Abstract
Although 20–40% of persons with acute HCV infection demonstrate spontaneous clearance, the time-course and factors associated with clearance remain poorly understood. We investigated the time to spontaneous clearance and predictors among participants with acute HCV using Cox proportional hazards analyses. Data for this analysis were drawn from an international collaboration of nine prospective cohorts evaluating outcomes following acute HCV infection. Among 632 participants with acute HCV, 35% were female, 82% were Caucasian, 49% had IL28B CC genotype (rs12979860), 96% had injected drugs ever, 47% were infected with HCV genotype 1 and 5% had HIV co-infection. Twenty-eight percent were HCV antibody negative/RNA positive at the time of acute HCV detection (early acute HCV). During follow-up, spontaneous clearance occurred in 173 of 632 and at one year following infection, 25% (95%CI: 21%, 29%) had cleared virus. Among those with clearance, the median time to clearance was 16.5 weeks (IQR: 10.5, 33.4 weeks), with 34%, 67% and 83% demonstrating clearance at three, six and twelve months. Adjusting for age, factors independently associated with time to spontaneous clearance included female sex [adjusted hazards ratio (AHR) 2.16; 95%CI 1.48, 3.18], IL28B CC genotype (vs. CT/TT, AHR 2.26; 95%CI 1.52, 3.34), and HCV genotype 1 (vs. non-genotype 1, AHR 1.56; 95%CI 1.06, 2.30). The effect of IL28B genotype and HCV genotype on spontaneous clearance was greater among females compared to males.
Conclusions
Female sex, favorable IL28B genotype and HCV genotype 1 are independent predictors of spontaneous clearance. Further research is required to elucidate the observed sex-based differences in HCV control.
Keywords: injection drug use, hepatitis C virus, HIV, incident infection, longitudinal studies
Although 20–40% of persons with acute hepatitis C virus (HCV) infection demonstrate spontaneous clearance (1), the time-course and predictors of clearance remain poorly understood. Knowledge of clearance following acute HCV infection is limited due to the generally asymptomatic nature of initial infection and the highly marginalised nature of at-risk populations, such as people who inject drugs (PWID). Understanding the time-course and predictors of clearance provides insight into HCV pathogenesis and improves clinical decision making regarding the need for early therapeutic intervention.
Host factors such as female sex (1–4), immune responses (5, 6), virus-specific neutralizing antibodies (7) and host genetics (8, 9) have been associated with clearance in prospective studies of acute HCV infection. The strongest host factor associated with clearance is polymorphisms in the interleukin-28 (IL28B) gene region which encodes the interferon-λ3 protein (IFN-λ3, IFNL3) (8–10) and is involved in viral control (11). Individuals with non-favourable IL28B genotypes (rs12979860 CT/TT alleles) are less likely to clear HCV infection compared to those with favorable genotypes (CC alleles) (4, 8, 9). Pathogen factors, such as diversity of the HCV viral quasispecies (12) and HCV genotype (13) might also be linked with clearance. The majority of studies of people with acute HCV and clearance are limited by the small number of cases, which restricts statistical power, inference and generalizability.
The International Collaboration of Incident HIV and Hepatitis C in Injecting Cohorts (InC3) Study, a collaborative of pooled data from nine prospective international cohorts mainly following PWID (14), provides a unique opportunity to assess clearance in a large number of well characterized HCV-infected participants with prospective follow-up. The aim of this study was to investigate time to and predictors of clearance following acute HCV infection.
METHODS
Study population and design
The InC3 Study, a collaboration of nine prospective cohorts evaluating HIV and HCV infection outcomes from Australia, Canada, the Netherlands, and the United States has been previously described (14). All cohorts follow participants at regular intervals using standardized methods. Participants were recruited and followed between 1985 and 2010. The InC3 Study includes both: 1) Participants without HCV infection (≥2 HCV negative antibody tests); and 2) Participants with documented acute HCV infection (≥2 HCV antibody or RNA tests).
For the current study, only individuals with documented acute HCV were included. Documented acute HCV is defined as either: 1) HCV seroconversion with an HCV antibody negative test followed by either an HCV antibody or RNA positive test within two years of the HCV antibody negative test; or 2) evidence of symptomatic HCV infection (defined by a positive HCV antibody/RNA test; jaundice or ALT elevation >400 U/L; and detection of HCV RNA or history of high-risk exposure within three months of clinical manifestation of acute HCV). Individuals who were HCV antibody negative/HCV RNA positive at the time of acute HCV detection (early acute HCV infection) were identified for sub-analyses, given the well-defined estimated time of infection in this sub-group. Individuals treated for HCV with an estimated duration of infection <26 weeks were excluded to reduce misclassification bias due to uncertainty around subsequent spontaneous clearance in the absence of treatment (n=37). All participants provided written informed consent and cohort protocols approved by local ethics committees.
Laboratory testing
Choice of qualitative and quantitative HCV RNA testing varied by cohort but consistent at each site. Qualitative HCV RNA testing was performed using the following assays: Versant TMA [Bayer, Australia;<10 IU/ml], COBAS AmpliPrep/COBAS TaqMan (Roche, Branchburg, NJ, USA;<15 IU/ml), COBAS AMPLICOR HCV Test v2.0 (Roche Diagnostics, Mannheim, Germany; <50 IU/ml) or discriminatory HCV transcription-mediated amplification component of the Procleix HIV-1/HCV (Gen-Probe, San Diego, CA, USA; <12 copies/mL). Quantitative HCV RNA testing was performed using the Versant HCV RNA 3.0 (Bayer, Australia;<615 IU/ml), COBAS AMPLICOR HCV MONITOR 2.0 (Roche Diagnostics, Mannheim, Germany; <600 IU/ml), COBAS AmpliPrep/COBAS TaqMan (Roche, Branchburg, NJ, USA;<15 IU/ml) or an in-house PCR (<1000 IU/ml) (15, 16). HCV genotype was determined by line-probe assay (Versant LiPa1/LiPa2, Bayer, Australia) or HCV sequencing at acute HCV detection. Among those with undetectable HCV RNA (no genotype) and available samples, Murex HCV serotyping was performed to determine HCV genotype (Murex Biotech Limited, Dartford, UK). IL28B genotype was determined by sequencing of the rs12979860 single nucleotide polymorphism [as previously described in (4, 9, 10)].
