Abstract
To determine if matrix metalloproteinase (MMP)-28 mediates cardiac aging, wild-type (WT) and MMP-28−/− young (7 ± 1 months, n = 9 each) and old (20 ± 2 months, n = 7 each) female mice were evaluated. MMP-28 expression in the left ventricle (LV) increased 42% in old WT mice compared to young controls (p < 0.05). By Doppler echocardiography, LV function declined at 20 ± 2 months of age for both groups. However, dobutamine stress responses were similar, indicating that cardiac reserve was maintained. Plasma proteomic profiling revealed that macrophage inflammatory protein (MIP)-1 α, MIP-1β and MMP-9 plasma levels did not change in WT old mice but were significantly elevated in MMP-28−/− old mice (all p < 0.05), suggestive of a higher inflammatory status when MMP-28 is deleted. RT2-PCR gene array and immunoblotting analyses demonstrated that MIP-1α and MMP-9 gene and protein levels in the LV were also higher in MMP-28−/− old mice (all p < 0.05). Macrophage numbers in the LV increased similarly in WT and MMP-28−/− old mice, compared to respective young controls (both p < 0.05). Collagen content was not different among the WT and MMP-28−/− young and old mice. In conclusion, LV inflammation increases with age, and MMP-28 deletion further elevates inflammation and extracellular matrix responses, without altering macrophage numbers or collagen content.
Keywords: MMP-28, cardiac aging, extracellular matrix, inflammation, macrophage, collagen, left ventricle, mice
Introduction
Cardiac aging involves a progressive deterioration in the performance of the left ventricle (LV), accompanied by a subtle age-related remodeling phenotype that includes cardiac myocyte apoptosis and hypertrophy as well as fibrosis (Lin et al., 2008; Nishimura et al., 2011). Cardiac aging contributes to an elevated cardiovascular morbidity and mortality, as aging is a major risk factor for cardiovascular disease (Lakatta & Levy, 2003; Dai & Rabinovitch, 2009). As the elderly population continues to rise in number, it becomes even more pivotal to dissect the precise mechanisms responsible for cardiac aging to better understand the interplay between aging and cardiac responses to injury. Cardiac fibrosis contributes to ventricular stiffness and dysfunction. Senescent hearts showed enhanced collagen deposition and thicker endomysial and perimysial collagen fibers, as well as increased collagen cross-linking (Thomas et al., 2000; Lin et al., 2008). Active fibrotic remodeling of myocardial interstitium involves excessive activation of matrix metalloproteinases (MMPs), resulting in the development of ventricular dilation and systolic pump failure (Iwanaga et al., 2002; Polyakova et al., 2010).
MMP-28, the newest member of the MMP family, was originally cloned from human keratinocyte and testis cDNA libraries, as well as from lung cDNA in 2001 (Lohi et al., 2001; Marchenko & Strongin, 2001). MMP-28 contains all of the typical MMP domains: an N-terminal signal hydrophobic sequence, a pro-domain, a zinc-binding catalytic domain, a hinge region, and a C-terminal hemopexin-like domain (Illman et al., 2008). In addition, a functional furin activation sequence is located in the C-terminal end of the pro-domain, suggesting that MMP-28 can be intracellularly activated by cleavage of its pro-domain with a furin-like proprotein convertase (Illman et al., 2003; Bassi et al., 2005; Pavlaki et al., 2011). Unlike most other MMPs, MMP-28 is constitutively expressed in multiple normal adult tissues, suggesting roles in tissue homeostasis. Previous evidence has revealed that in response to skin injury, MMP-28 expression is up-regulated at both proximal and distal sites from the wound edge to regulate wound healing (Lohi et al., 2001). MMP-28 also induces Transforming Growth Factor-β (TGF-β) mediated epithelial to mesenchymal transition in lung carcinoma cells (Illman et al., 2006). Manicone and co-workers demonstrated that MMP-28 functions as a negative regulator of macrophage infiltration and restrains early macrophage recruitment in bacteria-induced pneumonia (Manicone et al., 2009).
We hypothesized that MMP-28 could mediate cardiac aging through regulating inflammation and extracellular matrix (ECM) responses. MMP-28 expression in the LV significantly increased with age. LV function was deteriorated, but cardiac reserve was not impaired at the 20 ± 2 month age group in both WT and MMP-28−/− genotypes. Inflammatory markers and MMP-9 levels in the plasma and LV were statistically enhanced at old age with MMP-28 deletion, while macrophage numbers and collagen content in the LV were not affected by MMP-28 deficiency. These observations indicate that MMP-28 deletion augments inflammatory and ECM responses with age.
Materials and Methods
Mice
C57BL/6J WT young (7 ± 1 months, n = 9), old (20 ± 2 months, n = 7), and age-matched MMP-28−/− (n = 9 for young, n = 7 for old) female mice were used in this study. The MMP-28−/− mice were generated as described previously (Manicone et al., 2009). All mice were kept in a light-controlled environment with a 12:12 hour light-dark cycle and free access to standard mouse chow and water, and both the WT and MMP-28−/− colonies were bred in-house and maintained in the same room. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio in accordance with the “Guide for the Care and Use of Laboratory Animals.”
Blood Pressure Measurement
Blood pressure was noninvasively acquired with the MC4000 Blood Pressure Analysis System (Hatteras Instruments, Cary, NC, USA). To ensure accuracy and reproducibility, each mouse was trained for 3–5 days prior to the experiment. Conscious unanesthetized mice were placed on the specimen platform, and their tails were placed through tail cuffs and secured in place with tape. Following a 15 min warm-up period, 5 preliminary cycles were performed to allow the mice to adjust to the inflating cuff. For each mouse, 10 cycles were recorded and averaged.
Doppler Echocardiography
Mitral and aortic blood flow velocities were measured with a Doppler signal processing workstation (Indus Instruments, Webster, TX, USA). Mice were anesthetized with 1–2% isoflurane in a 100% oxygen mix. The 10 MHz Doppler probe was located just under the sternum and angled toward the left ventricular inflow and outflow tracks, respectively (Reddy et al., 2005). At each measurement, the probe position and sample volume depth were adjusted to acquire the correct direction, timing, and maximal velocity of mitral and aortic blood flows. All images were taken at heart rates of 400–500 bpm, as E and A waves fuse at rates >500 bpm and rates <400 bpm are not physiologically relevant. For each mouse, 10 measurements were analyzed and averaged.
Dobutamine Stress Echocardiography
Transthoracic echocardiography was performed using a Visual Sonics Vevo 770 system (VisualSonics, Toronto, Ontario, Canada) with a 30 MHz image transducer. Mice were anesthetized with 1–2% isoflurane in a 100% oxygen mix. Electrocardiogram and heart rate were monitored throughout the imaging procedure. Measurements were taken from the parasternal long axis B- and M-mode views. For each parameter, three images from consecutive cardiac cycles were measured and averaged (Zamilpa et al., 2011). All baseline images were acquired at heart rates >400 bpm to achieve physiologically relevant measurements. Following the acquisition of baseline images, stress echocardiographic measurements were acquired at 30 min after intraperitoneal injection of dobutamine (3 µg/g body weight).
