Abstract
Background
Atrial fibrillation (AF) is common among patients with heart failure and preserved ejection fraction (HFpEF) but its clinical profile and impact on exercise capacity remains unclear. RELAX was a multicenter randomized trial testing the impact of sildenafil on peak VO2 in stable outpatients with chronic HFpEF. We sought to compare clinical features and exercise capacity among HFpEF patients who were in sinus rhythm (SR) or AF.
Methods and Results
RELAX enrolled 216 HFpEF patients with 79 (37%) in AF, 124 (57%) in SR and 13 in other rhythms. Participants underwent baseline cardiopulmonary exercise testing (CPXT), echocardiogram, biomarker and rhythm status assessment prior to randomization. AF patients were older than those in SR but had similar symptom severity, co-morbidities and renal function. Betablocker use and chronotropic indices were also similar. Despite comparable LV size and mass, AF was associated with worse systolic (lower EF, stroke volume and cardiac index) and diastolic (shorter deceleration time and larger left atria) function compared to SR. Pulmonary artery systolic pressure was higher in AF. AF patients had higher NT-proBNP, aldosterone, endothelin-1, troponin I and CITP levels suggesting more severe neurohumoral activation, myocyte necrosis and fibrosis. Peak VO2 was lower in AF, even after adjustment for age, sex, and chronotropic response, and VE/VCO2 was higher.
Conclusions
AF identifies an HFpEF cohort with more advanced disease and significantly reduced exercise capacity. These data suggest that evaluation of the impact of different rate or rhythm control strategies on exercise tolerance in HFpEF patients with AF is warranted.
Keywords: atrial fibrillation, heart failure with preserved ejection fraction, exercise capacity
Atrial fibrillation (AF) and heart failure (HF) commonly co-exist and the presence of each worsens the outcome of the other1. The prevalence of AF in HF with preserved left ventricular ejection fraction (HFpEF; LVEF≥50%) is similar to that observed in patients with HF and reduced ejection fraction (HFrEF)2. In HFrEF, patients with AF have worse exercise capacity than those in sinus rhythm (SR)3 and small studies suggest that rhythm control improves exercise capacity4-6. However, HFpEF patients have been underrepresented in AF studies and it remains unclear whether the presence of AF further compromises exercise performance in HFpEF. Likewise, there is limited information regarding the profile of the HFpEF patient with AF, particularly in stringently defined HFpEF subjects with truly normal LVEF7.
The RELAX (Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in HFpEF) trial enrolled 216 HFpEF patients who met rigorous entry criteria designed to insure the presence of HF and cardiac limitation to exercise8. Rhythm status, echocardiography, biomarker assessment, and cardiopulmonary exercise testing (CPXT) were performed at baseline. We hypothesized that HFpEF patients with AF would display more advanced HF and greater impairment of exercise capacity when compared to HFpEF patients in SR. As HFpEF is associated with chronotropic incompetence9, while AF patients display an increased heart rate response during exercise10, we also evaluated the relationship between rhythm status and chronotropic response during exercise in HFpEF.
Methods
RELAX was a multicenter randomized (1:1) placebo-controlled trial testing the impact of chronic PDE5 inhibition (sildenafil) on exercise capacity in patients with HFpEF (ClinicalTrials.gov identifier NCT00763867)8. The trial was conducted by the Heart Failure Clinical Research Network (HFN) and funded by the National Heart, Lung, and Blood Institute. All patients provided written informed consent and the trial was approved by the institutional review board at each participating site. Notably, sildenafil did not improve exercise capacity in RELAX HFpEF patients and there was no evidence of an interaction between sildenafil and rhythm status8.
Study participants
RELAX enrolled patients with objective evidence of HF, LVEF≥50%, reduced exercise capacity, as evidenced by reduced (≤60% predicted) peak oxygen consumption (peak VO2) at screening CPXT11 and evidence of elevated filling pressures (elevated pulmonary capillary wedge pressure measured invasively or NT-proBNP≥400pg/ml). Additional entry criteria have been reported previously8. Rhythm status was classified as rhythm present on baseline electrocardiography (ECG). A history of AF was also recorded.