Estimating the date of acute HCV infection
The estimated date of acute HCV infection was calculated based on a hierarchy using the most precise information indicating the time of infection. Among individuals who were HCV antibody negative/HCV RNA positive at the time of acute HCV detection (early acute HCV infection undergoing HCV seroconversion), the estimated date of HCV infection was calculated as four weeks prior to the date of acute HCV detection [mid-point between HCV infection and detection of HCV antibodies (eight weeks) (17, 18)]. Among individuals with symptomatic acute HCV, the estimated date of infection was calculated as six weeks prior to its onset (jaundice or ALT >400 IU/mL) (19). Among individuals with HCV seroconversion, the estimated date of infection was calculated as the mid-point between the last negative HCV antibody and first positive HCV antibody or RNA test.
Study outcomes
Spontaneous clearance was defined by two consecutive undetectable HCV RNA test results ≥4 weeks apart following infection. The estimated date of clearance was defined as the midpoint between the first of two consecutive undetectable qualitative HCV RNA tests and either the last sample with detectable HCV RNA or the estimated date of infection, in the event that the sample collected at the time of acute detection was HCV RNA undetectable.
The time to clearance was calculated as the time from the estimated date of infection to the estimated date of clearance. For those without clearance, follow-up time was calculated from the estimated date of infection until the date of the last therapy-naïve detectable HCV RNA test. For participants with only one undetectable HCV RNA as their last measurement, follow-up time was calculated from the estimated date of infection until the date of the last positive HCV RNA test. Participants treated for HCV were censored at the date of treatment.
Statistical analyses
Time to and predictors of clearance were assessed. Hypothesized predictors were determined a priori and included age (categorized as <30, 30–39 and ≥40 years) (20), sex (1–4), symptomatic HCV infection (3, 8), ethnicity (21), IL28B genotype (SNP rs12979860; CC vs. CT/TT) (8–10), HIV infection (21), HCV genotype [genotype 1 versus genotype non-1 (those with unknown genotypes were not included)] (13, 22) and cohort site (given that site may introduce unmeasured confounders). The effects of these variables on time to clearance were assessed by Kaplan-Meier analyses (significance assessed by log-rank test). The median [interquartile range, (IQR)] time to clearance among those with clearance was also estimated.
Cox proportional hazards analyses were used to identify predictors of clearance. In multivariate analyses, all variables with P<0.20 in unadjusted analysis were considered as potential independent predictors. Initial models were adjusted for age and built using a backwards stepwise approach with factors sequentially eliminated according to the result of the likelihood ratio test. Additional models were also considered using a shared ‘frailty’ random effect model to provide improved variance estimates and control for potential confounding by site.
Given previous data demonstrating an interaction between sex and IL28B genotype in HCV clearance (4), it was hypothesized that sex would modify the effect of IL28B genotype and HCV genotype on clearance. Analyses were performed by investigating the separate effects of these factors and their joint effect. Differences in the susceptibility of the effect of sex and dichotomous exposures of interest (IL28B and HCV genotype) on clearance were explored by testing for interaction: a new composite variable with four categories (a−b−, a−b+, a+b− and a+b+) was redefined for sex and the dichotomous exposure of interest (a− and b− denote absence of exposure). Adjusted hazard ratios (AHR) were calculated for each category after adjustment for age and other variables significant in the final multivariate model. Further, given data suggesting different distributions of HCV genotypes according to IL28B genotype (23), similar analyses were performed to assess whether the effect of IL28B genotype on clearance would vary by HCV genotype. Analyses stratified by sex and IL28B genotype were also performed.
Sensitivity analyses were performed to assess HCV time to and predictors of clearance among individuals with early acute HCV infection. All analyses were repeated using the mid-point estimation method for calculating the estimated date of HCV infection for those with symptomatic acute symptomatic infection and excluding participants receiving HCV treatment. Statistically significant differences were assessed at P<0.05; p-values are two-sided. All analyses were performed using Stata v12.0 (College Station, TX, United States).
RESULTS
Participant characteristics
Among the 632 participants with acute HCV infection included, the median age was 26 years, 36% were female, 96% had a history of injecting drug use and 16% received HCV treatment during follow-up (all treated participants that were included started treatment at an estimated duration of infection >6 months) (Table 1, Supplementary Table 1). Among those with data on infecting HCV genotype (n=537), 55% had genotype 1. Among all genotypes, 42% were determined by InnoLipa, 48% by sequencing and 9% by serotyping. Forty-nine percent (266 of 542 with test results) were IL28B CC genotype favourable, with no differences among females and males (48% vs. 50%, P=0.606) or those with HCV genotype 1 and non-1 infection (48% vs. 50%, P=0.592). Untreated individuals had similar proportions with IL28B CC and HCV genotype 1 infection, but a greater proportion of females (38% vs. 26%, P=0.017, Supplemental Table 2).
Table 1.
Characteristics of participants with acute HCV infection in the InC3 Study (n=632)
| Overall (n=632), n (%)† | Spontaneous Clearance (n=173), n (%)‡ | |
|---|---|---|
| Site | ||
| UFO (United States) | 115 (18) | 31 (27) |
| ATAHC (Australia) | 119 (19) | 27 (23) |
| BAHSTION (United States) | 49 (8) | 14 (29) |
| BBAASH (United States) | 114 (18) | 41 (36) |
| HEPCO (Canada) | 75 (12) | 16 (21) |
| HITS-c (Australia) | 10 (2) | 3 (30) |
| HITS-p (Australia) | 89 (14) | 17 (19) |
| N2 (Australia) | 17 (3) | 4 (24) |
| ACS (the Netherlands) | 44 (7) | 20 (45) |
| Median age, yrs (IQR)* | 26 (23–32) | 26 (23–30) |
| Age, categorized* | ||
| <30 years | 403 (64) | 122 (30) |
| 30–39 years | 103 (16) | 28 (27) |
| ≥40 years | 75 (12) | 14 (19) |
| Missing | 51 (8) | 9 (18) |
| Female Sex# | 228 (36) | 86 (38) |
| Ethnicity | ||
| Caucasian | 516 (82) | 141 (27) |
| Aboriginal | 32 (5) | 10 (31) |
| Asian | 12 (2) | 3 (25) |
| Black | 24 (4) | 4 (17) |
| Other | 48 (8) | 15 (31) |
| History of injecting drug use | 608 (96) | 165 (27) |
| Symptomatic HCV infection* | ||
| No | 111 (18) | 15 (14) |
| Yes | 138 (22) | 37 (27) |
| Unknown | 383 (61) | 121 (32) |
| IL28B genotype (rs12979860) | ||
| TT | 63 (10) | 15 (24) |
| CT | 213 (34) | 46 (22) |
| CC | 266 (42) | 98 (37) |
| Missing | 90 (14) | 14 (16) |
| HIV infection* | ||
| No | 531 (84) | 147 (28) |
| Yes | 29 (5) | 7 (24) |
| Missing | 72 (11) | 19 (26) |
| HCV genotype* | ||
| Genotype 1 | 297 (47) | 79 (27) |
| Genotype 2 | 32 (5) | 7 (22) |
| Genotype 3 | 183 (29) | 33 (18) |
| Genotype 4 | 7 (1) | 1 (14) |
| Genotype 6 | 4 (1) | 0 (0) |
| Mixed genotype | 14 (2) | 2 (14) |
| Unknown genotype | 95 (15) | 51 (54) |
Percentages indicate column percentages,
Percentages indicate row percentages,
At the time of incident HCV infection,
Includes 2 missing.
