genes or loci identified probably exerts a major effect.2 Genetic factors for immune-mediated diseases (e.g. HLA genotyping) have not been explored in TS. However, microarray gene expression profiling of peripheral blood cells is helping the search for disease-specific gene expression fingerprints. In preliminary reports, a subgroup of TS patients over-expressed genes controlling the function of NK and/or CD8+ T cells40; moreover, this subgroup showed a higher rate of ADHD comorbidity.41 Recent work documented that a subset of TS differ from healthy children in the age-related gene expression patterns emphasizing NK and CD8+ lymphocyte activity, interferon response and viral processing.42 Overall, these results suggest age-dependent over-activity of innate/inflammatory responses in a subgroup of TS patients.
Immune Cell Subpopulations and Immunophenotyping
Autoimmune diseases result from the breakdown of immune tolerance processes, which suppress the activity of potentially autoreactive T and B lymphocytes, thus limiting immune responses towards the “self”. One mechanism of peripheral tolerance involves a subset of T lymphocytes, defined natural regulatory T (Tregs) cells (or CD4+CD25+), which are potent inhibitors of B, CD4+, and CD8+ T lymphocytes.43,44 Reduced numbers of Tregs are detected in autoimmune conditions like lupus erythematosus,45 rheumatoid arthritis,46 type 1 diabetes,47 and multiple sclerosis,48 whereas increased levels occur in hepatitis B and C viruses and cytomegalovirus infections, and in several types of cancer.44
Using flow cytometry techniques, Kawikowa et al.49 found lower percentages of Tregs in the peripheral blood of 37 children with TS and/or OCD compared to healthy children; this reduction seemed to be more marked in patients with higher disease severity or during tic or OC symptom exacerbations. This finding might be explained by a genetic predisposition of TS/ OCD patients to reduced numbers of Tregs, and/or by prolonged reaction to persisting foreign antigens (e.g., microbial), potentially leading to compensatory exhaustion of Tregs, or to their functional ‘inversion’ into reactive lymphocytes.43 A third explanation was suggested by Ferrari et al.50 who reported increased expression of the D5 dopamine receptor on peripheral blood cells of TS patients; intriguingly, dopamine receptors have immunomodulating effects, and D5 activation, in particular, reduces the suppressive activity and the adhesive and migratory abilities of Tregs.51 Overall, TS and OCD appear to share with other auto-immune conditions decreased levels of peripheral Tregs, suggesting a predisposition for overriding auto-immune responses correlated to symptom severity. Kawikowa et al.49 observed also a 60% decrease in the number of CD8+ T lymphocytes bearing a specific variant of antigen receptor, i.e. β-18, which recognizes specific microbial antigens to which patients might have been persistently exposed, leading to activation-induced death of these cells.
Further support to increased peripheral immune activity came from an exploratory study of lymphocyte surface markers.52 Moller et al. found significantly increased numbers of CD69+ B lymphocytes and CD95+ T helper lymphocytes in 20 adults with TS, compared to healthy subjects, respectively suggesting increased B cell activation and increased activation-induced apoptosis of T lymphocytes.52 An increased frequency of activated B lymphocytes is also supported by prior research pointing towards a higher density of immunoglobulin receptors on the surface of B cells in these patients.53 Novel studies are warranted to analyse the effect of age and medications on the immunophenotype of patients with this disorder.
Effector Molecules
Effector molecules (cytokines, chemokines, adhesion molecules) modulate the activity of innate and adaptive immune-competent cells. Adaptive responses are regulated by type 1, type 2, type 3, and type 17 T-helper lymphocytes, which promote and control cell-mediated and antibody-mediated responses. Different cytokines convey the regulatory effect of these two different sub-populations.
A number of early reports on serum and cerebrospinal fluid cytokine levels in OCD, one of the main comorbidities of TS, yielded discrepant results, probably due to methodological heterogeneity, and were mostly limited to adult patients.54–57 Leckman and coworkers measured plasma levels of a broad array of cytokines in 46 TS patients and 31 healthy controls, reporting increased baseline levels of tumour necrosis factor-α (TNF-α) and interleukin-12 (IL-12).58 Interestingly, there was a 50–60% rise of these two cytokines, plus a general increase of all the main cytokines explored, at symptom exacerbation. These combined immune-clinical fluctuations were more frequent in non-PANDAS than in PANDAS patients, and seemed more evident in drug-naive than in medicated patients. Another recent prospective study on 12 children with PANDAS found no correlation between clinical exacerbations (associated or not with GABHS infection) and several type 1 cytokines, type 2 cytokines, and chemokines.18
Both TNF-α and IL-12 are involved in cell-mediated inflammatory responses. Specifically, IL-12 promotes both innate and adaptive responses by activating respectively NK and T-helper-1 cells.59 On balance, these results suggest that both innate and adaptive cell-mediated mechanisms may be overactive in TS. It also needs to be pointed out that, in line with previous criticism,8 the stringent working criteria for PANDAS4 may not isolate a specific subgroup of TS/OCD patients in whom clinical features are associated with immune markers.
Further support for the presence of pro-inflammatory mechanisms in TS is given by the observed increase in baseline plasma levels of neopterin, a soluble marker of T cell activation by interferon-gamma,13,60 and of two soluble adhesion molecules (vascular cell adhesion molecule-1 and E-selectin), which are involved in the recruitment of lymphocytes towards sites of inflammation.61
Autoantibodies
The current hypothesis on the pathophysiology of SC, the prototype of poststreptococcal CNS disorders, suggests that GABHS induces auto-reactive T and B lymphocytes (including antibody-producing plasma cells). 62 These B cells (or their antibodies, or cytokines) cross the blood-brain barrier and alter neurotransmission, resulting in the characteristic phenomenology. An analogous autoantibody hypothesis has been under investigation for TS and OCD symptoms. Despite much recent research, the role of autoantibodies is contentious.
Most pathogenic autoantibodies in neurological disorders bind to proteins or receptors on the cell surface of neurons, axons, or endothelium. The conformation of these auto-antigens is important in autoantibody–auto-antigen binding. Therefore, autoantibody detection methods that disrupt the conformation of proteins during preparation could lead to misleading results. To date, most of the immunological methods used in the investigation of SC, TS, and PANDAS have employed methods that disrupt protein structure. For example, Western blotting involves homogenisation and detergents which release cytoplasmic antigens, unravel proteins and disrupt disulphide bonds. By contrast, recent discoveries of pathogenic autoantibodies have used live cell systems which express candidate auto-antigens in their natural conformational state on the cell surface.63
Indirect immunofluorescence (IF) studies have involved the use of fine slices of brain tissue. Patient serum is incubated on the tissue, washed and patient antibody binding is detected using a secondary antihuman antibody with a fluorescent label and microscopy. This method can demonstrate the presence of autoantibodies, and the regional and cellular localisation of auto-antigens. In SC, there is general consensus that patients have auto-antibodies to cytoplasmic proteins of neurons (particularly basal ganglia neurons).64–66 These cytoplasmic proteins may not be available for auto-antibody binding in vivo unless there has been preceding cellular damage or disruption. Several IF case-control studies of TS and PANDAS have shown significantly elevated autoantibody binding in cytoplasm of cortical and striatal neurons,67–69 but, as shown in Table 1, findings in other IF studies did not support an autoantibody hypothesis.