Tissue Harvest
For tissue harvest, mice were anesthetized with 2% isoflurane in a 100% oxygen mix. Five minutes after heparin administration (i.p., 100 USP Units/mouse), the blood was collected from the common carotid artery, centrifuged for collection of plasma, and sent to Rules Based Medicine (Austin, TX, USA) for multianalyte profiling. The heart and vasculature were flushed with cardioplegic solution (NaCl, 69 mM; NaHCO3, 12 mM; glucose, 11 mM; 2,3-butanedione monoxime, 30 mM; EGTA, 10 mM; Nifedipine, 0.001 mM; KCl, 50 mM and Heparin 100 Units were dissolved in 0.9% saline adjusting pH to 7.4 ± 0.5 and volume to 1 L). The hearts were resected and the LV and right ventricle (RV) were separated and weighed individually. The LV was sliced into apex, middle, and base sections. The apex and base section were snap frozen and stored at −80°C for reverse transcriptase–polymerase chain reaction (RT-PCR) and immunoblotting analyses. The middle section was fixed in 10% zinc formalin for histological examination.
Real Time RT2-PCR
RNA extraction was performed using TRIzol® Reagent (Invitrogen 15596; Invitrogen Corp., Carlsbad, CA, USA) according to the manufacturer’s instruction. RNA levels were quantified using the NanoDrop ND-1000 Spectrophotometer (Thermo Scientific, Waltham, MA, USA). Reverse transcription of equal RNA content (1 µg) was performed using the RT2 First Strand Kit (Qiagen 330401; Qiagen, Valencia, CA, USA). Real-time RT2-PCR gene array for inflammatory cytokines and receptors (Qiagen PAMM-011A) and for extracellular matrix and adhesion molecules (Qiagen PAMM-013A) were performed to quantify gene expression levels.
Histology
The middle section of the LV was embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin. Picrosirius red (PSR) staining was performed using the Picrosirius Red Staining Kit (EMS 26357) and collagen content was analyzed by Image-Pro software (MediaCybernetics, Bethesda, MD, USA). Six random scans per section were analyzed and averaged. Immunohistochemistry was conducted using the Vectastain ABC Kit (Vector Laboratories, Marion, IA, USA). HistoMark Black (KPL 54-75-00) was used to visualize positive staining, with eosin as a counterstain. An antibody specific for macrophages (Mac-3, Cedarlane CL8943AP; 1:100) was used to stain macrophages. Quantification was calculated as the numbers of positive cells per 40× high magnification field. Eight random scans per section were analyzed and averaged.
Protein Extraction
Total protein was extracted by homogenizing the samples in protein extraction reagent type 4 (Sigma-Aldrich, Milwaukee, WI, USA; 7 M urea, 2 M thiourea, 40 mM Trizma® base and the detergent 1% C7BzO) and 1× protease inhibitor cocktail (Roche). Protein concentrations were determined by the Quick Start™ Bradford Protein Assay (Bio-Rad, Hercules, CA, USA).
Immunoblotting
Protein expression levels were quantified by western blot using the following antibodies: MMP-28 (Sigma, M5066) and MMP-9 (Abcam, ab38898). Total protein (10 µg) for all samples was separated on 4–12% Criterion™ XT Bis-Tris gels (Bio-Rad), transferred to nitrocellulose membrane (Bio-Rad), and stained with MemCode™ Reversible Protein Stain Kit (Thermo Scientific) to verify protein concentration and loading accuracy. After blocking with 5% nonfat milk (Bio-Rad), the membrane was incubated with primary antibody (1:1000), secondary antibody (Vector Laboratories, PI-1000, 1:5000), and detected with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific). Molecular Imaging Software (Kodak, Rochester, NY, USA) was used to measure densitometry. The relative expression for each immunoblot was calculated as the densitometry of the protein of interest divided by the densitometry of the entire lane of the total protein stained membrane.
Statistical Analyses
Data are presented as mean ± SEM. Two group comparisons were analyzed by unpaired t-test. Multiple group comparisons were analyzed using one-way ANOVA, followed by the Student Newman-Keuls when the Bartlett’s variation test was passed, or using the nonparametric Kruskal-Wallis test, followed by Dunn post-hoc test when the Bartlett’s variation test did not pass. A value of p < 0.05 was considered statistically significant.
Results
MMP-28 Protein Levels in the LV Increased with Age
MMP-28 is expressed in many normal adult tissues, including the heart (Lohi et al., 2001). Our findings revealed that MMP-28 protein expression increased 42% in WT old mice compared to young controls (Fig. 1; p < 0.05).
Figure 1.
MMP-28 protein expression in the LV increased with age. MMP-28 levels were up-regulated in WT old mice, compared to WT young mice. All values are mean ± SEM. n = 6/group. *p < 0.05 versus WT young. The data were analyzed by unpaired t-test.
Blood Pressure and LV Weight
We measured systolic and diastolic blood pressure noninvasively in WT and MMP-28−/− mice. The old mice in both genotypes showed no differences in blood pressure, compared to young controls, suggesting that hypertension did not occur at this old age time point. As shown in Table 1, old WT and MMP-28−/− mice weighed significantly more than respective young mice (both p < 0.05). LV mass statistically increased in WT and MMP-28−/− old mice, compared with respective young mice (both p < 0.05). In contrast, the LV to tibia length ratio in old mice of both genotypes was markedly increased, compared to respective young controls (both p < 0.05), indicating LV hypertrophy with age.
Table 1.
Blood Pressure, Echocardiography, and Necropsy Parameters.