UFO, UFO STUDY; ATAHC, Australian Trial in Acute Hepatitis C; BAHSTION, Boston Acute HCV Study: Transmission, Immunity and Outcomes Network; BBAASH, Baltimore Before and After Acute Study of Hepatitis; HEPCO, St. Luc Cohort, HEPCO; HITS-c, Hepatitis C Incidence and Transmission Study-Community; HITS-p, Hepatitis C Incidence and Transmission Study-Prison; N2, Networks 2; ACS, Amsterdam Cohort Studies.
Acute HCV infection was documented by HCV seroconversion in 98% (n=621) of participants, with 2% (n=11) identified by acute symptomatic infection and a recent history of high-risk exposure. Among those with available data on symptomatic infection (n=249, 383 missing), 54% (n=138) had symptomatic HCV infection. Twenty-nine percent (n=183) were HCV antibody negative/HCV RNA positive at acute HCV detection and were defined as having early acute HCV infection.
Following the estimated date of HCV infection, participants had a median of five HCV RNA tests (IQR:2, 9; range:1–55), with a median of 60 days (IQR;28, 120) between tests. The overall median follow-up time from the estimated date of infection to the last HCV RNA measurement was 1.51 years (IQR;0.72, 2.99). Across cohorts, the median follow-up ranged from 0.63 years (HITS-c) to 9.42 years (ACS). The median interval from the estimated date of infection to the first positive HCV antibody or RNA test at the time of acute HCV detection was 9.0 weeks (IQR;4.0, 20.4).
Spontaneous clearance of acute HCV infection
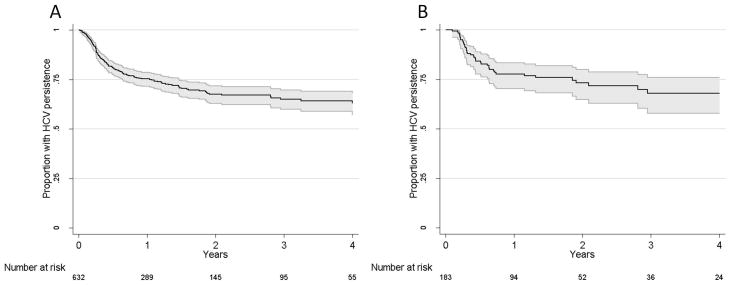
During follow-up, clearance was observed in 173/632 overall and 43/183 in those with early acute HCV infection. At one year following infection, 25% [95% confidence interval(95%CI): 21%, 29%] had cleared virus overall and 22% (95%CI 17%, 30%; 43/183) had cleared virus among those with early acute HCV. Figure 1 shows the proportion with viral persistence as a function of number of years following HCV infection among the overall population (Figure 1A) and those with early acute HCV infection (Figure 1B). Among those who cleared HCV, the median time to clearance was 16.5 weeks (IQR, 10.5, 33.4) overall and 18.9 weeks (IQR, 13.3, 33.4) among those with early acute HCV.
Figure 1.
Kaplan-Meier graphs of time to spontaneous clearance in A) the overall population with acute HCV infection (n=632) and B) those with early acute HCV infection (n=183). 95% confidence intervals are in shaded grey.
Among those with clearance, 34% (95%CI, 27%, 42%, n=59) and 67% (95%CI, 60%, 74%, n=116) had cleared infection by three months and six months following infection, respectively. At 12, 18 and 24 months following infection, 83% (95%CI, 77%, 88%, n=144), 92% (95%CI, 87%, 96%, n=160) and 97% (95%CI, 93%, 99%, n=167) of those who did clear, had cleared infection. Among those with early acute HCV infection and clearance (n=43), the proportion of participants with clearance by 3, 6, 12, 18 and 24 months were 23% (95%CI, 12%, 39%, n=10), 63% (95%CI, 47%, 77%, n=27), 84% (95%CI, 69%, 93%, n=36), 88% (95%CI, 75%, 96%, n=38) and 93% (95%CI, 81%, 99%, n=40). The rate of spontaneous clearance was 42 per 100 p-yrs (95%CI, 35, 51) within the first six months of infection and 10 per 100 p-yrs (95%CI, 8, 13) six months following infection.
Factors predicting spontaneous clearance of acute HCV infection
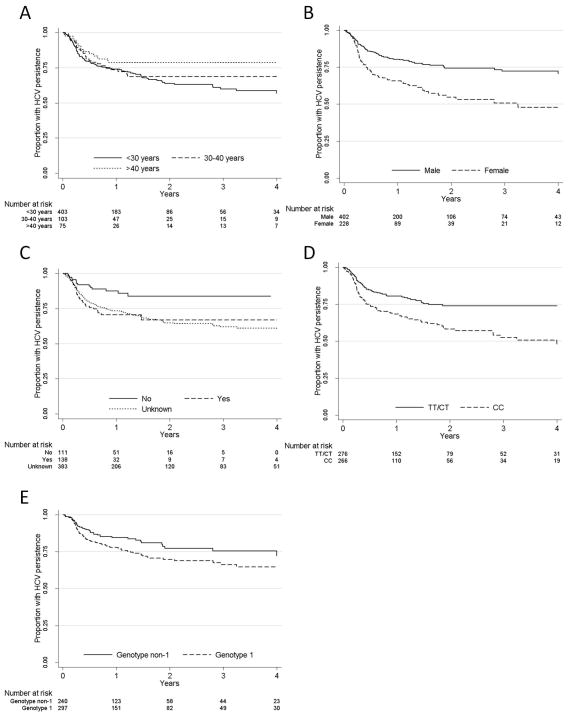
In Kaplan-Meier and unadjusted Cox proportional hazards analyses, clearance was associated with female sex, symptomatic HCV infection, IL28B CC genotype and HCV genotype 1 (Figure 2, Table 2). Given that for 61% (n=383) of participants data were missing on symptomatic infection, this variable was not explored in multivariate analyses. Due to the a priori hypothesis that HCV genotype 1 would have higher clearance (13, 22), the small numbers within some genotype categories (2/4/6/mixed) and the observation that all genotypes other than genotype 1 demonstrated lower clearance in unadjusted analysis (Table 2), all HCV genotype non-1 infections were grouped together. There was no difference in clearance by HCV genotyping assay. Neither ethnicity nor HIV status was associated with clearance.