TABLE 1.
Autoantibody findings in PANDAS
| Reference | Method | Clinical syndrome (number of patients) | Finding |
|---|---|---|---|
| 70 | IF | PANDAS (N = 22) vs. uncomplicated Strep. infection (n = 22) | Positive binding in 64% vs. 9% |
| 71 | ELISA | PANDAS (n = 15) vs. control (n = 15) | No difference in patients compared to controls |
| 72 | ELISA | PANDAS (n = 40) vs. controls (n = 190) | Elevated mean ELISA in PANDAS vs. controls |
| 69 | ELISA | PANDAS (n = 48) vs. TS (N = 46) vs. control (n = 43) | No difference in patients compared to controls |
| 72 | WB | PANDAS (n = 40) vs. controls (n = 190) | Positive binding in 92% vs. 5% |
| 71 | WB | PANDAS (n = 15) vs. controls (n = 15) | No difference in binding between groups |
| 69 | WB | PANDAS (n = 48) vs. controls (n = 43) | No difference in binding between groups |
| 73 | Cell surf. | PANDAS (n = 16) vs. TS, OCD, ADHD (n = 25) | Positive binding to cell surface in PANDAS more than TS, OCD, ADHD |
IF, immunofluorescence; ELISA, enzyme linked immunosorbent assay; WB, Western blotting; Cell surf, cell surface binding to live neurons; PANDAS, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection; TS, Tourette syndrome; OCD, obsessive-compulsive disorder; ADHD, attention deficit hyperactivity disorder.
Enzyme-linked immunoabsorbent assay (ELISA) studies have typically employed homogenates of neuroblastoma cells, rat brain or human striatum. An optical signal (such as chromogen) is used to quantify antibody binding. This assay can define the total antibody binding to brain-derived proteins but is unable to identify subcellular localization or differentiate specific antigens. In SC there is significantly elevated ELISA antibody binding.66,68 However, ELISA does not consistently differentiate TS and PANDAS cohorts from controls, possibly due to significant methodological differences between the studies, both in the antigens used and the antibody concentrations (see Table 1).67,69,71,72,74,75
Western immunoblotting studies have utilized homogenisation of brain tissue (either whole brain, basal ganglia or rat brain) followed by the use of detergents and heat to unravel proteins and break disulphide bonds. The proteins are next electrophoresed, separating them by charge, and then transferred to a membrane. The membrane is incubated with diluted patient serum, and antibody binding is demonstrated using an antihuman IgG. The advantages of this method are that antibody binding can be compared between patients (run in parallel) and the molecular weight of candidate auto-antigens can be defined. However, the Western blotting method is vulnerable to methodological problems related to unravelling and alteration of protein structure. In SC, patients have increased autoantibody binding compared to controls.65,66,68 Candidate auto-antigens were defined by Church as having molecular weight 40, 45, and 60 kDa.66 In PANDAS, the findings were far more equivocal, and Singer et al. found no difference using complex discriminant analysis (see Table 1).69,71 In TS, the findings are divergent and confusing (see Table 2).67,69,74,80 Finally, quantification of antibody binding to neuroblastoma or animal brain-derived live cell lines have theoretical advantages over methods that disrupt cell and protein structure. Two small but compelling studies using this technology (see Tables 1 and 2),73,79 and found that SC, TS, and PANDAS sera all contained auto-antibodies that bound to the cell surface of neuroblastoma cell lines or rat striatal neurons.73,79
TABLE 2.
Autoantibody findings in tic disorders and Tourette syndrome
| Reference | Method | Clinical syndrome (number of patients) | Finding |
|---|---|---|---|
| 76 | IF | Tics/OCS (n = 19) vs. ADHD (N = 19) | Positive binding in 63% vs. 37% |
| 11 | IF | TS (N = 100) vs. controls (n = 190) | Similar binding in TS compared to SC |
| 65 | IF | TS (N = 81) vs. controls (n = 119) | Elevated mean IF in TS cohort |
| 77 | IF | TS and OCD (n = 53) vs. controls (n = 19) | No difference: 0% vs. 0% |
| 78 | IF | TS (n = 69) vs. controls (n = 72) | Positive binding in 32% vs. 10% |
| 79 | IF | TS (n = 4) vs. TS family members (n = 11) vs. controls (n = 8) | Positive binding in 100% vs. 100% vs. 0% |
| 67 | ELISA | TS (n = 41) vs. controls (n = 39) | Elevated mean absorbance in TS vs. controls |
| 74 | ELISA | TS (n = 41) vs. controls (n = 39) | Elevated median absorbance (but not mean) in TS vs. controls |
| 75 | ELISA | TS (n = 41) vs. controls (n = 38) | No difference in absorbance between groups |
| 69 | ELISA | TS (N = 46) vs. controls (n = 43) | No difference ELISA between groups |
| 67 | WB | TS (n = 41) vs. controls (n = 39) | Positive binding more common in TS vs. controls |
| 74 | WB | TS (n = 41) vs. controls (n = 39) | No difference in binding between groups |
| 80 | WB | TS (n = 20) vs. controls (n = 21) | Positive binding more common in TS vs. controls |
| 69 | WB | TS (N = 46) vs. controls (n = 43) | No difference in binding between groups |
| 11 | WB | TS (n = 100) vs. controls (n = 190) | Positive binding in TS (20–27%) vs. controls (2–4%) |
| 79 | WB | TS (n = 4) vs. TS family (n = 11) vs. controls (n = 8) | Positive binding in TS (100%) vs. controls (0%) |
| 77 | WB | TS and OCD (n = 53) vs. controls (n = 19) | Positive binding in TS (13%) v. controls (0%) |
| 79 | Cell surf. | TS (n = 4) vs. TS family (n = 11) vs. controls (n = 8) | Positive binding to cell surface in patients not controls |
IF, immunofluorescence; ELISA, enzyme linked immunosorbent assay; WB, Western blotting; Cell surf., cell surface binding to live neurons; PANDAS, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection; TS, Tourette syndrome; SC, Sydenham’s chorea; OCD, obsessive-compulsive disorder; ADHD, attention deficit hyperactivity disorder.
The true frequency of antineuronal antibodies in TS and PANDAS is still controversial. Part of the reason for the disparate findings is due to the different laboratory methodologies used as well as different patient recruitment methods. In order to shed more light on this issue, general recommendations would be to move away from antibody detection methodologies which alter protein conformation and structure, and at the same time to orientate research efforts towards studies that assess pathogenic effects of antibodies on cell function and animal behaviour.
Following this line, preliminary work on the putative auto-antigens targeted by these antineuronal antibodies has been published in recent years. The auto-antigens of 40, 45, and 60 kDa identified using the Church method were subsequently identified as glycolytic enzymes (aldolase C, neuron-specific enolase, non-neuronal enolase and pyruvate kinase M1).81 These glycolytic enzymes are present in the neuronal cytoplasm and on the cell surface of neurons. They are involved in energy metabolism and support ion channels.