Baseline studies
The RELAX study design has been reported previously8, 12. Pertinent to this analysis, patients underwent baseline transthoracic echocardiography performed according to a standard protocol13 with measurements performed at the HFN echocardiography core laboratory (Mayo Clinic, Rochester, MN). Values reported in AF represent an average of 3 to 5 beats.
Plasma biomarker measurement included markers of neurohumoral activation (aldosterone, N-terminal pro-B-type natriuretic peptide [NT-proBNP], endothelin-1), cardiac injury or inflammation (troponin I, c-reactive peptide [CRP]), renal function (cystatin C, uric acid), and fibrosis (procollagen III n-terminal peptide [NT-procollagen III], galectin 3, c-telopeptide for type I collagen [CITP]). Assays were performed at the HFN biomarker core laboratory (University of Vermont, Burlington, VT)8, 12. Details of the RELAX CPXT protocol and HFN CPXT core laboratory (Massachusetts General Hospital, Boston, MA) methodologies have been reported12.
Briefly, symptom-limited CPXT with simultaneous expired ventilatory gas analysis was performed by treadmill or bicycle ergometry. Percent predicted peak VO2 was calculated according to published age and sex-normalized values11. Age, sex, body weight and mode-specific predicted peak VO2 was also calculated using the Wasserman equation14, 15. Entry criteria specified maximal effort as evidenced by a peak respiratory exchange ratio (RER)>1.0. The ventilatory anaerobic threshold (VAT) was determined by the V-slope method16. Peak oxygen pulse, reflecting oxygen consumption per heart beat during exercise, was obtained from the ratio of peak VO2 to peak exercise heart rate (HR). Peak circulatory power was defined as the product of peak VO2 and peak systolic blood pressure (SBP), while circulatory stroke work (SW) was derived from the ratio of circulatory power to peak exercise HR17. The VE/VCO2 slope for the entire duration of exercise was calculated based on 10s averaged VE (L/min) and VCO2 (L/min) data18.
Resting HR was documented after 5 minutes in a stationary position prior to CPXT. Peak HR was defined as HR at peak VO2. Chronotropic response was expressed as the change in HR from rest to peak exercise. Age-predicted maximal HR (APMHR) was defined with the Astrand (220-age)19, and Brawner (164-[0.7*age])20 formulae, and each used to calculate a chronotropic index (CI) reflecting the percentage of HR reserve used (change in HR from rest to peak exercise/ APMHR minus resting HR). Clinically significant chronotropic incompetence was defined as a CI<0.8 for patients not taking betablockers, and <0.62 in patients reporting active betablocker use (Astrand formula). No betablocker correction was employed for chronotropic incompetence defined by the Brawner formula. HR recovery (HRR) was taken as the absolute difference in HR between peak exercise and at 1 minute during exercise unloading or cessation. A HRR≤18bpm was considered abnormal21.
Statistical analysis
Continuous data are presented as mean ±standard deviation or median (25th, 75th percentiles) as appropriate; categorical data are presented as frequency (%) within each group (AF vs. SR). Baseline characteristics and CPXT parameters for the RELAX study population stratified by rhythm status were compared using the t test or Wilcoxon rank sum test for continuous variables, and chi-squared test for categorical variables. Univariable and multivariable linear regression analyses for pre-specified pertinent variables were performed to define the association between rhythm status and peak VO2. To adjust for the pathophysiological role of chronotropic response to exercise, a linear regression model was used to examine the relationship between CI and peak VO2 or peak workload with an interaction term included for rhythm status, thereby comparing the slope of the VO2-CI or workload-CI relationship between patients in AF and SR. Normality of model residuals was tested using the Kolmogorov-Smirnov test and visually assessed for symmetry. Analyses were performed using SAS version 9.2.; p<0.05 (2-sided) was considered statistically significant.