Figure 2.
Kaplan-Meier graphs of time to viral clearance by A) Age; B) Sex; C) Symptomatic HCV infection; D) IL28B genotype; and E) HCV genotype.
Table 2.
Cox proportional hazards analysis of predictors of time to spontaneous HCV clearance among participants with acute HCV infection (n=632).
| Clearance Rate (/100 pyo) | Unadjusted HR (95% CI) | P | P overall | Model 1 Adjusted for age HR¥ (95% CI) | P | Model 2 Adjusted for age and site HR‡ (95% CI) | P | |
|---|---|---|---|---|---|---|---|---|
| Site | ||||||||
| UFO | 21.3 | 1.00 | - | 0.313 | - | - | - | - |
| ATAHC | 26.5 | 0.89 (0.53, 1.49) | 0.645 | - | - | - | - | - |
| BAHSTION | 33.8 | 1.23 (0.65, 2.31) | 0.527 | - | - | - | - | - |
| BBAASH | 21.4 | 1.19 (0.75, 1.90) | 0.464 | - | - | - | - | - |
| HEPCO | 18.2 | 0.80 (0.44, 1.47) | 0.476 | - | - | - | - | - |
| HIT-c | 48.7 | 1.51 (0.46, 4.94) | 0.497 | - | - | - | - | - |
| HIT-p | 11.2 | 0.63 (0.35, 1.13) | 0.123 | - | - | - | - | - |
| N2 | 14.4 | 0.75 (0.26, 2.13) | 0.589 | - | - | - | - | - |
| ACS | 21.1 | 1.36 (0.77, 2.39) | 0.289 | - | - | - | - | - |
| Age categorized* | ||||||||
| <30 years | 23.3 | 1.00 | - | 0.185 | 1.00 | - | 1.00 | - |
| 30–39 years | 20.0 | 0.86 (0.57, 1.29) | 0.456 | - | 0.99 (0.61, 1.62) | 0.976† | 1.05 (0.63, 1.74) | 0.862†† |
| ≥40 years | 14.8 | 0.61 (0.35, 1.06) | 0.077 | - | 0.80 (0.41, 1.57) | 0.522 | 0.84 (0.42, 1.69) | 0.630 |
| Female sex (vs. male sex) | 34.3 | 2.03 (1.51, 2.74) | <0.001 | - | 2.16 (1.48, 3.18) | <0.001 | 2.11 (1.43, 3.10) | <0.001 |
| Symptomatic HCV infection* | ||||||||
| No | 11.5 | 1.00 | - | 0.007 | - | - | - | - |
| Yes | 32.5 | 2.48 (1.36, 4.52) | 0.003 | - | - | - | - | - |
| Unknown | 20.0 | 2.29 (1.34, 3.92) | 0.003 | - | - | - | - | - |
| Ethnicity | ||||||||
| Caucasian | 20.8 | 1.00 | - | 0.763 | - | - | - | - |
| Aboriginal | 20.4 | 1.11 (0.58, 2.10) | 0.760 | - | - | - | - | - |
| Asian | 21.7 | 0.90 (0.29, 2.82) | 0.852 | - | - | - | - | - |
| Black | 9.8 | 0.54 (0.20, 1.47) | 0.231 | - | - | - | - | - |
| Other | 22.5 | 1.13 (0.67, 1.93) | 0.643 | - | - | - | - | - |
| IL28B CC genotype (vs. CT/TT) | 29.5 | 1.90 (1.38, 2.62) | <0.001 | - | 2.26 (1.52, 3.34) | <0.001 | 2.20 (1.48, 3.26) | <0.001 |
| HIV infection (vs. no HIV infection) | 23.1 | 0.88 (0.41, 1.88) | 0.744 | - | - | - | - | - |
| HCV genotype 1 (vs. genotype non-1)£,ρ | 18.1 | 1.49 (1.03, 2.16) | 0.035 | - | 1.56 (1.06, 2.30) | 0.025 | 1.44 (0.97, 2.14) | 0.074 |
At the time of incident HCV infection,
includes 448 participants in the final adjusted model,
overall P=0.813,
overall P=0.856,
includes 448 participants in the final adjusted model and is adjusted for site using a random-effects model including a frailty term to adjust for site,
among those with available genotypes.
pyo, person-years observation, UFO, UFO STUDY; ATAHC, Australian Trial in Acute Hepatitis C; BAHSTION, Boston Acute HCV Study: Transmission, Immunity and Outcomes Network; BBAASH, Baltimore Before and After Acute Study of Hepatitis; HEPCO, St. Luc Cohort, HEPCO; HITS-c, Hepatitis C Incidence and Transmission Study-Community; HITS-p, Hepatitis C Incidence and Transmission Study-Prison; N2, Networks 2; ACS, Amsterdam Cohort Studies.
HCV genotype 2 (vs 1; HR 0.84; 95%CI, 0.39, 1.82), HCV genotype 3 (vs 1; HR 0.68; 95%CI, 0.45, 1.02), HCV genotype 4 (vs 1; HR 0.41; 95%CI, 0.06, 2.98), mixed HCV genotype (vs 1; HR 0.57; 95%CI, 0.14, 2.32), unknown genotype (vs. 1; HR 3.40, 95%CI, 2.38, 4.87).
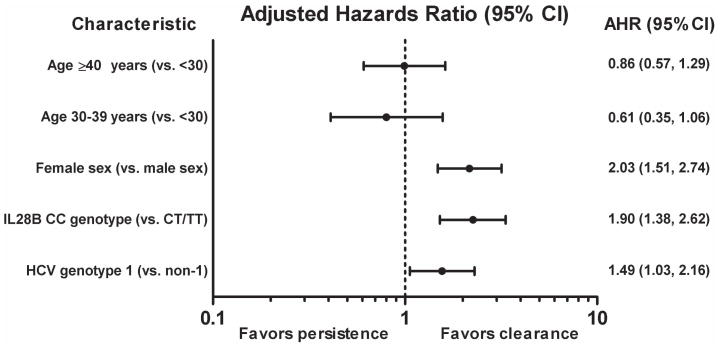
Factors independently predicting clearance included female sex [vs. male, AHR 2.16; 95%CI 1.48, 3.18. P<0.001], IL28B CC genotype (vs. CT/TT, AHR 2.26; 95%CI 1.52, 3.34, P<0.001) and HCV genotype 1 (vs. non-genotype 1, AHR 1.56; 95%CI 1.06, 2.30, P=0.025) (Table 2, Figure 3). In sensitivity analyses, results did not substantially change when analyses were restricted to individuals with early acute HCV infection, when the mid-point method was used to estimate the date of HCV infection or when participants treated for HCV infection were excluded (Supplementary Table 2). Adjustment for site also did not change the results (Table 2).