Furthermore, the same glycolytic enzymes exist on the cell surface of streptococcal bacteria. Non-neuronal enolase has been previously incriminated as an auto-antigen in rheumatic fever.82 Pyruvate kinase M1 was subsequently identified as an auto-antigen in TS by an independent group of investigators, which found elevated antipyruvate kinase antibodies during streptococcal-induced exacerbations of tics.83 However, these findings were not confirmed by Singer et al. in a study of TS, PANDAS, and controls,69 and the pathogenic potential of these autoantibodies has not been demonstrated to date.
Kirvan et al. found cross-reactivity of autoantibodies between streptococcal N-acetyl-glucosamine and brain lysoganglioside83; antibodies against lysoganglioside were elevated in SC and PANDAS. Furthermore, these auto-antibodies appeared to cause alteration in calcium-calmodulin kinase-II activation and possibly up-regulation of tyrosine hydroxylase (and therefore dopamine synthesis), which could theoretically result in SC or TS symptoms.73,84 This eloquent work utilized a hybridoma cell line produced from a single patient with SC, and requires replication.
“NEUROPATHOGENICITY” OF ABNORMAL IMMUNE RESPONSES IN TOURETTE’S SYNDROME
Autoantibodies
The presence of autoantibodies is not sufficient to prove causality. For example, antibrain antibodies have been identified in Huntington’s disease85 and genetic Parkinson’s disease,86 where they may be produced in response to the disease rather than being causal (i.e. an epiphenomenon). For autoantibodies to be considered pathogenic, they should also be present in the target organ, passive transfer of autoantibodies should induce disease in animals, and patients’ symptoms should improve after removal of autoantibodies.87 Due to the relative paucity of postmortem data, the presence of autoantibodies in the brain of TS patients has never been explored.
Researchers have attempted to create rodent models of tics and stereotyped behaviours using immunological triggers. Induction of disease in animals after human immunoglobulin infusion has been attempted with inconsistent results. Initial studies reported that infusion of TS sera with high autoantibodies induced stereotypies in rodents.88,89 Two subsequent studies have been inconclusive or negative.90,91 Most recently, a third study replicated the earlier studies reporting induction of stereotypies after infusion of TS sera, and also reported that the subsequent infusion of neural stem cells reduced the severity of the stereotypies.92 Another fascinating approach has been proposed by Hoffman et al., who immunized female SJL/J mice with a GABHS homogenate.93 Immunoreactive animals had increased IgG deposits in the deep cerebellar nuclei, which correlated with rearing and ambulatory behaviour and with serum immunoreactivity to GABHS proteins. This result suggests that active immunization may represent a better animal model strategy than antibody passive transfer to explore the involvement of antibody-mediated mechanisms in the pathogenesis of stereotypical behaviours resembling tics and related symptoms.
No human studies have demonstrated that removal of specific antistreptococcal or antibrain autoantibodies improves patient symptoms. Nonspecific immune modulation with plasma exchange, intravenous immunoglobulin (IVIg), and steroids has generally been reported beneficial in SC.94,95 Hoekstra found no benefit of IVIg in TS adult patients,96 whereas Perlmutter et al. demonstrated 1-month and a 1-year benefit of plasma exchange and IVIg compared to placebo in children and adolescent patients with PANDAS.97 However, in the absence of more robust and consistent evidence, current recommendations do not support the use of these agents in TS.
Antibody-mediated damage might be inflammatory in nature; alternatively, autoantibodies might act as ‘neurotoxins’, causing an impairment of intracellular neuronal signalling. Consistent with the hypothesis that an autoimmune inflammatory response in the basal ganglia might be occurring in a subgroup of patients with tics or OC symptoms, volumetric magnetic resonance imaging studies have demonstrated enlargement of the basal ganglia in both SC and PANDAS cases.98,99 Similar findings have been reported following the abrupt onset of OCD associated with a newly acquired Mycoplasma pneumoniae infection.37 These findings might be related to acute inflammatory changes occurring shortly after onset, as volumetry did not differ with respect to the presence of autoantibodies in patients with longer disease duration.100 Consistent with the ‘neurotoxic’ hypothesis, Teixeira et al. found increased calcium influx in neuroblastoma cell lines after SC antibody incubation,101 whereas antilysoganglioside antibodies, described by Kirvan et al.,73 promote neuronal cell signalling via calcium-calmodulin kinase-II activation, as mentioned above.
Despite the general consensus that antineuronal antibodies are present in SC and that these auto-antibodies are probably pathogenic, at present there is no general consensus on their pathogenicity in TS and PANDAS.
Cytokines
Apart from antibody-mediated mechanisms, symptoms observed in TS patients, such as tics, OC symptoms, and depressive/anxiety symptoms, might also be directly or indirectly precipitated by cytokines. A pathological model of these processes is sickness behaviour and ‘cytokine-induced depression’ (for a detailed review of this condition, see ref. 102). Pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 may modulate catecholaminergic transmission,103 alter tryptophan metabolism thus leading to abnormal serotonin metabolism and over-production of potentially toxic tryptophan metabolites,104 alter gene expression,105 and activate the hypothalamic-pituitary-adrenal axis, thus leading to abnormal responsiveness to stress.106 However, currently clear evidence of cytokine-induced neural dysfunction in TS is lacking and should be addressed in future studies.
AN EMERGING MODEL INTEGRATING IMMUNE ALTERATIONS AND CURRENT PATHOPHYSIOLOGICAL CONCEPTS IN TS
For a better insight of the role of abnormal immune responses in the pathogenesis of TS, it is necessary to integrate the reviewed findings (summarized in Table 3) with the current views on the pathophysiology of the disorder. TS, similarly to SC and tic-related forms of OCD, have traditionally been viewed as hyperkinetic disorders in which central dopamine systems play an important etiological role.107,108 There is compelling evidence of a hyperdopaminergic state in some cases of TS with increased ventral striatal dopaminergic innervation109 and elevated intrasynaptic dopamine release in the striatum following amphetamine administration.110 It is also well known that dopamine receptor blocking agents are among the most effective and efficacious in the treatment of TS, SC, and tic-related forms of OCD.111
TABLE 3.
Summary of findings supporting increased activation of immune responses in patients with tic disorders and Tourette syndrome
| References of studies supporting the finding | |
|---|---|
| Changes in gene expression | |
| Over-expression of genes modulating cytotoxicity and antigen presentation of NK and CD8+ T lymphocytes in PBMC | 40–42 |
| Over-expression of the D5 dopamine receptor gene in PBMC | 50 |
| Changes in lymphocyte subpopulation numbers | |
| Reduced percentage of CD4+CD25+ natural regulatory | 49a |
| T lymphocytes (Tregs) in moderate/severe patients | |
| Correlation of Tregs percentage with disease severity | 49a |
| Decreased percentage of β18+ CD8+ T lymphocytes in moderate/severe patients | 49a |
| Increased number of CD95+ T lymphocytes | 52 |
| Increased number of CD69+ B lymphocytes | 52 |
| Changes in the synthesis of effector molecules by immune competent cells | |
| Increased plasma levels of TNFα and IL-12 | 58a |
| Correlation of TNFα and IL-12 plasma levels with disease severity | 58a |
| Increased plasma levels of sVCAM-1 and sE-selectin | 61 |
| Increased plasma levels of neopterin | 13a |
| Increased synthesis of anti-neuronal antibodies (CONTROVERSIAL) | 11, 65, 67, 74, 76–80 |
| Increased density of Fc μ receptors (receptors for IgM) on B lymphocytes | 53 |
NK, natural killer cells; PBMC, peripheral blood mononuclear cells; TNFα, tumor necrosis factor-α; IL-12, interleukin-12; sVCAM-1, soluble vascular cell adhesion molecule-1; sE-selectin, soluble E-selectin; Fc, Fragment crystallizable; IgM, immunoglobulin M.