Results
Patient characteristics
RELAX enrolled 216 patients with HFpEF (mean age 69±10 years, 48% female) of whom 79 (37%) had AF, 124 (57%) were in SR, and 13 (6%) were in other rhythms (excluded from this analysis). Patients in AF were older than those in SR, but had similar reported symptom severity (NYHA class, MLWHFQ score), distribution of co-morbidities, hemoglobin and renal function (Table 1). Loop diuretic and digoxin therapy were more frequent, angiotensin converting-enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) use less frequent, and betablocker use similar among AF patients compared to those in SR.
Table 1. Baseline characteristics by rhythm status at enrollment.
| Variable | Atrial Fibrillation n=79 |
Sinus rhythm n=124 |
p-value |
|---|---|---|---|
| Age, y | 72.7±9.17 | 65.7±10.3 | <0.0001 |
| Female sex | 32 (41) | 67 (54) | 0.06 |
| BMI, kg/m2 | 33.2±6.4 | 34.7±8.2 | 0.14 |
| Rest Systolic BP, mmHg | 126±15 | 129±18 | 0.17 |
| Rest Diastolic BP, mmHg | 71±10 | 69±10 | 0.18 |
| Rest HR, bpm | 72±13 | 68±11 | 0.04 |
| NYHA III/IV | 42 (53) | 65 (52) | 0.92 |
| MLWHFQ score | 46±21 | 45±24 | 0.70 |
| Ischemic etiology | 33 (42) | 44 (36) | 0.37 |
| Comorbidities | |||
| Hypertension | 65 (82) | 108 (87) | 0.35 |
| Diabetes | 28 (35) | 56 (45) | 0.17 |
| COPD | 15 (19) | 24 (19) | 0.95 |
| History of AF | 79 (100) | 28 (23) | <0.0001 |
| GFR, mL/min/1.73m2 | 65.3 (51.6,77.2) | 65.6 (43.5,86.0) | 0.77 |
| Cystatin C, mg/L | 1.4 (1.1,1.7) | 1.3 (1.0,1.7) | 0.07 |
| Hb, mg/dL | 12.6 (12.1,13.8) | 13.0 (11.9,13.9) | 0.58 |
| NT-procollagen III, μg/L | 8.1 (6.4,10.7) | 7.5 (5.5,9.5) | 0.10 |
| CITP, μg/L | 6.7 (5.5,10.2) | 5.7 (4.2,9.0) | 0.003 |
| Galectin 3, ng/mL | 14.3 (11.7,19.7) | 13.6 (10.8,16.9) | 0.15 |
| Medication at enrollment | |||
| ACEI/ARB | 49 (62) | 97 (78) | 0.01 |
| Beta blocker | 63 (80) | 91 (73) | 0.30 |
| Aldosterone antagonist | 10 (13) | 11 (9) | 0.39 |
| Loop diuretic | 77 (98) | 78 (63) | <0.0001 |
| Digoxin | 18 (23) | 3 (2) | <0.0001 |
| Calcium channel blocker | 28 (35) | 36 (29) | 0.34 |
| Amiodarone | 1 (1) | 7 (6) | 0.15 |
| Other antiarrhythmic | 3 (4) | 6 (5) | 1.00 |
| Aspirin/Thienopyridine | 31 (39) | 90 (73) | <0.0001 |
| Warfarin | 69 (87) | 22 (18) | <0.0001 |
| Echocardiography | |||
| LVEF, % | 59±8 | 63±7 | 0.002 |
| Rest cardiac index, L/min/m2 | 2.4±0.6 | 2.6±0.7 | 0.04 |
| Stroke volume, mL | 70±20 | 84±24 | 0.0001 |
| eFS, % | 36±7 | 40±6 | 0.0002 |
| mFS, % | 19±4 | 21±3 | 0.0009 |
| LV mass index, g/m2 | 84±33 | 82±33 | 0.77 |
| LV diastolic dimension, cm | 4.6±0.7 | 4.7±0.6 | 0.71 |
| Deceleration time, ms | 172±48 | 203±44 | <0.0001 |
| LAVI, mL/m2 | 62±25 | 41±13 | <0.0001 |
| PASP, mmHg | 45±12 | 41±12 | 0.045 |
| RA pressure, mmHg | 11±5 | 7±4 | <0.0001 |
| E/e’ (medial) | 18.6±9.2 | 18.2±9.7 | 0.74 |
Continuous variables shown as mean ±SD (median [25th,75th percentiles] for biomarkers); categorical variables shown as count (percentage).