Figure 3.
Predictors of time to spontaneous HCV clearance among participants with acute HCV infection.
The effect of sex and IL28B genotype on spontaneous clearance
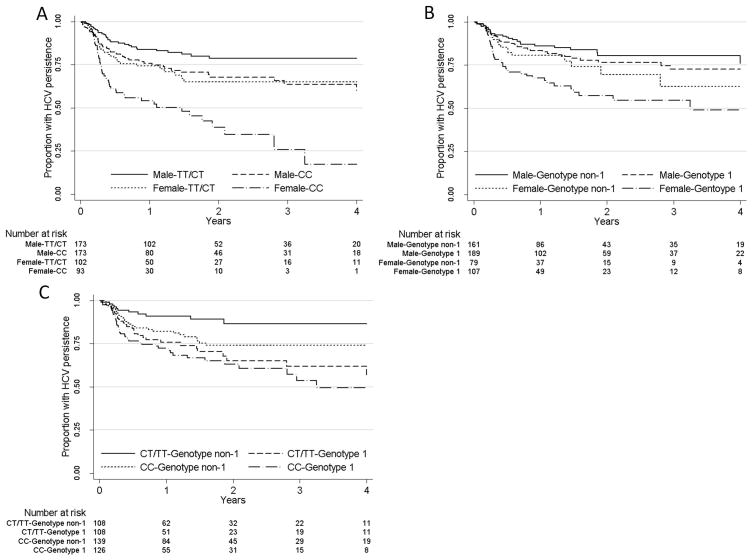
Clearance rates were highest among females with the IL28B CC genotype (Figure 4A). After adjusting for age and HCV genotype (Table 3), females with the IL28B CC genotype had the greatest probability of clearance as compared to males with the CT/TT genotype (AHR 4.65; 95%CI 2.71, 7.96, P<0.001). The AHR for males with the CC genotype was close to the AHR for female with the CT/TT genotype (Table 3). The interaction between sex and IL28B was not significant on the multiplicative scale (P=0.265).
Figure 4.
Kaplan-Meier graphs of time to viral clearance by A) Sex and IL28B genotype; B) Sex and HCV genotype; and C) IL28B genotype and HCV genotype.
Table 3.
Cox proportional hazards models of predictors of time to spontaneous HCV clearance among participants with acute HCV infection.
| Model | Adjusted HR¥ (95% CI) | P | |
|---|---|---|---|
| 1 | Age, sex/IL28B genotype and HCV genotype¥ | ||
| Sex and IL28B genotype | |||
| Male, CT/TT | 1.00 | - | |
| Male, CC | 1.81 (1.06, 3.10) | 0.029 | |
| Female, CT/TT | 1.64 (0.87, 3.08) | 0.129 | |
| Female, CC | 4.65 (2.71, 7.96) | <0.001 | |
| HCV genotype 1 (vs. genotype non-1) | 1.53 (1.04, 2.27) | 0.031 | |
| 2 | Age, sex/HCV genotype and IL28B genotype¥ | ||
| Sex and HCV genotype | |||
| Male, genotype non-1 | 1.00 | - | |
| Male, genotype 1 | 1.40 (0.82, 2.37) | 0.217 | |
| Female, genotype non-1 | 1.87 (0.99, 3.54) | 0.053 | |
| Female, genotype 1 | 3.30 (1.94, 5.62) | <0.001 | |
| IL28B CC genotype (vs. CT/TT) | 2.23 (1.50, 3.30) | <0.001 | |
| 3 | Age, HCV genotype/IL28B genotype and sex¥ | ||
| HCV genotype and IL28B genotype | |||
| Genotype non-1, CT/TT | 1.00 | - | |
| Genotype non-1, CC | 3.82 (1.86, 7.84) | <0.001 | |
| Genotype 1, CT/TT | 2.66 (1.30, 5.47) | 0.008 | |
| Genotype 1, CC | 4.56 (2.28, 9.12) | <0.001 | |
| Female sex (vs. male sex) | 2.23 (1.52, 3.28) | <0.001 | |
| 4 | Females: Age, IL28B genotype and HCV genotype┼ | ||
| IL28B CC genotype (vs. CT/TT) | 2.89 (1.60, 5.22) | <0.001 | |
| HCV genotype 1 (vs. genotype non-1) | 1.78 (1.00, 3.17) | 0.052 | |
| 5 | Males: Age, IL28B genotype and HCV genotype‡ | ||
| IL28B CC genotype (vs. CT/TT) | 1.79 (1.05, 3.06) | 0.033 | |
| HCV genotype 1 (vs. genotype non-1) | 1.40 (0.82, 2.38) | 0.220 | |
| 6 | IL28B CT/TT genotype: Age, sex and HCV genotypeα | ||
| Female sex (vs. male sex) | 1.74 (0.92, 3.30) | 0.089 | |
| HCV genotype 1 (vs. genotype non-1) | 2.65 (1.29, 5.46) | 0.008 | |
| 7 | IL28B CC genotype: Age, sex and HCV genotypeβ | ||
| Female sex (vs. male sex) | 2.64 (1.62, 4.31) | <0.001 | |
| HCV genotype 1 (vs. genotype non-1) | 1.18 (0.74, 1.90) | 0.488 | |
All models adjusted for age, Tests for interaction on the multiplicative scale: Model 1: P = 0.265; Model 2: P = 0.560; Model 3: P = 0.068.
includes 448 participants in the final adjusted model,
includes 151 participants in the final adjusted model,
includes 297 participants in the final adjusted model,
includes 227 participants in the final adjusted model,
includes 221 participants in the final adjusted model.
The effect of sex and HCV genotype on spontaneous clearance
Clearance rates were highest among females with HCV genotype 1 (Figure 4B). After adjusting for age and IL28B genotype (Table 3), females with HCV genotype 1 had the greatest probability of clearance as compared to males with HCV genotype non-1 (AHR 3.30; 95%CI 1.94, 5.62, P<0.001). The AHR for males with the HCV genotype 1 was close to the AHR for female with HCV genotype non-1 (Table 3). The interaction between sex and HCV genotype was not statistically significant on the multiplicative scale (P=0.560).