The study combined patients with Tourette’s syndrome (or other chronic tic disorder) and patients with pediatric-onset obsessive-compulsive disorder.
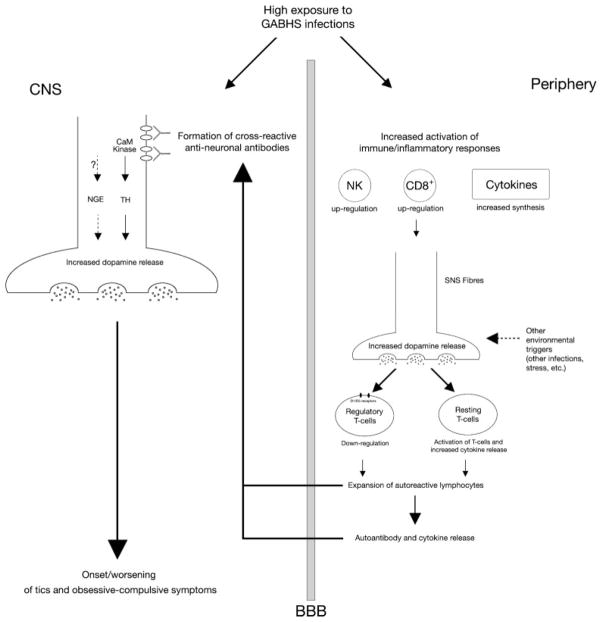
Expanding upon an idea originally articulated by Cunningham, Kawikowa, Bothwell and Leckman (Leckman JF et al., personal communication) we propose a pathophysiological model to explain the involvement of immune-mediated mechanisms in the pathophysiology of TS and related disorders. The increased exposure of TS patients to GABHS (or other) infections might induce the synthesis of cross-reactive antineural antibodies, e.g. antilysoganglioside antibodies,112 which promote dopamine synthesis and exocytosis via CaM kinase II activation.113,114 Antiglycolytic enzymes antibodies might also modify neuronal excitability and affect neurotransmitter release, although evidence to support this hypothesis is currently lacking. At the same time, an increased exposure to GABHS infections might promote first-line inflammatory responses, which may potentiate dopamine release by autonomic fibres and enhance dopaminergic modulation of peripheral immune cells. Dopamine exerts its immunomodulatory effects via activation of D1/D5 dopamine receptors on the lymphocyte cell surface,50,51 causing functional down-regulation of Tregs,49 activation of resting T cells and increased cytokine (particularly, TNF-α) release.58,115 Treg down-regulation would skew immune regulatory mechanisms in favour of autoimmunity, whereas an increase in TNFα would increase the permeability of the blood-brain barrier. These changes may lead to an enhanced autoimmune response and even greater dopamine release in the basal ganglia which in turn contribute to the clinical symptoms of TS and related disorders. Figure 1 summarizes the proposed mechanism.
FIG. 1.
Proposed hypothetical model of immune-mediated basal ganglia dysfunction in tics and obsessive-compulsive symptoms. Group A β-hemolytic streptococcal (GABHS) infections induce the formation of cross-reactive autoantibodies which bind cell surface neuronal antigens. This binding might lead to increased calcium-calmodulin-dependent (CaM) kinase II activity, subsequent increase of tyrosine hydroxylase activity and enhanced dopamine release at the level of central synapses; alternatively autoantibodies might modulate the activity of membrane-bound neuronal glycolytic enzymes, thus leading to alterations in membrane excitability and to abnormal neurotransmitter release. In the periphery, GABHS infections may activate inflammatory responses, leading to Natural Killer and cytotoxic T cell functional up-regulation, and to increased synthesis of pro-inflammatory cytokines. This inflammatory background may enhance dopamine synthesis and release from sympathetic nervous system fibres, with the possible contribution of other environmental triggers, e.g. psychosocial stressors. Higher peripheral levels of dopamine may down-regulate the function of regulatory T cells and amplify the release of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α. These changes may ultimately lead to an enhanced autoimmune response and even greater dopamine release in the basal ganglia, which in turn contribute to onset or worsening of tics and obsessive-compulsive symptoms.
Psychosocial stressors, another environmental exposure shown to be relevant in TS, is known to directly cause release of striatal dopamine.116 At the same time, chronic exposure to stressors in other stress-related disorders (e.g. depression or post-traumatic stress disorders) may be associated with reduced responsiveness to glucocorticoids, which may facilitate abnormal immune activation.106 Increased pro-inflammatory cytokine levels in turn may enhance HPA axis activation, possibly contributing to the abnormally enhanced response to stress observed in these patients.106 Further investigation on responsiveness to glucocorticoids and reactivity of the HPA axis in patients with TS might help clarifying the relationship between exposure to stressors and regulation of immune responses in these patients.
In conclusion, the body of evidence in favor of a contribution of the immune system on the pathophysiology of TS and related disorders is constantly growing. Recent research is progressively unveiling a complex interplay between neural transmission, immune response regulation and endocrine systems, which might give further insight into the natural history of TS and the typical vulnerability of TS-related symptomatology to environmental changes.
Acknowledgments
The Authors wish to acknowledge Prof. Madeleine W. Cunningham for her precious comments during the preparation of this article.
Footnotes
Author Roles: Davide Martino: Conception, organization, and execution of the research project; writing of the first draft and review of the manuscript; Russell C Dale: Organization and execution of the research project; writing of the first draft, review and critique of the manuscript; Donald L Gilbert: Review and critique of the manuscript; Gavin Giovannoni: Review and critique of the manuscript; James F Leckman: Organization and execution of the research project; writing of the first draft, review and critique of the manuscript.
Potential conflict of interest: None reported.