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CITP, c-telopeptide for type I collagen; COPD, chronic obstructive pulmonary disease; eFS, endocardial fractional shortening; GFR, glomerular filtration rate; HR, heart rate; LAVI, left atrial volume index; LV, left ventricular; LVEF, left ventricular ejection fraction; mFS, midwall fractional shortening; MLWHFQ, Minnesota living with heart failure questionnaire total score; NT-procollagen III, procollagen III n-terminal peptide; NYHA, New York Heart Association class; PASP, pulmonary arterial systolic pressure; RA, right atrial.
LV dimensions and LV mass index (LVMI) were comparable between AF and SR; however AF was associated with worse systolic function at rest (lower LVEF, stroke volume [SV], endocardial [eFS] and midwall fractional shortening [mFS]). Although E/e’ was similar between groups, other parameters of LV diastolic function were significantly worse in AF (shorter deceleration time, higher right atrial pressure [RAP], larger left atrial volume index [LAVI]). Pulmonary artery systolic pressures [PASP] were also higher in AF. Neurohumoral activation was more severe in AF relative to SR (elevated plasma NT-proBNP, aldosterone, endothelin-1; Figure 1). Troponin I levels were higher in AF than SR, consistent with greater myocardial necrosis (Figure 1). Plasma markers of fibrosis (NT-procollagen III, CITP, galectin 3) were higher in AF than SR, however only CITP reached statistical significance (Table 1).
Figure 1. Biomarkers of neurohumoral activity in HFpEF patients in atrial fibrillation and sinus rhythm.
(a) Plasma NT-proBNP, (b) aldosterone, (c) endothelin-1, (d) troponin I, (e) uric acid, (f) c-reactive protein. White bars (atrial fibrillation), black bars (sinus rhythm). Median (75th percentile) shown.
Exercise performance
Fewer patients in AF performed bicycle ergometry (52% AF vs. 68% SR, p=0.02). Both groups performed a maximal or near-maximal CPXT, inferable from subjective (Borg score) and objective (RER) measures of exertion at peak exercise (Table 2). The most common reason for exercise cessation was dyspnea in AF (49% AF vs. 37% SR) and fatigue among patients in SR (31% AF vs. 52% SR). Exercise duration was shorter for AF than SR (mean 9.0 vs. 10.1min, p=0.02) but not after age-sex adjustment (p=0.14).
Table 2. Cardiopulmonary exercise test data.