| WT | MMP-28−/− | |||
|---|---|---|---|---|
| Young (n = 9) |
Old (n = 7) |
Young (n = 9) |
Old (n = 7) |
|
| Blood pressure | ||||
| Heart rate (beats/min)a | 715 ± 10 | 726 ± 10 | 713 ± 13 | 735 ± 6 |
| Systolic BP (mmHg)b | 108 ± 3 | 106 ± 3 | 104 ± 1 | 102 ± 1 |
| Diastolic BP (mmHg)b | 92 ± 3 | 96 ± 3 | 90 ± 1 | 90 ± 1 |
| Cardiac Doppler indices | ||||
| Heart rate (beats/min)a | 457 ± 11 | 428 ± 4 | 444 ± 7 | 442 ± 9 |
| Mean aortic flow velocity (cm/s)a | 27 ± 1 | 22 ± 1 | 28 ± 2 | 21 ± 2* |
| Peak E-flow velocity (cm/s)b | 77 ± 2 | 64 ± 3* | 74 ± 2 | 61 ± 2* |
| Peak A-flow velocity (cm/s)a | 53 ± 2 | 49 ± 2 | 51 ± 2 | 44 ± 2* |
| E/A ratiob | 1.46 ± 0.04 | 1.31 ± 0.05 | 1.47 ± 0.05 | 1.40 ± 0.07 |
| Baseline echocardiography | ||||
| Heart rate (beats/min)a | 461 ± 8 | 449 ± 4 | 456 ± 8 | 459 ± 6 |
| End diastolic dimension (mm)a | 3.22 ± 0.08 | 3.34 ± 0.09 | 3.49 ± 0.07 | 3.53 ± 0.08 |
| End systolic dimension (mm)a | 1.91 ± 0.09 | 2.08 ± 0.14 | 2.13 ± 0.08 | 2.16 ± 0.08 |
| Fractional shortening (%)a | 41 ± 1 | 38 ± 3 | 39 ± 1 | 38 ± 2 |
| Stress echocardiography | ||||
| Heart rate (beats/min)a | 530 ± 9† | 526 ± 8† | 523 ± 7† | 560 ± 8† |
| End diastolic dimension (mm)a | 2.99 ± 0.06 | 2.99 ± 0.10† | 3.32 ± 0.07 | 3.29 ± 0.11 |
| End systolic dimension (mm)a | 1.49 ± 0.08† | 1.32 ± 0.07† | 1.63 ± 0.09† | 1.54 ± 0.08† |
| Fractional shortening (%)a | 50 ± 2† | 55 ± 1† | 51 ± 2† | 50 ± 3† |
| Necropsy | ||||
| Body weight (g)a | 22 ± 1 | 26 ± 1* | 22 ± 1 | 28 ± 1* |
| LV weight (mg)a | 71 ± 3 | 81 ± 3* | 66 ± 2 | 82 ± 3* |
| RV weight (mg)b | 16 ± 1 | 19 ± 1 | 17 ± 1 | 19 ± 1 |
| LV/tibia length (mg/mm)a | 4.1 ± 0.2 | 4.5 ± 0.1* | 3.9 ± 0.1 | 4.7 ± 0.2* |
Note that blood pressure heart rates are unanesthetized values, while echocardiography heart rates are acquired under 1–2% isoflurane. Stress echocardiographic parameters were acquired 30 min after dobutamine injection. BP = blood pressure. LV = left ventricle. RV = right ventricle. All values are mean ± SEM.
p < 0.05 versus respective young control;
p < 0.05 versus baseline value.
Analyzed by ANOVA.
Analyzed by Kruskal-Wallis test.
Baseline LV Function
We studied systolic and diastolic function with pulsed Doppler and two-dimensional echocardiography in WT and MMP-28−/− mice (Table 1). Although MMP-28 is constitutively expressed in normal adult LV, MMP-28−/− mice showed no overt cardiac dysfunction. Mean aortic flow velocity, a systolic function index, was significantly decreased in MMP-28−/− old mice (p < 0.05), but not in WT old mice, indicating systolic dysfunction may start earlier in the MMP-28−/− mice. Peak E-flow velocity declined in the old mice of both genotypes, compared to young controls (both p < 0.05). The marked decline in a LV diastolic index peak A-flow velocity, however, only appeared in MMP-28−/− old mice (p < 0.05 versus young controls), suggesting diastolic dysfunction may occur earlier in the absence of MMP-28 (Angomachalelis et al., 1994). However, there was no significant difference in baseline LV function in the old mice of both genotypes.
Response to Dobutamine Stress
Heart rate and fractional shortening increased significantly at 30 min after dobutamine injection, indicating that all four groups responded to dobutamine. LV end systolic and end diastolic dimensions decreased significantly after dobutamine administration, in the WT and MMP-28−/− young and old mice (Table 1). The dobutamine stress response was similar among the four groups, indicating that cardiac reserve was not impaired at the old age time point or with MMP-28 deletion.
MMP-28 Deletion Increased Inflammatory Markers in the Plasma
To determine the role of MMP-28 in cardiac aging, we performed multianalyte proteomic profiling on plasma collected from the four groups. As shown in Table 2, 20 analytes out of 59 showed statistical difference between WT and MMP-28−/− groups (Table 2). Of these, 8 analytes showed statistical difference between WT and MMP-28−/− young mice. These analytes were apolipoprotein A-1, macrophage-derived chemokine, myeloperoxidase, serum amyloid P-component, stem cell factor, tissue factor, vascular cell adhesion molecule (VCAM)-1, and vascular endothelial growth factor (VEGF)-A. In addition, 12 analyte levels were increased with age. The analytes that were increased in both WT and MMP-28−/− old mice were fibrinogen, immunoglobulin A, serum amyloid P-component, and VCAM-1. The analytes that were only increased in MMP-28−/− old mice were CD40 ligand, macrophage inflammatory protein (MIP)-1α, MIP-1β, MMP-9, stem cell factor, tissue inhibitor of metalloproteinase (TIMP)-1, VEGF-A, and von Willbrand factor (vWF). Interestingly, MIP-1α, MIP-1β, MMP-9, stem cell factor, VEGF-A, and vWF were increased to a greater extent in the MMP-28−/− old mice, compared with age-matched WT (all p < 0.05). The above results demonstrated that inflammation increased with age and MMP-28 deficiency further elevated the inflammatory status.
Table 2.
Multianalyte Proteomic Profiling in Plasma Reveals Aging and MMP-28 Effects.