This article is part of the journal’s online CME program. The CME activity including form, can be found online at http://www.movementdisorders.org/education/journalcme/
References
- 1.Leckman JF. Tourette’s syndrome. Lancet. 2002;360:1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- 2.Swain JE, Scahill L, Lombroso PJ, King RA, Leckman JF. Tourette syndrome and tic disorders: a decade of progress. J Am Acad Child Adolesc Psychiatry. 2007;46:947–968. doi: 10.1097/chi.0b013e318068fbcc. [DOI] [PubMed] [Google Scholar]
- 3.Kiessling LS, Marcotte AC, Culpepper L. Antineuronal antibodies in movement disorders. Pediatrics. 1993;92:39–43. [PubMed] [Google Scholar]
- 4.Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155:264–271. doi: 10.1176/ajp.155.2.264. [DOI] [PubMed] [Google Scholar]
- 5.Swedo SE, Rapoport JL, Cheslow DL, et al. High prevalence of obsessive-compulsive symptoms in patients with Sydenham’s chorea. Am J Psychiatry. 1989;146:246–249. doi: 10.1176/ajp.146.2.246. [DOI] [PubMed] [Google Scholar]
- 6.Mercadante MT, Busatto GF, Lombroso PJ, et al. The psychiatric symptoms of rheumatic fever. Am J Psychiatry. 2000;157:2036–2038. doi: 10.1176/appi.ajp.157.12.2036. [DOI] [PubMed] [Google Scholar]
- 7.Murphy TK, Snider LA, Mutch PJ, et al. Relationship of movements and behaviors to Group A Streptococcus infections in elementary school children. Biol Psychiatry. 2007;61:279–284. doi: 10.1016/j.biopsych.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 8.Kurlan R, Kaplan EL. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) etiology for tics and obsessive-compulsive symptoms: hypothesis or entity? Practical considerations for the clinician. Pediatrics. 2004;113:883–886. doi: 10.1542/peds.113.4.883. [DOI] [PubMed] [Google Scholar]
- 9.Swedo SE, Leonard HL, Rapoport JL. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) subgroup: separating fact from fiction. Pediatrics. 2004;113:907–911. doi: 10.1542/peds.113.4.907. [DOI] [PubMed] [Google Scholar]
- 10.Cardona F, Orefici G. Group A Streptococcal infections and tic disorders in an Italian pediatric population. J Pediatr. 2001;138:71–75. doi: 10.1067/mpd.2001.110325. [DOI] [PubMed] [Google Scholar]
- 11.Church AJ, Dale RC, Lees AJ, Giovannoni G, Robertson MM. Tourette’s syndrome: a cross sectional study to examine the PANDAS hypothesis. J Neurol Neurosurg Psychiatry. 2003;74:602–607. doi: 10.1136/jnnp.74.5.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy ML, Pichichero ME. Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group A Streptococcal infection (PANDAS) Arch Pediatr Adolesc Med. 2002;156:356–361. doi: 10.1001/archpedi.156.4.356. [DOI] [PubMed] [Google Scholar]
- 13.Luo F, Leckman JF, Katsovich L, et al. Prospective longitudinal study of children with tic disorders and/or obsessive-compulsive disorder: relationship of symptom exacerbations to newly acquired streptococcal infections. Pediatrics. 2004;113:e578–e585. doi: 10.1542/peds.113.6.e578. [DOI] [PubMed] [Google Scholar]
- 14.Murphy TK, Sajid M, Soto O, et al. Detecting pediatric autoimmune neuropsychiatric disorders associated with Streptococcus in children with obsessive-compulsive disorder and tics. Biol Psychiatry. 2004;55:61–68. doi: 10.1016/s0006-3223(03)00704-2. [DOI] [PubMed] [Google Scholar]
- 15.Mell LK, Davis RL, Owens D. Association between streptococcal infection and obsessive-compulsive disorder, Tourette’s syndrome, and tic disorder. Pediatrics. 2005;116:56–60. doi: 10.1542/peds.2004-2058. [DOI] [PubMed] [Google Scholar]
- 16.Leslie DL, Kozma L, Martin A, et al. Neuropsychiatric disorders associated with streptococcal infection: A case-control study among privately insured children. J Am Acad Child Adolesc Psychiatry. 2008;47:1162–1172. doi: 10.1097/CHI.0b013e3181825a3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurlan R, Johnson D, Kaplan EL the Tourette Syndrome Study Group. Streptococcal infection and exacerbations of childhood tics and obsessive-compulsive symptoms: a prospective blinded cohort study. Pediatrics. 2008;121:1188–1197. doi: 10.1542/peds.2007-2657. [DOI] [PubMed] [Google Scholar]
- 18.Singer HS, Gause C, Morris C, Lopez P Tourette Syndrome Study Group. Serial immune markers do not correlate with clinical exacerbations in pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Pediatrics. 2008;121:1198–1205. doi: 10.1542/peds.2007-2658. [DOI] [PubMed] [Google Scholar]
- 19.Perrin EM, Murphy ML, Casey JR, et al. Does group A beta-hemolytic streptococcal infection increase risk for behavioral and neuropsychiatric symptoms in children? Arch Pediatr Adolesc Med. 2004;158:848–856. doi: 10.1001/archpedi.158.9.848. [DOI] [PubMed] [Google Scholar]
- 20.Bornstein RA. Neuropsychological performance in children with Tourette’s syndrome. Psychiatry Res. 1990;33:73–81. doi: 10.1016/0165-1781(90)90150-4. [DOI] [PubMed] [Google Scholar]
- 21.Chappell P, Riddle M, Anderson G, et al. Enhanced stress responsivity of Tourette syndrome patients undergoing lumbar puncture. Biol Psychiatry. 1994;36:35–43. doi: 10.1016/0006-3223(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 22.Hoekstra PJ, Steenhuis MP, Kallenberg CGM, Minderaa RB. Association of small life events with self reports of tic severity in pediatric and adult tic disorder patients: a prospective longitudinal study. J Clin Psychiatry. 2004;65:426–431. doi: 10.4088/jcp.v65n0320. [DOI] [PubMed] [Google Scholar]
- 23.Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric stress: hormonal mediators and human development. Horm Res. 2003;59:161–179. doi: 10.1159/000069325. [DOI] [PubMed] [Google Scholar]
- 24.Jaffee SR, Moffitt TE, Caspi A, Fombonne E, Poulton R, Martin J. Differences in early childhood risk factors for juvenile-onset and adult-onset depression. Arch Gen Psychiatry. 2002;59:215–222. doi: 10.1001/archpsyc.59.3.215. [DOI] [PubMed] [Google Scholar]
- 25.Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry. 2004;161:631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- 26.Biederman J, Milberger S, Faraone SV, et al. Impact of adversity on functioning and comorbidity in children with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34:1495–1503. doi: 10.1097/00004583-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Biederman J, Faraone S, Milberger S, et al. Predictors of persistence and remission of ADHD into adolescence: results from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1996;35:343–351. doi: 10.1097/00004583-199603000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Sandyk R, Bamford CR. Heightened cortisol response to administration of naloxone in Tourette’s syndrome. Int J Neurosci. 1988;39:225–227. doi: 10.3109/00207458808985707. [DOI] [PubMed] [Google Scholar]