| Variable | Atrial Fibrillation n=79 |
Sinus rhythm n=124 |
p-value |
|---|---|---|---|
| Exercise duration, min | 9.0±3.0 | 10.1±3.0 | 0.02 |
| 6MWD, m | 283±107 | 313±109 | 0.055 |
| Rest VO2, mL/kg/min | 3.1±0.6 | 2.9±0.8 | 0.049 |
| Rest pulse pressure, mmHg | 51±15 | 59±19 | 0.001 |
| Peak RER | 1.1±0.1 | 1.1±0.1 | 0.62 |
| Peak Borg score | 6.8±2.5 | 6.8±2.3 | 0.91 |
| Peak SBP, mmHg | 138±30 | 163±29 | <0.0001 |
| Peak DBP, mmHg | 69±14 | 74±15 | 0.02 |
| Peak pulse pressure, mmHg | 69±26 | 89±24 | <0.0001 |
| Peak VO2, mL/kg/min | 11.7±2.7 | 12.8±3.2 | 0.008 |
| VO2 at VAT, mL/kg/min | 7.2±1.8 | 7.7±1.9 | 0.07 |
| VO2 at VAT (% of peak VO2) | 62.6±9.2 | 60.6±8.6 | 0.12 |
| % age-sex predicted VO2 | |||
| Wasserman, % | 63.6±14.2 | 68.8±16.0 | 0.02 |
| Standard, % | 40.4±8.6 | 42.8±9.6 | 0.11 |
| Peak O2 pulse, mL/kg/bpm | 0.11±0.03 | 0.12±0.03 | 0.07 |
| Peak O2 pulse, mL/bpm | 10.5±3.2 | 11.6±4.3 | 0.04 |
|
Peak circulatory power,
mmHg*mL/kg/min |
1644±588 | 2109±751 | <0.0001 |
|
Circulatory stroke work,
mmHg*mL/kg/bpm |
15.5±5.7 | 19.4±6.1 | <0.0001 |
| Peak workload, Watts | 67±29 | 77±32 | 0.03 |
| Peak VCO2, mL/kg/min | 12.8±3.3 | 14.1±3.8 | 0.01 |
| Peak VE, L/min | 43.6±12.7 | 47.0±16.0 | 0.12 |
| VE/VCO2 slope | 35.1±7.2 | 32.6±8.1 | 0.03 |
| Chronotropic indices | |||
| Rest HR (pre-exercise) | 72±12 | 69±14 | 0.14 |
| Peak HR, bpm | 109±27 | 111±24 | 0.69 |
| Chronotropic response, bpm | 37±23 | 42±20 | 0.17 |
| Age-predicted maximal HR, bpm | |||
| Astrand formula† | 147±9 | 154±10 | <0.0001 |
| Brawner formula‡ | 113±6 | 118±7 | <0.0001 |
| Chronotropic index | |||
| Astrand formula† | 0.51±0.34 | 0.50±0.25 | 0.73 |
| Brawner formula‡ | 1.10±1.08 | 0.92±0.61 | 0.19 |
| HRR, bpm | 11±15 | 10±9 | 0.51 |
| HRR≤18bpm, n (%) | 55 (73) | 94 (83) | 0.14 |
Continuous variables shown as mean ±SD; categorical variables shown as count (percentage).
HR, heart rate; HRR, absolute heart rate recovery at 1 minute post exercise; RER, respiratory exchange ratio; VAT, ventilatory anerobic threshold; VE, minute ventilation; VCO2, carbon dioxide output; VE/VCO2, slope of the relationship between minute ventilation and carbon dioxide output; VO2, oxygen consumption.
220-age19
164-[0.7*age]20
Resting VO2 was higher in AF patients compared to SR. However peak VO2, scaled to body mass (standard), was significantly reduced in AF, and confirmed by a lower percent-predicted peak VO2 (Wasserman formula; Table 2). VO2 at VAT tended to be lower in AF, though as a proportion of peak VO2 was similar between groups. Peak exercise workload was also lower in AF relative to SR. On multivariable analysis, AF was associated with a reduced peak VO2 even after adjustment for age, sex, LVEF, and exercise modality (Table 3).
Table 3. Relationship between atrial fibrillation and exercise capacity measured by peak VO2.
| Model | Linear regression analysis (peak VO2) | |||
|---|---|---|---|---|
| Sample size |
Estimated difference between AF and SR (mL/kg/min) |
95%CI | p-value | |
| Unadjusted | 202 | −1.2 | −2.0 to −0.3 | 0.008 |
| Adjusted for age/sex | 202 | −0.9 | −1.7 to −0.1 | 0.03 |
| Adjusted for | 199 | −0.9 | −1.8 to −0.1 | 0.03 |
| age/sex/EF | ||||
| Adjusted for | 199 | −1.0 | −1.9 to −0.2 | 0.02 |
| age/sex/EF and | ||||
| exercise modality* | ||||
| Adjusted for age/ sex/ | ||||
| chronotropic index | ||||
| Astrand formula | 200 | −1.2 | −2.2 to −0.3 | 0.01 |
| Brawner formula | 200 | −1.2 | −2.2 to −0.2 | 0.02 |
CI, confidence interval; EF, ejection traction.