The effect of HCV genotype and IL28B genotype on spontaneous clearance
Clearance rates were lowest among individuals with HCV genotype non-1 and CT/TT IL28B genotype, (Figure 4C). After adjusting for age and sex (Table 3), compared to those with HCV genotype non-1 and CT/TT IL28B genotype, individuals with HCV genotype non-1 and IL28B CC genotype (AHR 3.82; 95%CI 1.86, 7.84, P<0.001), HCV genotype 1 and CT/TT IL28B genotype (AHR 2.66; 95%CI 1.30, 5.47, P=0.008) and HCV genotype 1 and IL28B CC genotype (AHR 4.56; 95%CI 2.28, 9.12, P<0.001) all had an increased probability of clearance. The interaction between HCV genotype and IL28B genotype did not reach statistical significance on the multiplicative scale (P=0.068).
Spontaneous clearance in females and males
Given the differential effect of IL28B genotype and HCV genotype on time to clearance by sex, the impact of IL28B genotype and HCV genotype was also explored separately for females and males. Among females, after adjusting for age (Table 3), IL28B CC genotype (vs. CT/TT, AHR 2.89; 95%CI 1.60, 5.22, P<0.001) and HCV genotype 1 (vs. non-genotype 1, AHR 1.78; 95%CI 1.00, 3.17, P=0.052) independently predicted clearance. Among males, after adjusting for age, only IL28B CC genotype (vs. CT/TT, AHR 1.79; 95%CI 1.05, 3.06, P=0.033) independently predicted clearance but genotype did not (genotype 1 vs. non-1, AHR 1.40; 95%CI 0.82, 2.38, P=0.220).
Spontaneous clearance stratified by IL28B genotypes
Given the differential effect of HCV genotype on clearance by IL28B genotype, the impact of HCV genotype on clearance was examined separately for those with CT/TT and CC IL28B genotypes after adjusting for age and female sex. Among those with CT/TT IL28B genotype, HCV genotype 1 (vs. non-genotype 1, AHR 2.65; 95%CI 1.29, 5.46, P=0.008) independently predicted clearance. Among those with CC IL28B genotype, after adjusting for age and sex, there was no statistically significant effect of HCV genotype 1 on clearance (vs. non-genotype 1, AHR 1.18; 95%CI 0.74, 1.90, P=0.488).
DISCUSSION
This study describes the time-course and independent predictors of spontaneous clearance in a large sample of participants with well-defined acute HCV infection, the majority of whom were PWID. The proportion with clearance at one year was 25%. Among those with clearance, the median time to clearance was 16.5 weeks, with two-thirds clearing within the first six months of infection. Independent predictors of clearance included female sex, favorable IL28B genotype and HCV genotype 1 infection. The effect of both IL28B genotype and HCV genotype on clearance tended to be greater in females compared to males. This study provides important insights into factors affecting HCV viral control and offers guidance in clinical decision-making for the treatment of acute HCV infection.
The overall proportion with spontaneous clearance of 25% at one year is consistent with a weighted mean clearance of 26% reported in a systematic review of prospective acute HCV studies (1). Confirmation of this estimate is important, given that the systematic review by Micallef et al. was limited by heterogeneity of studies in terms of sample size, inclusion criteria and follow-up (1). The large sample size, well-defined population and frequent follow-up within the InC3 study provide a more precise estimate of the rate of clearance among individuals with acute HCV infection.
The median time from the estimated date of infection to clearance was 16.5 weeks. This is longer than previously reported (8–11 weeks) (19, 24, 25), but these studies had larger proportions of cases with symptomatic infection (19, 24, 25). Consistent with another study (3), symptomatic infection was associated with clearance in this study. Unfortunately, the majority of cohorts in the InC3 study did not systematically collect information on the presence of symptoms at the time of infection, precluding the ability to assess this factor in adjusted analyses. Previous studies are limited by short follow-up time (and thus less likely to include late clearance), small sample sizes and heterogeneous definitions for infection and clearance, which may also impact estimates of time to clearance.
The finding that one-third of participants who demonstrated clearance did so more than six months following infection must be interpreted with caution. This is greater than reported in previous studies (19, 24, 25). Although one explanation for this difference might be longer follow-up in InC3 (19, 24, 25), it is more likely that there is imprecise characterisation of time of clearance in InC3, particularly in cases with broader intervals of HCV RNA testing.
Female sex independently predicted spontaneous clearance, after adjusting for IL28B genotype and HCV genotype, consistent with previous reports (1–4). The effect of IL28B genotype on clearance was greater among females than among males. While this is consistent with previous data demonstrating an interaction between female sex and IL28B genotype on clearance (4), this interaction was not statistically significant in the current study. The impact of HCV genotype 1 on clearance was also greater among females. This is consistent with very high proportions of females with clearance (52–54%) following HCV genotype 1 infection through contaminated anti-D immune globulin (26, 27). Taken together, these results are striking and suggestive of the potential role of sex in modifying factors important in HCV clearance.
Mechanisms behind the association of female sex and clearance may be linked to sex-based differences in immunity. Females have a lower burden of infections (28), a higher prevalence of several autoimmune diseases (28) and an increased number and magnitude of immune and inflammatory responses (29), as compared to males. The prevailing hypothesis to explain immunological differences between males and females is that sex steroids bind to specific receptors expressed in lymphoid tissue cells, macrophages, dendritic cells and lymphocytes, thereby influencing the function of immune cells (29). However, despite considerable research demonstrating differences in immune function between females and males, there are little data on sex-based differences in immune profiles in those with HCV. Further studies should focus on mechanisms explaining differences in clearance between males and females as this may contain important information for understanding HCV viral control.
Genetic variation in the IL28B gene independently predicted spontaneous clearance, consistent with previous reports (4, 8–10). The large number of cases and detailed demographic and clinical information in InC3 provided sufficient power to adjust for multiple factors. The molecular mechanism linking IL28B genotype to clearance remains to be elucidated.
HCV genotype 1 was independently associated with spontaneous clearance. Few studies have investigated the impact of HCV genotype on clearance in acute HCV infection, partly due to the difficulties in identifying people early during infection to detect and genotype HCV RNA. Limitations of previous studies include small numbers, the potential misclassification of genotype by HCV serotyping assays (13, 30), the large proportion with an unknown HCV genotype status among those with clearance who could not be genotyped/serotyped and a lack of genotypic diversity in some groups (22). Among studies that have investigated the impact of HCV genotype on spontaneous clearance, results are conflicting (13, 22, 30), with some studies demonstrating lower (30), higher (13, 22) or comparable (4) proportions with clearance among individuals with HCV genotype 1 when compared to other genotypes. The association between HCV genotype 1 and clearance observed in InC3 is convincing due to the well-defined nature of acute HCV infection, and the fact that the large majority of HCV genotyping was performed via either line-probe assay or HCV sequencing at the time of acute HCV detection.