- 29.Chappell P, Leckman JF, Goodman W, et al. Elevated cerebrospinal fluid corticotropin-releasing factor in Tourette’s syndrome: comparison to obsessive-compulsive disorder and normal controls. Biol Psychiatry. 1996;39:776–783. doi: 10.1016/0006-3223(95)00221-9. [DOI] [PubMed] [Google Scholar]
- 30.Corbett BA, Mendoza SP, Baym CL, Bunge SA, Levine S. Examining cortisol rhythmicity and responsivity to stress in children with Tourette syndrome. Psychoneuroendocrinology. 2008;33:810–820. doi: 10.1016/j.psyneuen.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin H, Katsovich L, Ghebremichael M, et al. Psychosocial stress predicts future symptom severities in children and adolescents with Tourette syndrome and/or obsessive-compulsive disorder. J Child Psychol Psychiatry. 2007;48:157–166. doi: 10.1111/j.1469-7610.2006.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jankovic J, Kwak C. Tics in other neurological disorders. In: Kurlan R, editor. Handbook of Tourette’s syndrome and related tic and behavioural disorders. New York: Marcel Dekker; 2004. [Google Scholar]
- 33.Dale RC, Church AJ, Heyman I. Striatal encephalitis after Varicella zoster infection complicated by tourettism. Mov Disord. 2003;18:1554–1556. doi: 10.1002/mds.10610. [DOI] [PubMed] [Google Scholar]
- 34.Cardoso F. Infectious and transmissible movement disorders. In: Jankovic J, Tolosa E, editors. Parkinson’s disease and movement disorders. 4. Baltimore: Williams and Wilkins; 2002. pp. 930–940. [Google Scholar]
- 35.Hoekstra PJ, Manson WL, Steenhuis MP, Kallenberg CG, Minderaa RB. Association of common cold with exacerbations in pediatric but not adult patients with tic disorder: a prospective longitudinal study. J Child Adolesc Psychopharm. 2005;15:285–292. doi: 10.1089/cap.2005.15.285. [DOI] [PubMed] [Google Scholar]
- 36.Müller N, Riedel M, Blendinger C, Oberle K, Jacobs E, Abele-Horn M. Mycoplasma pneumoniae infection and Tourette’s syndrome. Psychiatry Res. 2004;129:119–125. doi: 10.1016/j.psychres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Termine C, Uggetti C, Veggiotti P, et al. Long-term follow-up of an adolescent who had bilateral striatal necrosis secondary to Mycoplasma pneumoniae infection. Brain Dev. 2005;27:62–65. doi: 10.1016/j.braindev.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Ercan TE, Ercan G, Severge B, Arpaozu M, Karasu G. Mycoplasma pneumoniae infection and obsessive-compulsive disease: a case report. J Child Neurol. 2008;23:338–340. doi: 10.1177/0883073807308714. [DOI] [PubMed] [Google Scholar]
- 39.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 40.Tang Y, Gilbert DL, Glauser TA, Hershey AD, Sharp FR. Blood gene expression profiling of neurologic diseases: a pilot microarray study. Arch Neurol. 2005;62:210–215. doi: 10.1001/archneur.62.2.210. [DOI] [PubMed] [Google Scholar]
- 41.Lit L, Gilbert DL, Walker W, Sharp FR. A subgroup of Tourette’s patients overexpress specific natural killer cell genes in blood: a preliminary report. Am J Med Genet B. 2007;144B:958–963. doi: 10.1002/ajmg.b.30550. [DOI] [PubMed] [Google Scholar]
- 42.Lit L, Enstrom A, Sharp FR, Gilbert DL. Age-related gene expression in Tourette syndrome. J Psychiat Res. 2009;43:319–330. doi: 10.1016/j.jpsychires.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81–88. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Cools N, Ponsaerts P, Van Tendeloo VFI, Berneman ZN. Regulatory T cells and human disease. Clin Dev Immunol. 2007;2007:89–195. doi: 10.1155/2007/89195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crispin JC, Martinez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2003;21:273–276. doi: 10.1016/s0896-8411(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 46.de Kleer IM, Wedderburn LR, Taams LS, et al. CD4+CD25 bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol. 2004;172:6435–6443. doi: 10.4049/jimmunol.172.10.6435. [DOI] [PubMed] [Google Scholar]
- 47.Kukreja G, Cost J, Marker C, et al. Multiple immunoregulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matarese G, Carrieri PB, La Cava A, et al. Leptin increase in multiple sclerosis associates with reduced number of CD4+CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2005;102:5150–5155. doi: 10.1073/pnas.0408995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawikova I, Leckman JF, Kronig H, et al. Decreased numbers of regulatory T cells suggest impaired immune tolerance in children with Tourette syndrome: a preliminary study. Biol Psychiatry. 2007;61:273–278. doi: 10.1016/j.biopsych.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 50.Ferrari M, Termine C, Franciotta D, et al. Dopaminergic receptor D5 mRNA expression is increased in circulating lymphocytes of Tourette syndrome patients. J Psychiat Res. 2008;43:24–29. doi: 10.1016/j.jpsychires.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 51.Kipnis J, Cardon M, Avidan H, et al. Dopamine, through the extracellular signal-regulated kinase pathway, downregulates CD4+CD25+ regulatory T-cell activity: implications for neurodegeneration. J Neurosci. 2004;24:6133–6143. doi: 10.1523/JNEUROSCI.0600-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moller JC, Tackenberg B, Heinzel-Gutenbrunner M, et al. Immunophenotyping in Tourette syndrome—a pilot study. Eur J Neurol. 2008;15:749–753. doi: 10.1111/j.1468-1331.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 53.Hoekstra PJ, Bijzet J, Limburg PC, Kallenberg CG, Minderaa RB. Elevated binding of D8/17-specific monoclonal antibody to B lymphocytes in tic disorder patients. Am J Psychiatry. 2004;161:1501–1502. doi: 10.1176/appi.ajp.161.8.1501-a. [DOI] [PubMed] [Google Scholar]
- 54.Brambilla F, Perna G, Bellodi L, et al. Plasma interleukin-1 beta and tumor necrosis factor concentrations in obsessive-compulsive disorders. Biol Psychiatry. 1997;42:976–981. doi: 10.1016/s0006-3223(96)00495-7. [DOI] [PubMed] [Google Scholar]
- 55.Mittleman BB, Castellanos FX, Jacobsen LK, Rapoport JL, Swedo SE, Shearer GM. Cerebrospinal fluid cytokines in pediatric neuropsychiatric disease. J Immunol. 1997;159:2994–2999. [PubMed] [Google Scholar]
- 56.Monteleone P, Catapano F, Fabrazzo M, Tortorella A, Maj M. Decreased blood levels of tumor necrosis factor-alpha in patients with obsessive-compulsive disorder. Neuropsychobiology. 1998;37:182–185. doi: 10.1159/000026500. [DOI] [PubMed] [Google Scholar]