Bicycle vs. treadmill
Minute ventilation was similar in AF and SR but the VE/VCO2 slope was elevated in AF. Even after age-sex adjustment, patients with AF demonstrated a significantly reduced peak exercise SBP (p<0.0001; Table 3). AF patients had lower pulse pressure, lower circulatory power and stroke work; O2 pulse was also or tended to be lower (Table 3). Six-minute walk distance, representing submaximal exercise capacity, also displayed a lower trend in AF versus SR (Table 2).
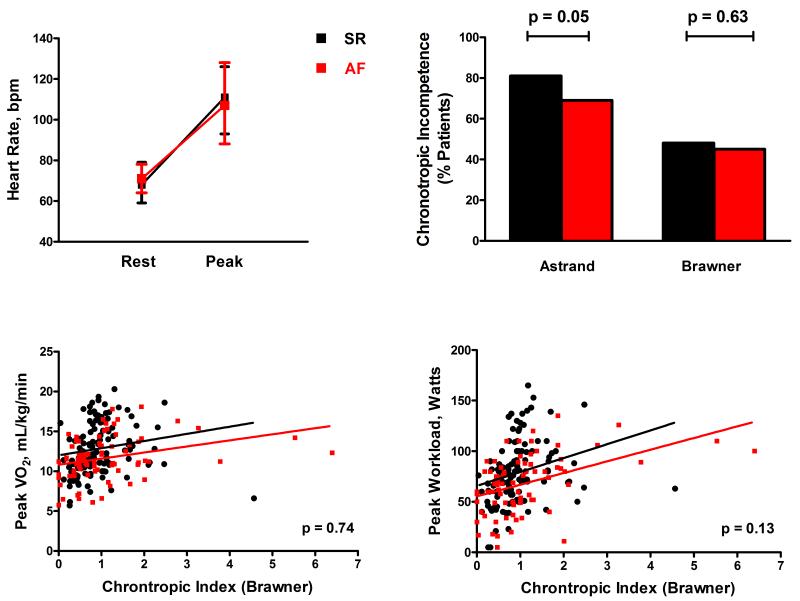
Heart rates at rest and at peak exercise were similar between patients in AF and SR (Table 2, Figure 2A). Likewise, despite a lower mean APMHR (Astrand/Brawner formula) for AF patients, the chronotropic response as reflected by the CI (Table 2) and the prevalence of chronotropic incompetence (Figure 2B) were equal between groups. Albeit a majority of patients reported betablocker use (Table 1), the median CI was similar among AF patients with and without betablockers (0.85 vs. 0.9, p=0.90), and higher in SR patients on betablockers compared to those without (0.74 vs. 0.42, p<0.0001). The relationship between peak VO2 or peak workload and CI did not differ between HFpEF patients in AF or SR (Figure 2C-D) and AF remained associated with a lower peak VO2 even after adjustment for CI (Table 3).
Figure 2. Chronotropic response to exercise in HFpEF patients in atrial fibrillation and sinus rhythm.
(a) Heart rates at rest and at peak exercise, (b) prevalence of chronotropic incompetence during exercise (calculated using standard [Astrand] or Brawner formulae; p-values AF vs. SR), (c) relationship between chronotropic index (Brawner formula) and peak VO2, (d) relationship between chronotropic index and peak workload. Results for unadjusted linear regression shown for patients in atrial fibrillation (red line) and sinus rhythm (black line). p-values (c) and (d) refer to interaction terms between rhythm status and chronotropic index.
While a majority of HFpEF patients had abnormal HRR consistent with autonomic dysfunction, the severity of impaired HRR was similar between groups.