Those with CT/TT IL28B genotypes and HCV genotype non-1 infection demonstrated a lower likelihood of spontaneous clearance, suggesting a biological interaction between HCV genotype and IL28B genotype. Although it has been demonstrated that the expression of interferon-stimulated genes (ISG) is higher among individuals with HCV genotype 1 infection with chronic infection (31), there are no studies investigating ISG expression during acute infection stratified by IL28B and HCV genotype. The role of a potential interaction between the effect of HCV genotype and IL28B on clearance during acute HCV requires further investigation.
There are several limitations to this study. Nine cohorts of individuals with acute HCV (mainly PWID) were combined. Participating cohorts bring a range of data types and structures presenting issues surrounding both inconsistent measurement and biological data testing protocols (e.g. HCV RNA assays differed across cohorts). As such, there was some heterogeneity across the cohorts with respect to the availability of data on certain variables known to be associated with clearance (e.g. symptomatic infection) and it was not possible to adjust our analyses for these factors. The availability of only one HCV RNA negative test at last follow-up limited the ability to assess late clearance outcome in a small minority (<5%). In contrast, some instances of late clearance may be due to very early clearance and subsequent reinfection (with clearance of the second infection detected) (32). Broad intervals of HCV RNA testing in some individuals reduced the precision of estimated time of clearance. There were also small numbers for some categorized variables in this study (HIV and ethnicity) and the absence of an observed effect does not imply the absence of an association. Further, potential unmeasured confounding factors may have influenced the results.
In conclusion, female sex, favorable IL28B genotype and HCV genotype 1 infection are independent predictors of spontaneous HCV clearance following acute infection. Further research is required to better understand the mechanism behind the potential effect of female sex on HCV viral control.
Supplementary Material
Acknowledgments
Financial Support: The InC3 Study is supported by the National Institute on Drug Abuse Award Number R01DA031056. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government. JGr is supported by a National Health and Medical Research Council (NHMRC) Career Development Fellowship. JB and NHS are supported by Fonds de la Recherche du Québec – Santé Research Career Awards. BH is supported by an Australian Postgraduate PhD Award. GD and AL are supported by NHMRC Practitioner Research Fellowships. MH and LM were supported by NHMRC Senior Research Fellowships and MH additionally by a VicHealth Senior Research Fellowship. RSD was supported by an NHMRC postgraduate scholarship and a Centre for Research Excellence into Injecting Drug Use postgraduate top-up scholarship. Other research support includes NIH U19 AI088791 (AC), NIH U19 AI066345 (AYK, GML and BHM), U19 AI082630 (NIAID; GML), R01 DA033541 (NIDA; AYK), MOP-103138, MOP-210232 (CIHR, JB and NHS) and the Netherlands National Institute for Public Health and the Environment (MSvdL, MP). JGe is supported by the Sydney Medical Foundation and grants from the NHMRC.
List of abbreviations
- HCV
hepatitis C virus
- PWID
people who inject drugs
- IL28B
interleukin-28
- InC3
The International Collaboration of Incident HIV and Hepatitis C in Injecting Cohorts
- IQR
interquartile range
- HR
hazard ratio
- AHR
adjusted hazard ratio
- ISG
interferon-stimulated genes
- UFO
UFO STUDY
- ATAHC
Australian Trial in Acute Hepatitis C
- BAHSTION
Boston Acute HCV Study
- Transmission
Immunity and Outcomes Network
- BBAASH
Baltimore Before and After Acute Study of Hepatitis
- HEPCO
St. Luc Cohort, HEPCO
- HITS-c
Hepatitis C Incidence and Transmission Study-Community
- HITS-p
Hepatitis C Incidence and Transmission Study-Prison
- N2
Networks 2
- ACS
Amsterdam Cohort Studies
InC3 Study Group
Steering Committee
Kimberly Page (Chair, UFO STUDY), Julie Bruneau (HEPCO), Andrea L. Cox (BBAASH), Gregory J. Dore (ATAHC), Jason Grebely (ATAHC), Margaret Hellard (N2), Georg Lauer (BAHSTION), Arthur Y. Kim (BAHSTION), Andrew R. Lloyd (HITS-p), Lisa Maher (HITS-c), Barbara H. McGovern (BAHSTION), Maria Prins (ACS) and Naglaa H. Shoukry (HEPCO).
Coordinating Centre
Meghan Morris (Study Co-ordinator), Judy Hahn (Co-Investigator), Thomas M. Rice (Data Manager) and Megan Rilla (Administration).
Site Data Managers
Maryam Alavi (ATAHC), Rachel Bouchard (HEPCO), Jennifer Evans (UFO Study), Bart Grady (ACS), Jasneet Aneja (BAHSTION), Rachel Sacks-Davis (Networks 2), Suzy Teutsch (HITS-p), Bethany White (HITS-c), Brittany Wells (BBAASH) and Geng Zang (HEPCO).
InC3 Researcher Acknowledgements
ATAHC – Tanya Applegate, Gail Matthews and Barbara Yeung; ACS – Bart Grady and Janke Schinkel; BAHSTION – Jasneet Aneja and Leslie Erin Prince; HEPCO – Elise Roy and Geng Zang; HITS-c – Anna Bates, Jarliene Enriquez, Sammy Chow, Bethany White; HITS-p - Luke McCredie and Suzy Teutsch; N2 – Campbell Aitken, Joseph Doyle and Tim Spelman; 9) UFO – Jennifer Evans.
Footnotes
Disclosures: The authors have nothing to disclose in relation to this work.
Author Contributions: All authors contributed to the design of the InC3 Study. All authors have contributed data to the InC3 Study. Authors JGr, GD, MS and MP compiled the first draft of the manuscript, which was reviewed by KP. The primary statistical analysis was conducted by RSD, BH, MS and JGr. MS, MP, GD and KP reviewed the data analysis. All authors contributed to and have approved the final manuscript.