- 57.Denys D, Fluitman S, Kavelaars A, Heijnen K, Westenberg H. Decreased TNF-a and NK activity in obsessive-compulsive disorder. Psychoneuroendocrinology. 2004;29:945–952. doi: 10.1016/j.psyneuen.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Leckman JF, Katsovich L, Kawikova I, et al. Increased serum levels of tumour necrosis factor-alpha and IL-12 in Tourette’s syndrome. Biol Psychiatry. 2005;57:667–673. doi: 10.1016/j.biopsych.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 60.Hoekstra PJ, Anderson GM, Troost PW, Kallenberg CG, Minderaa RB. Plasma kynurenine and related measures in tic disorder patients. Eur Child Adolesc Psychiatry. 2007;16 (Suppl 1):71–77. doi: 10.1007/s00787-007-1009-1. [DOI] [PubMed] [Google Scholar]
- 61.Martino D, Church AJ, Defazio G, et al. Soluble adhesion molecules in Gilles de la Tourette’s syndrome. J Neurol Sci. 2005;234:79–95. doi: 10.1016/j.jns.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 62.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blaes F, Beeson D, Plested P, Lang B, Vincent A. IgG from “seronegative” myasthenia gravis patients binds to a muscle cell line, TE671, but not to human acetylcholine receptor. Ann Neurol. 2000;47:504–510. [PubMed] [Google Scholar]
- 64.Husby G, van de Rijn I, Zabriskie JB, Abdin ZH, Williams RC., Jr Antibodies reacting with cytoplasm of subthalamic and caudate nuclei neurons in chorea and acute rheumatic fever. J Exp Med. 1976;144:1094–1110. doi: 10.1084/jem.144.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morshed SA, Parveen S, Leckman JF, et al. Antibodies against neural, nuclear, cytoskeletal, and streptococcal epitopes in children and adults with Tourette’s syndrome, Sydenham’s chorea, and autoimmune disorders. Biol Psychiatry. 2001;50:566–577. doi: 10.1016/s0006-3223(01)01096-4. [DOI] [PubMed] [Google Scholar]
- 66.Church AJ, Cardoso F, Dale RC, Lees AJ, Thompson EJ, Giovannoni G. Anti-basal ganglia antibodies in acute and persistent Sydenham’s chorea. Neurology. 2002;59:227–231. doi: 10.1212/wnl.59.2.227. [DOI] [PubMed] [Google Scholar]
- 67.Singer HS, Giuliano JD, Hansen BH, et al. Antibodies against human putamen in children with Tourette syndrome. Neurology. 1998;50:1618–1624. doi: 10.1212/wnl.50.6.1618. [DOI] [PubMed] [Google Scholar]
- 68.Singer HS, Loiselle CR, Lee O, Garvey MA, Grus FH. Anti-basal ganglia antibody abnormalities in Sydenham chorea. J Neuroimmunol. 2003;136:154–161. doi: 10.1016/s0165-5728(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 69.Singer HS, Hong JJ, Yoon DY, Williams PN. Serum autoantibodies do not differentiate PANDAS and Tourette syndrome from controls. Neurology. 2005;65:1701–1707. doi: 10.1212/01.wnl.0000183223.69946.f1. [DOI] [PubMed] [Google Scholar]
- 70.Pavone P, Bianchini R, Parano E, et al. Anti-brain antibodies in PANDAS versus uncomplicated streptococcal infection. Pediatr Neurol. 2004;30:107–110. doi: 10.1016/S0887-8994(03)00413-2. [DOI] [PubMed] [Google Scholar]
- 71.Singer HS, Loiselle CR, Lee O, Minzer K, Swedo S, Grus FH. Anti-basal ganglia antibodies in PANDAS. Mov Disord. 2004;19:406–415. doi: 10.1002/mds.20052. [DOI] [PubMed] [Google Scholar]
- 72.Church AJ, Dale RC, Giovannoni G. Anti-basal ganglia antibodies: a possible diagnostic utility in idiopathic movement disorders? Arch Dis Childhood. 2004;89:611–614. doi: 10.1136/adc.2003.031880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol. 2006;179:173–179. doi: 10.1016/j.jneuroim.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 74.Singer HS, Giuliano JD, Hansen BH, et al. Antibodies against a neuron-like (HTB-10 neuroblastoma) cell in children with Tourette syndrome. Biol Psychiatry. 1999;46:775–780. doi: 10.1016/s0006-3223(98)00384-9. [DOI] [PubMed] [Google Scholar]
- 75.Loiselle CR, Wendlandt JT, Rohde CA, Singer HS. Antistreptococcal, neuronal, and nuclear antibodies in Tourette syndrome. Pediatr Neurol. 2003;28:119–125. doi: 10.1016/s0887-8994(02)00507-6. [DOI] [PubMed] [Google Scholar]
- 76.Kiessling LS, Marcotte AC, Culpepper L. Antineuronal antibodies: tics and obsessive-compulsive symptoms. J Dev Behav Pediatr. 1994;15:421–425. [PubMed] [Google Scholar]
- 77.Morer A, Lazaro L, Sabater L, Massana J, Castro J, Graus F. Antineuronal antibodies in a group of children with obsessive-compulsive disorder and Tourette syndrome. J Psychiatr Res. 2008;42:64–68. doi: 10.1016/j.jpsychires.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 78.Rizzo R, Gulisano M, Pavone P, Fogliani F, Robertson MM. Increased antistreptococcal antibody titers and anti-basal ganglia antibodies in patients with Tourette syndrome: controlled cross-sectional study. J Child Neurol. 2006;21:747–753. doi: 10.1177/08830738060210091001. [DOI] [PubMed] [Google Scholar]
- 79.Yeh CB, Wu CH, Tsung HC, Chen CW, Shyu JF, Leckman JF. Antineural antibody in patients with Tourette’s syndrome and their family members. J Biomed Sci. 2006;13:101–112. doi: 10.1007/s11373-005-9033-y. [DOI] [PubMed] [Google Scholar]
- 80.Wendlandt JT, Grus FH, Hansen BH, Singer HS. Striatal antibodies in children with Tourette’s syndrome: multivariate discriminant analysis of IgG repertoires. J Neuroimmunol. 2001;119:106–113. doi: 10.1016/s0165-5728(01)00370-8. [DOI] [PubMed] [Google Scholar]
- 81.Dale RC, Candler PM, Church AJ, Wait R, Pocock JM, Giovannoni G. Neuronal surface glycolytic enzymes are autoantigen targets in post-streptococcal autoimmune CNS disease. J Neuroimmunol. 2006;172:187–197. doi: 10.1016/j.jneuroim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 82.Fontan PA, Pancholi V, Nociari MM, Fischetti VA. Antibodies to streptococcal surface enolase react with human alpha-enolase: implications in poststreptococcal sequelae. J Infect Dis. 2000;182:1712–1721. doi: 10.1086/317604. [DOI] [PubMed] [Google Scholar]
- 83.Kansy JW, Katsovich L, McIver KS, et al. Identification of pyruvate kinase as an antigen associated with Tourette syndrome. J Neuroimmunol. 2006;181:165–176. doi: 10.1016/j.jneuroim.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirvan CA, Swedo SE, Kurahara D, Cunningham MW. Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham’s chorea. Autoimmunity. 2006;39:21–29. doi: 10.1080/08916930500484757. [DOI] [PubMed] [Google Scholar]
- 85.Husby G, Li L, Davis LE, Wedege E, Kokmen E, Williams RC., Jr Antibodies to human caudate nucleus neurons in Huntington’s chorea. J Clin Invest. 1977;59:922–932. doi: 10.1172/JCI108714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van de Warrenburg BP, Church AJ, Martino D, et al. Antineuronal antibodies in Parkinson’s disease. Mov Disord. 2008;23:958–963. doi: 10.1002/mds.21929. [DOI] [PubMed] [Google Scholar]