Discussion
This analysis of the RELAX HFpEF cohort demonstrates that important phenotypic differences exist between HFpEF patients with and without AF and is the first comprehensive analysis of the impact of AF on exercise capacity in HFpEF. Consistent with our hypothesis, HFpEF patients in AF were older and exhibited worse LV systolic and diastolic function at rest, more severe neurohumoral activation, and greater impairment of exercise capacity compared to HFpEF patients in SR. Peak VO2 was lower in AF despite adjustment for pertinent variables, and was not accompanied by a higher chronotropic response as has been seen in HFrEF patients with AF22. Ventilatory efficiency was lower (steeper VE/VCO2 relationship) in AF, suggesting greater impairment of pulmonary perfusion during exercise. Peak exercise SBP, circulatory power, and circulatory stroke work were lower and peak O2 pulse tended to be lower in AF suggesting impaired systolic reserve function. These findings demonstrate that mechanisms beyond altered chronotropic response mediate the more impaired exercise tolerance in HFpEF patients with AF and suggest that AF is a marker of a more advanced HFpEF phenotype.
In patients with HFrEF, AF is associated with more severe symptoms and LV systolic impairment23. In HFrEF, a rhythm control strategy does not result in better outcomes than rate control24, and betablockers may have reduced pharmacological efficacy contrary to established benefits for patients in SR25. Importantly, characteristics associated with AF in HFpEF are less well documented, despite the high prevalence of AF in HFpEF and evidence of equivalent26 or perhaps stronger association with morbidity and mortality in HFpEF than HFrEF2, 27. Existing studies are highly discrepant, have limited phenotypic characterization and enrolled patients during a hospitalization for HF which may not accurately reflect the HFpEF population at large27-29. Although the CHARM-Preserved trial studied stable HFpEF outpatients with and without AF2, the prevalence of coronary artery disease was higher than typically observed in HFpEF and HFpEF was defined by a LVEF>40% thereby including patients with potential HFrEF pathophysiology.
RELAX enrolled stable chronic HFpEF patients in SR and AF, at least 3 months out from any HF hospitalization, exhibiting a LVEF≥50% consistent with current diagnostic criteria7. In this setting AF was associated with older age and worse LV systolic and diastolic function, mirroring observations in HFrEF23 but contrary to sparse available data in HFpEF and AF, where systolic function (LVEF) has been reported as similar to SR27-29. Furthermore, in contrast to the findings of Linssen et al.27, and perhaps due to the exclusion of patients with LVEF40-49%, we found NT-proBNP to be significantly elevated in AF relative to HFpEF-SR patients. This corroborates baseline findings in the I-Preserve population30, and may either pertain to AF and/or more severe HF in HFpEF-AF patients. More severe HF is also suggested by elevation in other heretofore unstudied biomarkers of HF severity in HFpEF-AF patients (aldosterone, ET-1, troponin I), greater diuretic use and a lower cardiac index. A higher resting VO2 was observed in AF which, as in HFrEF, may signify increased resting energy demands or hypermetabolism corresponding to increased HF severity 31, 32. These findings support the rationale for aggressive upstream therapy addressing the atrial substrate in HF patients with AF, which is currently under investigation33.
AF has been associated with reduced functional capacity in HFrEF22 and in patients without structural heart disease34, however prior studies of exercise tolerance in HFpEF have mostly excluded patients in AF9, 35, 36. Similarly, HFpEF has been underrepresented in AF intervention trials4, 24, 37, thus the impact of AF on exercise capacity and cardiopulmonary function during exercise in HFpEF remains unclear. Fung et al. reported the mean 6MWD to be lower in hospitalized HFpEF patients with AF (279±66m, n=42) compared to SR (338±86m, n=104)28, however formal cardiopulmonary stress testing was not performed. In the present analysis we confirm and extend this observation in several respects.