| WT | MMP-28−/− | |||
|---|---|---|---|---|
| Analytes | Young (n = 9) |
Old (n = 7) |
Young (n = 9) |
Old (n = 7) |
| Apolipoprotein A-I (µg/mL)a | 29 ± 1 | 28 ± 1 | 25 ± 1*§ | 24 ± 1† |
| CD40 ligand (pg/mL)b,c | 3,480 ± 310 | 3,620 ± 112 | 2,519 ± 177 | 4,310 ± 423* |
| Endothelin-1 (pg/mL)a,c | 49 ± 9 | 26 ± 5* | 32 ± 3 | 39 ± 3 |
| Fibrinogen (mg/mL)a | 48 ± 3 | 69 ± 5* | 41 ± 3 | 58 ± 5*† |
| Immunoglobulin A (µg/mL)b,c | 43 ± 5 | 90 ± 11* | 75 ± 9 | 141 ± 31 |
| Leukemia inhibitory factor (pg/mL)b,c | 826 ± 85 | 684 ± 37 | 979 ± 98 | 1165 ± 80† |
| Macrophage inflammatory protein-1α (ng/mL)a,c | 2.83 ± 0.32 | 2.82 ± 0.22 | 3.28 ± 0.24 | 4.25 ± 0.27*† |
| Macrophage inflammatory protein-1β (pg/mL)a,c | 90 ± 10 | 69 ± 14 | 78 ± 13 | 146 ± 12*† |
| Macrophage inflammatory protein-2 (pg/mL)a,c | 6.74 ± 1.05 | 3.26 ± 0.30 | 6.56 ± 1.73 | 8.84 ± 1.51† |
| Macrophage inflammatory protein-3β (ng/mL)a,c | 1.37 ± 0.17 | 1.57 ± 0.11 | 1.73 ± 0.13 | 2.10 ± 0.12† |
| Macrophage-derived chemokine (pg/mL)a,c | 1,640 ± 237 | 1,782 ± 227 | 2,763 ± 265*§ | 2,914 ± 189† |
| Matrix metalloproteinase-9 (ng/mL)a,c | 72 ± 4 | 82 ± 6 | 66 ± 5 | 108 ± 7*† |
| Myeloperoxidase (ng/mL)b,c | 44 ± 3 | 78 ± 6 | 81 ± 6*§ | 125 ± 11 |
| Serum amyloid P-component (µg/mL)a,c | 19 ± 1 | 28 ± 1* | 11 ± 2*§ | 19 ± 3*† |
| Stem cell factor (pg/mL)a | 402 ± 14 | 330 ± 18* | 307 ± 13*§ | 409 ± 20*† |
| Tissue factor (ng/mL)a | 5.47 ± 0.46 | 4.82 ± 0.53 | 8.53 ± 1.22*§ | 9.82 ± 0.64† |
| Tissue inhibitor of metalloproteinase-1 (ng/mL)b,c | 0.69 ± 0.04 | 0.82 ± 0.09 | 0.59 ± 0.08 | 0.92 ± 0.09* |
| Vascular cell adhesion molecule-1 (ng/mL)a | 1,211 ± 60 | 1,475 ± 59* | 1,023 ± 37*§ | 1,424 ± 67* |
| Vascular endothelial growth factor-A (pg/mL)a | 333 ± 33 | 202 ± 18* | 185 ± 17*§ | 343 ± 44*† |
| Von Willebrand factor (ng/mL)a | 187 ± 32 | 259 ± 40 | 198 ± 20 | 449 ± 46*† |
Of 59 analytes measured, the 20 listed were statistically different among the groups. All values are mean ± SEM.
p < 0.05 versus respective young control;
p < 0.05 versus WT young;
p < 0.05 versus WT old.
Analyzed by ANOVA.
Analyzed by Kruskal-Wallis test.
Inflammation-associated protein.
MMP-28 Deletion Elevated the Inflammatory Response in LV with Age
To investigate the LV inflammatory status, we conducted Real-Time RT2-PCR gene array analysis for inflammatory cytokines and receptors. Out of 84 genes evaluated, 16 listed in the table showed significant difference among these groups (Table 3). Expression of six genes was increased in the MMP-28−/− young LV compared to WT young LV (all p < 0.05). These included Abcf1, Ccr7, Il10rb, Mif, Scye1, and Tollip. C3 mRNA expression similarly increased in WT and MMP-28−/− old mice, compared with respective young controls (both p < 0.05). Gene expression levels of Ccl3 (MIP-1α), Ccl11, and Cx3cl1 increased statistically more in MMP-28−/− old mice, compared with WT old mice (all p < 0.05). In contrast, Il13ra1 and Itgam mRNA levels were significantly lower in MMP-28−/− old mice, compared with age-matched WT (both p < 0.05).
Table 3.
Inflammatory Array Analysis Uncovers Differences in Inflammation with Aging and MMP-28 Deletion.
| WT | MMP-28−/− | |||
|---|---|---|---|---|
| Genes | Young (n = 3) |
Old (n = 3) |
Young (n = 9) |
Old (n = 7) |
| Abcf1a | 0.163 ± 0.001 | 0.152 ± 0.007 | 0.248 ± 0.009*§ | 0.229 ± 0.019† |
| C3a | 0.174 ± 0.009 | 0.309 ± 0.035* | 0.114 ± 0.013 | 0.236 ± 0.027* |
| Ccl3a | 0.0010 ± 0.0001 | 0.0009 ± 0.0002 | 0.0007 ± 0.0002 | 0.0023 ± 0.0005*† |
| Ccl8b | 0.009 ± 0.001 | 0.047 ± 0.019 | 0.009 ± 0.002 | 0.054 ± 0.019* |
| Ccl11a | 0.008 ± 0.001 | 0.011 ± 0.002 | 0.010 ± 0.001 | 0.017 ± 0.002*† |
| Ccl25b | 0.0024 ± 0.0002 | 0.0020 ± 0.0002 | 0.0034 ± 0.0006 | 0.0049 ± 0.0013† |
| Ccr7b | 0.00017 ± 0.00002 | 0.00019 ± 0.00003 | 0.00225 ± 0.00106*§ | 0.00335 ± 0.00221 |
| Cx3cl1a | 0.0347 ± 0.0024 | 0.0289 ± 0.0032 | 0.0395 ± 0.0033 | 0.0587 ± 0.0068*† |
| Cxcl13b | 0.0018 ± 0.0002 | 0.0060 ± 0.0024 | 0.0017 ± 0.0008 | 0.0069 ± 0.0027* |
| Il10rba | 0.284 ± 0.024 | 0.317 ± 0.015 | 0.424 ± 0.020*§ | 0.364 ± 0.034 |
| Il13ra1a | 0.079 ± 0.008 | 0.096 ± 0.004 | 0.065 ± 0.004 | 0.065 ± 0.007† |
| Itgama | 0.014 ± 0.001 | 0.020 ± 0.002 | 0.009 ± 0.001 | 0.012 ± 0.002† |
| Mifa | 0.370 ± 0.007 | 0.337 ± 0.006 | 0.919 ± 0.068*§ | 0.909 ± 0.098† |
| Scye1a | 0.311 ± 0.022 | 0.277 ± 0.008 | 0.428 ± 0.019*§ | 0.426 ± 0.031† |
| Tnfrsf1aa | 0.033 ± 0.001 | 0.031 ± 0.002 | 0.046 ± 0.004 | 0.055 ± 0.006† |
| Tollipa | 0.076 ± 0.005 | 0.069 ± 0.005 | 0.114 ± 0.007*§ | 0.092 ± 0.009 |
Of 84 genes analyzed, the 16 genes shown were statistically different among the groups. Gene levels are reported as 2−ΔCT units. Ccl3 = macrophage inflammatory protein-1α. All values are mean ± SEM.
p < 0.05 versus respective young control;
p < 0.05 versus WT young;
p < 0.05 versus WT old.
Analyzed by ANOVA.
Analyzed by Kruskal-Wallis test.