- 87.Archelos JJ, Hartung HP. Pathogenetic role of autoantibodies in neurological diseases. Trends Neurosci. 2000;23:317–327. doi: 10.1016/s0166-2236(00)01575-7. [DOI] [PubMed] [Google Scholar]
- 88.Hallett JJ, Harling-Berg CJ, Knopf PM, Stopa EG, Kiessling LS. Anti-striatal antibodies in Tourette syndrome cause neuronal dysfunction. J Neuroimmunol. 2000;111:195–202. doi: 10.1016/s0165-5728(00)00320-9. [DOI] [PubMed] [Google Scholar]
- 89.Taylor JR, Morshed SA, Parveen S, et al. An animal model of Tourette’s syndrome. Am J Psychiatry. 2002;159:657–660. doi: 10.1176/appi.ajp.159.4.657. [DOI] [PubMed] [Google Scholar]
- 90.Loiselle CR, Lee O, Moran TH, Singer HS. Striatal microinfusion of Tourette syndrome and PANDAS sera: failure to induce behavioral changes. Mov Disord. 2004;19:390–396. doi: 10.1002/mds.10522. [DOI] [PubMed] [Google Scholar]
- 91.Singer HS, Mink JW, Loiselle CR, et al. Microinfusion of antineuronal antibodies into rodent striatum: failure to differentiate between elevated and low titers. J Neuroimmunol. 2005;163:8–14. doi: 10.1016/j.jneuroim.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 92.Liu X, Wang Y, Li D, Ju X. Transplantation of rat neural stem cells reduces stereotypic behaviors in rats after intrastriatal microinfusion of Tourette syndrome sera. Behav Brain Res. 2008;186:84–90. doi: 10.1016/j.bbr.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 93.Hoffman KL, Hornig M, Yaddanapudi K, Jabado O, Lipkin WI. A murine model for neuropsychiatric disorders associated with group A beta-hemolytic streptococcal infection. J Neurosci. 2004;24:1780–1791. doi: 10.1523/JNEUROSCI.0887-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garvey MA, Snider LA, Leitman SF, Werden R, Swedo SE. Treatment of Sydenham’s chorea with intravenous immunoglobulin, plasma exchange, or prednisone. J Child Neurol. 2005;20:424–429. doi: 10.1177/08830738050200050601. [DOI] [PubMed] [Google Scholar]
- 95.Walker AR, Tani LY, Thompson JA, Firth SD, Veasy LG, Bale JF., Jr Rheumatic chorea: relationship to systemic manifestations and response to corticosteroids. J Pediatr. 2007;151:679–683. doi: 10.1016/j.jpeds.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 96.Hoekstra PJ, Minderaa RB, Kallenberg CG. Lack of effect of intravenous immunoglobulins on tics: a double-blind placebo-controlled study. J Clin Psychiatry. 2004;65:537–542. doi: 10.4088/jcp.v65n0413. [DOI] [PubMed] [Google Scholar]
- 97.Perlmutter SJ, Leitman SF, Garvey MA, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet. 1999;354:1153–1158. doi: 10.1016/S0140-6736(98)12297-3. [DOI] [PubMed] [Google Scholar]
- 98.Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am J Psychiatry. 2000;157:281–283. doi: 10.1176/appi.ajp.157.2.281. [DOI] [PubMed] [Google Scholar]
- 99.Giedd JN, Rapoport JL, Kruesi MJP, et al. Sydenham’s chorea: magnetic resonance imaging of the basal ganglia. Neurology. 1995;45:2199–2202. doi: 10.1212/wnl.45.12.2199. [DOI] [PubMed] [Google Scholar]
- 100.Martino D, Draganski B, Cavanna A, et al. Anti-basal ganglia antibodies and Tourette’s syndrome: a voxel-based morphometry and diffusion tensor imaging study in an adult population. J Neurol Neurosurg Psychiatry. 2008;79:820–822. doi: 10.1136/jnnp.2007.136689. [DOI] [PubMed] [Google Scholar]
- 101.Teixeira AL, Jr, GM, Romano-Silva MA, Cardoso F. Serum from Sydenham’s chorea patients modifies intracellular calcium levels in PC12 cells by a complement-independent mechanism. Mov Disord. 2005;20:843–845. doi: 10.1002/mds.20418. [DOI] [PubMed] [Google Scholar]
- 102.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dunn AJ. Effects of cytokines and infections on brain neurochemistry. Clin Neurosci Res. 2006;6:52–68. doi: 10.1016/j.cnr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wirleitner B, Neurauter G, Schroksnadel K, Frick B, Fuchs D. Interferon-gamma-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Curr Med Chem. 2003;10:1581–1591. doi: 10.2174/0929867033457179. [DOI] [PubMed] [Google Scholar]
- 105.Stone EA, Lehmann ML, Lin Y, Quartermain D. Depressive behaviour in mice due to immune stimulation is accompanied by reduced neural activity in brain regions involved in positively motivated behaviour. Biol Psychiatry. 2006;60:803–811. doi: 10.1016/j.biopsych.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 106.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 107.Goodman WR, McDougle CJ, Price LH, Riddle MA, Pauls DL, Leckman JF. Beyond the serotonin hypothesis: a role for dopamine in some forms of obsessive compulsive disorder? J Clin Psychiatry. 1990;51:36–43. [PubMed] [Google Scholar]
- 108.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 109.Albin RL, Koeppe RA, Bohne A, et al. Increased ventral striatal monoaminergic innervation in Tourette syndrome. Neurology. 2003;61:310–315. doi: 10.1212/01.wnl.0000076181.39162.fc. [DOI] [PubMed] [Google Scholar]
- 110.Singer HS, Szymanski S, Giuliano J, et al. Elevated intrasynaptic dopamine release in Tourette’s syndrome measured by PET. Am J Psychiatry. 2002;159:1329–1336. doi: 10.1176/appi.ajp.159.8.1329. [DOI] [PubMed] [Google Scholar]
- 111.Scahill L, Erenberg G, Berlin CM, Jr, et al. Tourette syndrome association medical advisory board: practice committee. Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx. 2006;3:192–206. doi: 10.1016/j.nurx.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med. 2003;9:914–920. doi: 10.1038/nm892. [DOI] [PubMed] [Google Scholar]
- 113.Bevilacqua LR, Graham ME, Dunkley PR, von Nagy-Felsobuki EI, Dickson PW. Phosphorylation of Ser(19) alters the conformation of tyrosine hydroxylase to increase the rate of phosphorylation of Ser(40) J Biol Chem. 2001;276:40411–40416. doi: 10.1074/jbc.M105280200. [DOI] [PubMed] [Google Scholar]
- 114.Kantor L, Hewlett GH, Gnegy ME. Enhanced amphetamine-and K+-mediated dopamine release in rat striatum after repeated amphetamine: differential requirements for Ca2+and calmodulin-dependent phosphorylation and synaptic vesicles. J Neurosci. 1999;19:3801–3808. doi: 10.1523/JNEUROSCI.19-10-03801.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNFalpha or both. J Neuroimmunol. 2005;169:161–171. doi: 10.1016/j.jneuroim.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 116.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C] raclopride. J Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]