References
- 1.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 2.Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, Tobler L, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis. 2009;200:1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang CC, Krantz E, Klarquist J, Krows M, McBride L, Scott EP, Shaw-Stiffel T, et al. Acute hepatitis C in a contemporary US cohort: modes of acquisition and factors influencing viral clearance. J Infect Dis. 2007;196:1474–1482. doi: 10.1086/522608. [DOI] [PubMed] [Google Scholar]
- 4.van den Berg CH, Grady BP, Schinkel J, van de Laar T, Molenkamp R, van Houdt R, Coutinho RA, et al. Female sex and IL28B, a synergism for spontaneous viral clearance in hepatitis C virus (HCV) seroconverters from a community-based cohort. Plos One. 2011;6:e27555. doi: 10.1371/journal.pone.0027555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Post J, Ratnarajah S, Lloyd AR. Immunological determinants of the outcomes from primary hepatitis C infection. Cell Mol Life Sci. 2009;66:733–756. doi: 10.1007/s00018-008-8270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemon SM. Induction and evasion of innate antiviral responses by hepatitis C virus. J Biol Chem. 2010;285:22741–22747. doi: 10.1074/jbc.R109.099556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller JL, et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–582. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 8.Tillmann HL, Thompson AJ, Patel K, Wiese M, Tenckhoff H, Nischalke HD, Lokhnygina Y, et al. A polymorphism near IL28B is associated with spontaneous clearance of acute hepatitis C virus and jaundice. Gastroenterology. 2010;139:1586–1592. 1592 e1581. doi: 10.1053/j.gastro.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Grebely J, Petoumenos K, Hellard M, Matthews GV, Suppiah V, Applegate T, Yeung B, et al. Potential role for interleukin-28B genotype in treatment decision-making in recent hepatitis C virus infection. Hepatology. 2010;52:1216–1224. doi: 10.1002/hep.23850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcello T, Grakoui A, Barba-Spaeth G, Machlin ES, Kotenko SV, MacDonald MR, Rice CM. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131:1887–1898. doi: 10.1053/j.gastro.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 12.Ray SC, Wang YM, Laeyendecker O, Ticehurst JR, Villano SA, Thomas DL. Acute hepatitis C virus structural gene sequences as predictors of persistent viremia: hypervariable region 1 as a decoy. J Virol. 1999;73:2938–2946. doi: 10.1128/jvi.73.4.2938-2946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris HE, Eldridge KP, Harbour S, Alexander G, Teo CG, Ramsay ME. Does the clinical outcome of hepatitis C infection vary with the infecting hepatitis C virus type? J Viral Hepat. 2007;14:213–220. doi: 10.1111/j.1365-2893.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- 14.Grebely J, Morris MD, Rice TM, Bruneau J, Cox AL, Kim AY, McGovern BH, et al. Cohort Profile: The International Collaboration of Incident HIV and Hepatitis C in Injecting Cohorts (InC3) Study. Int J Epidemiol. 2012 [Google Scholar]
- 15.Badr G, Bedard N, Abdel-Hakeem MS, Trautmann L, Willems B, Villeneuve JP, Haddad EK, et al. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. J Virol. 2008;82:10017–10031. doi: 10.1128/JVI.01083-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van de Laar TJ, Molenkamp R, van den Berg C, Schinkel J, Beld MG, Prins M, Coutinho RA, et al. Frequent HCV reinfection and superinfection in a cohort of injecting drug users in Amsterdam. J Hepatol. 2009;51:667–674. doi: 10.1016/j.jhep.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Cox AL, Netski DM, Mosbruger T, Sherman SG, Strathdee S, Ompad D, Vlahov D, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis. 2005;40:951–958. doi: 10.1086/428578. [DOI] [PubMed] [Google Scholar]
- 18.Page-Shafer K, Pappalardo BL, Tobler LH, Phelps BH, Edlin BR, Moss AR, Wright TL, et al. Testing strategy to identify cases of acute hepatitis C virus (HCV) infection and to project HCV incidence rates. J Clin Microbiol. 2008;46:499–506. doi: 10.1128/JCM.01229-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofer H, Watkins-Riedel T, Janata O, Penner E, Holzmann H, Steindl-Munda P, Gangl A, et al. Spontaneous viral clearance in patients with acute hepatitis C can be predicted by repeated measurements of serum viral load. Hepatology. 2003;37:60–64. doi: 10.1053/jhep.2003.50019. [DOI] [PubMed] [Google Scholar]
- 20.Zhang M, Rosenberg PS, Brown DL, Preiss L, Konkle BA, Eyster ME, Goedert JJ. Correlates of spontaneous clearance of hepatitis C virus among people with hemophilia. Blood. 2006;107:892–897. doi: 10.1182/blood-2005-07-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, Nolt K, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. Jama. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 22.Thomson EC, Fleming VM, Main J, Klenerman P, Weber J, Eliahoo J, Smith J, et al. Predicting spontaneous clearance of acute hepatitis C virus in a large cohort of HIV-1-infected men. Gut. 2010 doi: 10.1136/gut.2010.217166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neukam K, Nattermann J, Rallon N, Rivero A, Caruz A, Macias J, Vogel M, et al. Different distributions of hepatitis C virus genotypes among HIV-infected patients with acute and chronic hepatitis C according to interleukin-28B genotype. HIV Med. 2011;12:487–493. doi: 10.1111/j.1468-1293.2011.00912.x. [DOI] [PubMed] [Google Scholar]
- 24.Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, Schraut WW, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–88. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 25.Santantonio T, Medda E, Ferrari C, Fabris P, Cariti G, Massari M, Babudieri S, et al. Risk factors and outcome among a large patient cohort with community-acquired acute hepatitis C in Italy. Clin Infect Dis. 2006;43:1154–1159. doi: 10.1086/507640. [DOI] [PubMed] [Google Scholar]
- 26.Wiese M, Berr F, Lafrenz M, Porst H, Oesen U. Low frequency of cirrhosis in a hepatitis C (genotype 1b) single-source outbreak in germany: a 20-year multicenter study. Hepatology. 2000;32:91–96. doi: 10.1053/jhep.2000.8169. [DOI] [PubMed] [Google Scholar]
- 27.Fanning LJ, Levis J, Kenny-Walsh E, Wynne F, Whelton M, Shanahan F. Viral clearance in hepatitis C (1b) infection: relationship with human leukocyte antigen class II in a homogeneous population. Hepatology. 2000;31:1334–1337. doi: 10.1053/jhep.2000.7437. [DOI] [PubMed] [Google Scholar]
- 28.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 29.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehmann M, Meyer MF, Monazahian M, Tillmann HL, Manns MP, Wedemeyer H. High rate of spontaneous clearance of acute hepatitis C virus genotype 3 infection. J Med Virol. 2004;73:387–391. doi: 10.1002/jmv.20103. [DOI] [PubMed] [Google Scholar]
- 31.Sarasin-Filipowicz M, Oakeley EJ, Duong FH, Christen V, Terracciano L, Filipowicz W, Heim MH. Interferon signaling and treatment outcome in chronic hepatitis C. Proc Natl Acad Sci U S A. 2008;105:7034–7039. doi: 10.1073/pnas.0707882105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grebely J, Prins M, Hellard M, Cox AL, Osburn WO, Lauer G, Page K, et al. Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: towards a vaccine. Lancet Infect Dis. 2012;12:408–414. doi: 10.1016/S1473-3099(12)70010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.