MMP-28 Deletion Elevated MMP-9 mRNA Expression in the LV with Age
To evaluate how the change in inflammation could influence the cardiac ECM status, we performed Real-Time RT2-PCR gene array for ECM and adhesion molecules. Out of 84 ECM related genes examined, 17 shown in the table unexpectedly revealed significant differences in expression levels between WT and in MMP-28−/− young mice (Table 4). The mRNA expression of Col1a1, Col3a1, Col4a1, Col6a1, and Sparc were reduced and Postn was increased in the WT LV of old mice (all p < 0.05), compared to young controls. The mRNA levels of Lamb2 and Vtn were decreased in MMP-28−/− old mice compared to young mice (both p < 0.05). Interestingly, MMP-9 mRNA expression increased to a significantly greater extent in MMP-28−/− old mice than in age-matched old WT mice (p < 0.05), which was consistent with the plasma data.
Table 4.
Extracellular Matrix and Adhesion Molecule Array Analysis Uncovers ECM Differences with Aging and MMP-28 Deletion.
| WT | MMP-28−/− | |||
|---|---|---|---|---|
| Genes | Young (n = 3) |
Old (n = 3) |
Young (n = 9) |
Old (n = 7) |
| Ctnna1a | 1.07 ± 0.10 | 0.97 ± 0.03 | 1.58 ± 0.11*§ | 1.41 ± 0.06† |
| Ctnnb1a | 0.62 ± 0.04 | 0.60 ± 0.02 | 0.46 ± 0.02*§ | 0.40 ± 0.02† |
| Cdh4b | 0.008 ± 0.0004 | 0.012 ± 0.0005 | 0.003 ± 0.0004*§ | 0.006 ± 0.0007 |
| Col1a1a | 0.156 ± 0.003 | 0.095 ± 0.007* | 0.044 ± 0.003*§ | 0.042 ± 0.005† |
| Col3a1a | 0.88 ± 0.05 | 0.47 ± 0.07* | 0.44 ± 0.03*§ | 0.40 ± 0.04 |
| Col4a1a | 0.535 ± 0.051 | 0.335 ± 0.003* | 0.626 ± 0.046 | 0.568 ± 0.044† |
| Col6a1a | 0.124 ± 0.012 | 0.084 ± 0.004* | 0.139 ± 0.006 | 0.139 ± 0.007† |
| Vcana | 0.051 ± 0.004 | 0.043 ± 0.004 | 0.012 ± 0.001*§ | 0.016 ± 0.002† |
| Fn1a | 0.058 ± 0.004 | 0.051 ± 0.007 | 0.029 ± 0.003*§ | 0.028 ± 0.002† |
| Itgavb | 0.089 ± 0.006 | 0.089 ± 0.002 | 0.046 ± 0.005*§ | 0.050 ± 0.004 |
| Itgb1a | 0.86 ± 0.08 | 0.94 ± 0.04 | 0.63 ± 0.04*§ | 0.62 ± 0.04† |
| Lama2a | 0.086 ± 0.010 | 0.078 ± 0.009 | 0.115 ± 0.006 | 0.113 ± 0.007† |
| Lamb2a | 0.26 ± 0.02 | 0.22 ± 0.02 | 0.52 ± 0.04*§ | 0.41 ± 0.03*† |
| Mmp9b | 0.00176 ± 0.00006 | 0.00262 ± 0.00060 | 0.00214 ± 0.00041 | 0.00746 ± 0.00119*† |
| Mmp11b | 0.00036 ± 0.00003 | 0.00043 ± 0.0008 | 0.00177 ± 0.00053§ | 0.00107 ± 0.00011 |
| Mmp15a | 0.164 ± 0.002 | 0.146 ± 0.014 | 0.101 ± 0.010*§ | 0.086 ± 0.006† |
| Postna | 0.056 ± 0.003 | 0.074 ± 0.001* | 0.015 ± 0.001*§ | 0.022 ± 0.002*† |
| Seleb | 0.0090 ± 0.0008 | 0.0084 ± 0.0011 | 0.0033 ± 0.0005*§ | 0.0061 ± 0.0024 |
| Sparca | 0.597 ± 0.007 | 0.391 ± 0.013* | 0.342 ± 0.016*§ | 0.360 ± 0.008 |
| Spp1b | 0.0117 ± 0.0013 | 0.0055 ± 0.0018 | 0.0018 ± 0.0008*§ | 0.0037 ± 0.0019 |
| Thbs2a | 0.068 ± 0.007 | 0.075 ± 0.003 | 0.048 ± 0.004*§ | 0.056 ± 0.004† |
| Timp1b | 0.000054 ± 0.000004 | 0.000047 ± 0.000001 | 0.000314 ± 0.000204 | 0.000503 ± 0.000370† |
| Vtna | 0.138 ± 0.004 | 0.117 ± 0.014 | 0.118 ± 0.009 | 0.083 ± 0.007*† |
Of 84 genes analyzed, the 16 genes shown were statistically different among the groups. Gene levels are reported as 2−ΔCT units. All values are mean ± SEM.
p < 0.05 versus respective young control;
p < 0.05 versus WT young;
p < 0.05 versus WT old.
Analyzed by ANOVA.
Analyzed by Kruskal-Wallis test.
MMP-28 Deletion Increased MMP-9 Protein Levels in the LV with Age
The findings shown above revealed that MMP-9 gene expression in the LV and protein levels in the plasma dramatically increased in MMP-28−/− old mice compared to age-matched WT and MMP-28−/− young mice. To determine if MMP-9 protein expression was increased in the LV, we performed immunoblotting to quantify MMP-9 levels (Fig. 2). MMP-9 expression in the LV did not change in WT old mice, compared to WT young. However, MMP-9 levels were 58% and 79% significantly enhanced in MMP-28−/− old mice, compared to MMP-28−/− young controls and WT old mice, respectively (both p < 0.05).
Figure 2.
MMP-28 deficiency increased LV MMP-9 expression with aging. MMP-9 protein levels in WT old mice did not change, compared to WT young levels. In contrast, MMP-9 levels significantly increased in MMP-28−/− old mice, compared with both young controls and WT old mice. All values are mean ± SEM. n = 6/group. *p < 0.05 versus MMP-28−/− young; †p < 0.05 versus WT old. The data were analyzed by ANOVA.
MMP-28 Deletion Did Not Affect LV Macrophage Numbers
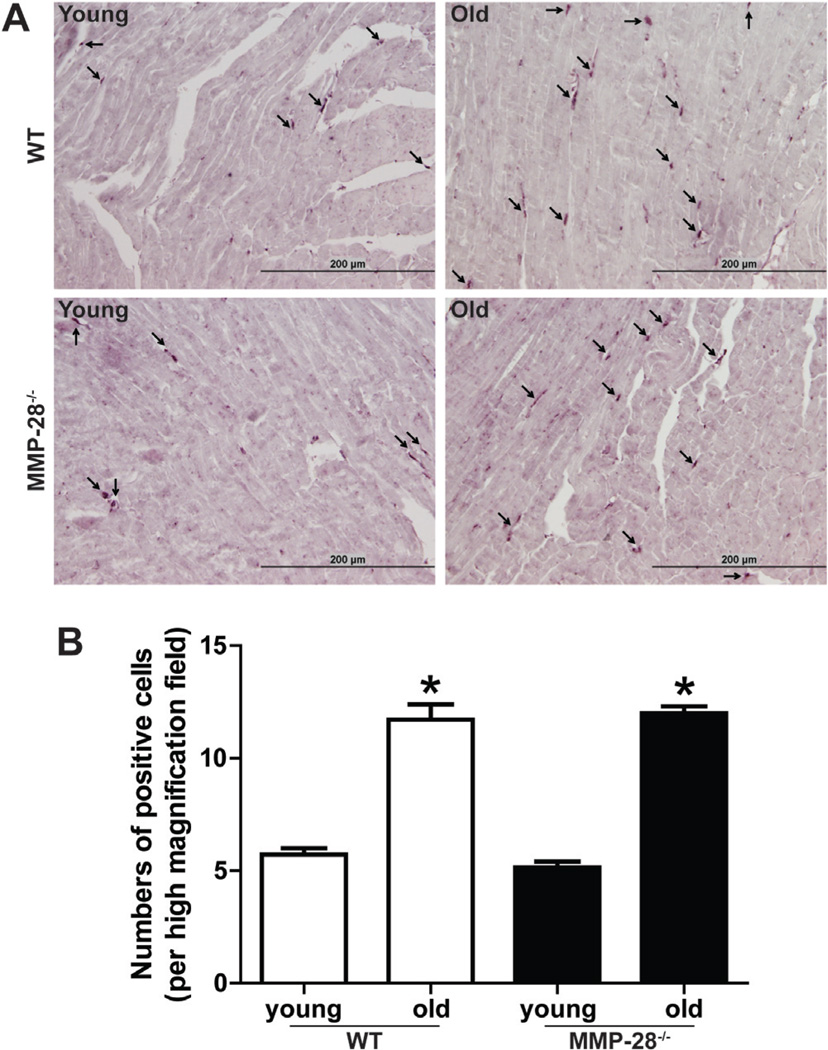
To test if the increased inflammatory markers and MMP-9 is due to increased macrophage infiltration, we stained the macrophages by immunohistochemistry. The results demonstrated that macrophage numbers in the LV increased in both WT and MMP-28−/− old mice, compared to respective young mice (Fig. 3, both p < 0.05). However, there was no statistical difference in macrophage counts between WT and MMP-28−/− old mice. MMP-28 deletion, therefore, did not alter LV macrophage numbers.
Figure 3.
MMP-28 deletion did not affect LV macrophage numbers. Immunohistochemical staining for mac-3 revealed that LV macrophage numbers increased with age similarly in both WT and MMP-28−/− mice. A: Representative images showed the positively stained macrophages (black staining). Scale = 200 µm. B: Quantitative analysis of positive cell numbers per high magnification field (magnification 400×). All values are mean ± SEM. n = 7/group. *p < 0.05 versus respective young control. The data were analyzed by ANOVA.
Collagen Content in the LV Did Not Change at This Old Age or with MMP-28 Deletion
To investigate the effect of MMP-28 deficiency on cardiac fibrosis with age, we quantified collagen content by PSR staining. The findings showed that there were similar collagen levels between WT and MMP-28−/− young and old mice (Fig. 4). Thus, collagen content in the LV did not increase at this old age, and MMP-28 deficiency did not have a marked effect on cardiac fibrosis.
Figure 4.
Total collagen content did not change between young and old mice. Total collagen quantity assessed by PSR staining was not different among the four groups. A: Representative images of PSR staining. B: Quantitative analysis of the ratio of collagen area to total area (magnification 200×). All values are mean ± SEM. n = 5/group. The data were analyzed by ANOVA.
Discussion
The goal of this study was to evaluate the effects of MMP-28 deletion on cardiac aging. The most significant findings were: (1) LV function deteriorated in both WT and MMP-28−/− old mice, but cardiac reserve was not impaired at the age points investigated; (2) plasma inflammatory proteins were significantly elevated with age, which was exacerbated in the absence of MMP-28; and (3) MIP-1α and MMP-9 showed increased gene and protein levels in the LV of old MMP-28−/− mice compared to young null and old WT controls. Combined, these results indicate that MMP-28 serves an important immune homeostatic function in the context of the aging myocardium.
Although MMP-28 is constitutively expressed in adult hearts, MMP-28−/− mice have no overt cardiac phenotype in the unstressed setting. Manicone and colleagues have demonstrated that MMP-28 functions in innate immunity and a phenotype would not be displayed until the mice are treated with an inflammatory or injury stimulus (Manicone et al., 2009). In this study, we observed that aging up-regulated LV MMP-28 protein expression. Studies have shown that MMP-28 expression was up-regulated in ulcerated pyogenic granulomas, suction blisters, hypertrophic scars, dermal wound repair, osteoarthritis, and tumors (Lohi et al., 2001; Saarialho-Kere et al., 2002; Kevorkian et al., 2004; Overall et al., 2004; Reno et al., 2005; Lin et al., 2006). In contrast, there are also several reports on the downregulation of this enzyme in malignant cells and after infection (Bister et al., 2004; Manicone et al., 2009). This discrepancy in the expression pattern of MMP-28 in a variety of diseases may be explained by the differential roles of MMP-28 in different diseases and different stages of the same disorder (Illman et al., 2008).
Cardiac aging involves the decline of LV function and the development of cardiac fibrosis. In this study, we observed that mean aortic flow velocity, a contractile index, and peak A-flow velocity, a diastolic parameter, declined in the MMP28−/− old mice but not in the WT old mice, possibly indicative of an earlier deterioration of LV function with MMP-28 deletion. The fact that there were similar magnitudes of response to dobutamine stress in WT old and MMP28−/− old mice suggests that at 20 ± 2 months age, cardiac reserve is not impaired. Reddy and co-workers demonstrated that 30 month old C57BL/6J WT mice had no change in fractional shortening versus young mice (Godfrey et al., 1993; Reddy et al., 2007). While the mice in the Reddy et al. study were older than the mice in our study, their study indirectly confirms our echocardiography results. Massive collagen deposition leads to cardiac fibrosis, which brings about LV wall stiffness and diastolic dysfunction (Chen & Frangogiannis, 2010). In this study, we did not observe significant difference in collagen content in the four groups of mice, indicating that the structural change in old hearts may not have yet reached statistical significance.
MIP-1α, a member of the chemokine superfamily, is expressed in ischemic myocardium and regulates monocyte and lymphocyte recruitment, which is involved in cardiac repair and remodeling post-myocardial infarction (Frangogiannis, 2004; de Jager et al., 2008). MMP-9, also known as gelatinase B, degrades multiple extracellular substrates and cleaves non-ECM cytokines and growth factors to regulate multiple cell functions (Lindsey, 2004, 2006). MMP-9 has been shown to be involved in many cardiovascular diseases, including atherosclerosis, myocardial infarction, heart failure, and hypertension (Lindsey et al., 2003, 2006; Onal et al., 2009). In this study, we showed that the levels of MIP-1α and MMP-9 in the plasma and LV were significantly elevated with MMP-28 deficiency. Since MMPs can cleave cytokines to increase or decrease activity, our results raise the possibility that MIP-1α and MMP-9 may be potential substrates of MMP-28 or may be indirectly regulated by MMP-28. We selected to focus on MIP-1α and MMP-9 for two reasons. First, MIP-1α and MMP-9 have been demonstrated to be involved in cardiac repair and remodeling post-injury (Spinale et al., 2000; Goser et al., 2005). Second, MIP-1α and MMP-9 had similar levels in young mice of WT and MMP-28 null in this study, indicating that MMP-28 deletion did not alter levels of these two proteins during development. Levels of MIP-1α and MMP-9 were significantly higher in MMP-28−/− old mice compared with age-matched WT, suggesting a role of MMP-28 in regulating these proteins in the setting of the aging myocardium.
Macrophages are a major cellular source of MIP-1α and MMP-9 (Kasama et al., 2006; Reynolds et al., 2011). We found that macrophage numbers in the LV increased with age; but interestingly, MMP-28 deficiency did not affect macrophage numbers with age. Thus, increased MIP-1α and MMP-9 could not be attributed to a more robust macrophage infiltration. We could not exclude the possibility that MMP-28 deletion may regulate macrophage function to increase its secretory capacity. Manicone and colleagues have shown that macrophages derived from MMP-28−/− mice migrated faster than did WT cells, lending support to this idea (Manicone et al., 2009).
In conclusion, MMP-28 expression levels in the LV increased with age. MMP-28 deletion may accelerate the decline of LV function with age. The inflammatory status within the myocardium was enhanced with aging and MMP-28 deficiency further amplifies inflammatory and extracellular matrix responses in cardiac aging, without affecting macrophage numbers within LV. Together, our results demonstrate an important role for MMP-28 in maintaining cardiac homeostasis with age.
Acknowledgments
We acknowledge support from NHLBI HHSN 268201000036C (N01-HV-00244) for the UTHSCSA Cardiovascular Proteomics Center and R01 HL-075360, the Max and Minnie Tomerlin Voelcker Fund, Health Resources and Services Administration, and the Veteran’s Administration (Merit) to M.L.L.; from NSF 0649172, NIH EB009496, and NIH 1SC2 HL101430 to Y.-F.J., and from NHLBI HL084385 and HHMI Physician-Scientist Early Career Award to A.M.M.
References
- Angomachalelis N, Hourzamanis A, Vakalis D, Vamvalis C, Serasli E, Siourthas D. Acoustic quantification: New diastolic indices of left ventricular function in hypertension correlation with Doppler echocardiography. Postgrad Med J. 1994;70(S1):S57–S66. [PubMed] [Google Scholar]
- Bassi DE, Fu J, Lopez de Cicco R, Klein-Szanto AJ. Proprotein convertases: “Master switches” in the regulation of tumor growth and progression. Mol Carcinog. 2005;44(3):151–161. doi: 10.1002/mc.20134. [DOI] [PubMed] [Google Scholar]
- Bister VO, Salmela MT, Karjalainen-Lindsberg ML, Uria J, Lohi J, Puolakkainen P, Lopez-Otin C, Saarialho-Kere U. Differential expression of three matrix metalloproteinases, MMP-19, MMP-26, and MMP-28, in normal and inflamed intestine and colon cancer. Dig Dis Sci. 2004;49(4):653–661. doi: 10.1023/b:ddas.0000026314.12474.17. [DOI] [PubMed] [Google Scholar]
- Chen W, Frangogiannis NG. The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Fail Rev. 2010;15(5):415–422. doi: 10.1007/s10741-010-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Rabinovitch PS. Cardiac aging in mice and humans: The role of mitochondrial oxidative stress. Trends Cardiovasc Med. 2009;19(7):213–220. doi: 10.1016/j.tcm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jager SC, Kraaijeveld AO, Grauss RW, de Jager W, Liem SS, van der Hoeven BL, Prakken BJ, Putter H, van Berkel TJ, Atsma DE, Schalij MJ, Jukema JW, Biessen EA. CCL3 (MIP-1 alpha) levels are elevated during acute coronary syndromes and show strong prognostic power for future ischemic events. J Mol Cell Cardiol. 2008;45(3):446–452. doi: 10.1016/j.yjmcc.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG. Chemokines in the ischemic myocardium: From inflammation to fibrosis. Inflamm Res. 2004;53(11):585–595. doi: 10.1007/s00011-004-1298-5. [DOI] [PubMed] [Google Scholar]
- Godfrey P, Rahal JO, Beamer WG, Copeland NG, Jenkins NA, Mayo KE. GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat Genet. 1993;4(3):227–232. doi: 10.1038/ng0793-227. [DOI] [PubMed] [Google Scholar]
- Goser S, Ottl R, Brodner A, Dengler TJ, Torzewski J, Egashira K, Rose NR, Katus HA, Kaya Z. Critical role for monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha in induction of experimental autoimmune myocarditis and effective anti-monocyte chemoattractant protein-1 gene therapy. Circulation. 2005;112(22):3400–3407. doi: 10.1161/CIRCULATIONAHA.105.572396. [DOI] [PubMed] [Google Scholar]
- Illman SA, Keski-Oja J, Parks WC, Lohi J. The mouse matrix metalloproteinase, epilysin (MMP-28), is alternatively spliced and processed by a furin-like proprotein convertase. Biochem J. 2003;375(Pt 1):191–197. doi: 10.1042/BJ20030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illman SA, Lehti K, Keski-Oja J, Lohi J. Epilysin (MMP-28) induces TGF-beta mediated epithelial to mesenchymal transition in lung carcinoma cells. J Cell Sci. 2006;119(Pt 18):3856–3865. doi: 10.1242/jcs.03157. [DOI] [PubMed] [Google Scholar]
- Illman SA, Lohi J, Keski-Oja J. Epilysin (MMP-28)— Structure, expression and potential functions. Exp Dermatol. 2008;17(11):897–907. doi: 10.1111/j.1600-0625.2008.00782.x. [DOI] [PubMed] [Google Scholar]
- Iwanaga Y, Aoyama T, Kihara Y, Onozawa Y, Yoneda T, Sasayama S. Excessive activation of matrix metalloproteinases coincides with left ventricular remodeling during transition from hypertrophy to heart failure in hypertensive rats. J Am Coll Cardiol. 2002;39(8):1384–1391. doi: 10.1016/s0735-1097(02)01756-4. [DOI] [PubMed] [Google Scholar]
- Kasama T, Yajima N, Matsukura S, Adachi M. Macrophage inflammatory protein 1 and CCR5 as attractive therapeutic targets for HIV infection. Recent Pat Antiinfect Drug Discov. 2006;1(3):275–280. doi: 10.2174/157489106778777655. [DOI] [PubMed] [Google Scholar]
- Kevorkian L, Young DA, Darrah C, Donell ST, Shep-stone L, Porter S, Brockbank SM, Edwards DR, Parker AE, Clark IM. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50(1):131–141. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lin J, Lopez EF, Jin Y, Van Remmen H, Bauch T, Han HC, Lindsey ML. Age-related cardiac muscle sarcopenia: Combining experimental and mathematical modeling to identify mechanisms. Exp Gerontol. 2008;43(4):296–306. doi: 10.1016/j.exger.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MH, Liu SY, Su HJ, Liu YC. Functional role of matrix metalloproteinase-28 in the oral squamous cell carcinoma. Oral Oncol. 2006;42(9):907–913. doi: 10.1016/j.oraloncology.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Lindsey ML. MMP induction and inhibition in myocardial infarction. Heart Fail Rev. 2004;9(1):7–19. doi: 10.1023/B:HREV.0000011390.44039.b7. [DOI] [PubMed] [Google Scholar]
- Lindsey ML. Novel strategies to delineate matrix metalloproteinase (MMP)-substrate relationships and identify targets to block MMP activity. Mini Rev Med Chem. 2006;6(11):1243–1248. doi: 10.2174/138955706778742777. [DOI] [PubMed] [Google Scholar]
- Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM, Jr, Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, Spinale FG. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;290(1):H232–H239. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- Lindsey ML, Mann DL, Entman ML, Spinale FG. Extracellular matrix remodeling following myocardial injury. Ann Med. 2003;35(5):316–326. doi: 10.1080/07853890310001285. [DOI] [PubMed] [Google Scholar]
- Lohi J, Wilson CL, Roby JD, Parks WC. Epilysin, a novel human matrix metalloproteinase (MMP-28) expressed in testis and keratinocytes and in response to injury. J Biol Chem. 2001;276(13):10134–10144. doi: 10.1074/jbc.M001599200. [DOI] [PubMed] [Google Scholar]
- Manicone AM, Birkland TP, Lin M, Betsuyaku T, van Rooijen N, Lohi J, Keski-Oja J, Wang Y, Skerrett SJ, Parks WC. Epilysin (MMP-28) restrains early macrophage recruitment in Pseudomonas aeruginosa pneumonia. J Immunol. 2009;182(6):3866–3876. doi: 10.4049/jimmunol.0713949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko GN, Strongin AY. MMP-28, a new human matrix metalloproteinase with an unusual cysteine-switch sequence is widely expressed in tumors. Gene. 2001;265(1–2):87–93. doi: 10.1016/s0378-1119(01)00360-2. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Ocorr K, Bodmer R, Cartry J. Drosophila as a model to study cardiac aging. Exp Gerontol. 2011;46(5):326–330. doi: 10.1016/j.exger.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onal IK, Altun B, Onal ED, Kirkpantur A, Gul Oz S, Turgan C. Serum levels of MMP-9 and TIMP-1 in primary hypertension and effect of antihypertensive treatment. Eur J Intern Med. 2009;20(4):369–372. doi: 10.1016/j.ejim.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Overall CM, Tam EM, Kappelhoff R, Connor A, Ewart T, Morrison CJ, Puente X, Lopez-Otin C, Seth A. Protease degradomics: Mass spectrometry discovery of protease substrates and the CLIP-CHIP, a dedicated DNA microarray of all human proteases and inhibitors. Biol Chem. 2004;385(6):493–504. doi: 10.1515/BC.2004.058. [DOI] [PubMed] [Google Scholar]
- Pavlaki M, Zucker S, Dufour A, Calabrese N, Bahou W, Cao J. Furin functions as a nonproteolytic chaperone for matrix metalloproteinase-28: MMP-28 propeptide sequence requirement. Biochem Res Int. 2011;2011:630319. doi: 10.1155/2011/630319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyakova V, Loeffler I, Hein S, Miyagawa S, Piotrowska I, Dammer S, Risteli J, Schaper J, Kostin S. Fibrosis in endstage human heart failure: Severe changes in collagen metabolism and MMP/TIMP profiles. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.04.053. [DOI] [PubMed] [Google Scholar]
- Reddy AK, Amador-Noguez D, Darlington GJ, Scholz BA, Michael LH, Hartley CJ, Entman ML, Taffet GE. Cardiac function in young and old Little mice. J Gerontol A Biol Sci Med Sci. 2007;62(12):1319–1325. doi: 10.1093/gerona/62.12.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AK, Taffet GE, Li YH, Lim SW, Pham TT, Pocius JS, Entman ML, Michael LH, Hartley CJ. Pulsed Doppler signal processing for use in mice: Applications. IEEE Trans Biomed Eng. 2005;52(10):1771–1783. doi: 10.1109/TBME.2005.855709. [DOI] [PubMed] [Google Scholar]
- Reno F, Sabbatini M, Stella M, Magliacani G, Cannas M. Effect of in vitro mechanical compression on Epilysin (matrix metalloproteinase-28) expression in hypertrophic scars. Wound Repair Regen. 2005;13(3):255–261. doi: 10.1111/j.1067-1927.2005.130307.x. [DOI] [PubMed] [Google Scholar]
- Reynolds JL, Mahajan SD, Aalinkeel R, Nair B, Sykes DE, Schwartz SA. Methamphetamine and HIV-1 gp120 effects on lipopolysaccharide stimulated matrix metalloproteinase-9 production by human monocyte-derived macrophages. Immunol Invest. 2011;40(5):481–497. doi: 10.3109/08820139.2011.559499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U, Kerkela E, Jahkola T, Suomela S, Keski-Oja J, Lohi J. Epilysin (MMP-28) expression is associated with cell proliferation during epithelial repair. J Invest Dermatol. 2002;119(1):14–21. doi: 10.1046/j.1523-1747.2002.01790.x. [DOI] [PubMed] [Google Scholar]
- Spinale FG, Coker ML, Bond BR, Zellner JL. Myocardial matrix degradation and metalloproteinase activation in the failing heart: a potential therapeutic target. Cardiovasc Res. 2000;46(2):225–238. doi: 10.1016/s0008-6363(99)00431-9. [DOI] [PubMed] [Google Scholar]
- Thomas DP, Zimmerman SD, Hansen TR, Martin DT, McCormick RJ. Collagen gene expression in rat left ventricle: Interactive effect of age and exercise training. J Appl Physiol. 2000;89(4):1462–1468. doi: 10.1152/jappl.2000.89.4.1462. [DOI] [PubMed] [Google Scholar]
- Zamilpa R, Kanakia R, Cigarroa JT, Dai Q, Escobar GP, Martinez H, Jimenez F, Ahuja SS, Lindsey ML. CC chemokine receptor 5 deletion impairs macrophage activation and induces adverse remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2011;300(4):H1418–H1426. doi: 10.1152/ajpheart.01002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]