Our novel principal finding is that, compared to SR, HFpEF-AF patients have a lower peak VO2 both in absolute terms and relative to age, sex, body-size and exercise mode-adjusted predicted values. Further, we demonstrate that the impaired exercise capacity in AF is not explained by differences in resting LVEF or altered chronotropic response, suggesting a specific effect of AF on cardiac reserve. Rhythm irregularity38 and loss of atrial contribution to LV filling39 are recognized to impair cardiac output in AF. Indeed, the lower pulse pressure and circulatory stroke work observed in HFpEF-AF patients suggest diminished SV at peak exercise. Additionally, since peak VO2 represents the product of cardiac output and arterial-venous oxygen content difference, a greater impairment of peripheral oxygen availability or utilization in AF may also be important. Endothelial dysfunction40 and peripheral muscle blood flow abnormalities have been correlated with exercise performance in patients with chronic AF, although in the absence of structural heart disease41.
The mean VE/VCO2 slope was significantly steeper in AF-HFpEF patients compared to SR, reflecting impaired ventilatory efficiency. As the prevalence of lung disease was similar between groups, this likely reflects impaired augmentation of pulmonary perfusion during exercise, a factor which may contribute to earlier exercise cessation and reduced functional capacity in AF42. An increased VE/VCO2 slope has been observed in patients with permanent AF compared to control subjects in SR43 and accompanying lone AF before cardioversion to SR34, 44. However, Ariansen et al.43 reported no difference in ventilatory efficiency between AF and SR when HF patients were excluded from their analysis. Further, Agostoni et al.22 did not observe an association between AF and VE/VCO2 slope in chronic HFrEF. Therefore it remains unclear whether the steeper VE/VCO2 slope can be ascribed to a specific effect of AF on exercise hemodynamics. More likely AF is a marker of reduced pulmonary vascular reserve as well as more impaired diastolic reserve function in HFpEF patients, as suggested by the differences in baseline characteristics.
Interestingly HFpEF-AF patients did not display an exaggerated chronotropic response to exercise compared to SR patients, contrary to previous findings in lone AF34 and AF with HFrEF3, 22. Peak HR and CI were similar between AF and SR, while peak VO2 and workload were lower in AF patients; however, the relationships between CI and peak VO2 or workload were not significantly different for AF than SR. Chronotropic incompetence is a common feature in HFpEF patients in SR9 and our data demonstrate that HFpEF patients in AF have a similar prevalence of chronotropic incompetence when their prevalence of BB use is equivalent to SR. Notably, no symptomatic or prognostic benefit has been demonstrated with strict versus lenient HR control in the general AF population45 including a post-hoc analysis of patients with HF46 although the latter did not examine the impact of rate control strategy on events stratified by HFpEF and HFrEF. Therefore, although current ACC/AHA guidelines recommend HR control in HFpEF patients with AF (class IC)47, the optimal level of HR control in HFpEF, particularly with respect to exercise capacity, has yet to be established.
Study Limitations
Rhythm was classified by the presence or absence of AF on enrollment ECG. Consequently HFpEF patients with previous or paroxysmal AF may have been classified as SR, though this would likely serve to underestimate the observed differences. Greater frequency of treadmill exercise versus bicycle ergometry in AF would also minimize any differences in peak VO2 from SR as bicycle ergometry is generally associated with a lower peak VO2. Information on right heart structure and function, along with duration of AF and decisions regarding rate or rhythm control were unavailable, though antiarrhythmic agents were used in <10% of the overall population. Therefore, these data pertain to patients with and without persistent AF in the context of heterogeneous AF and HF therapeutic strategies.
Conclusions
In patients with HFpEF, AF is associated with important phenotypic and functional differences: older age, impaired LV systolic and diastolic function and functional reserve, more severe neurohumoral activation, and impaired exercise tolerance, supporting AF as a marker of more advanced HF phenotype in HFpEF. Furthermore, in the context of betablocker therapy, chronotropic incompetence is equally common in HFpEF patients with AF or SR. Further study is required to determine whether different rate control strategies or indeed, rhythm control in HFpEF patients with AF may favorably impact exercise tolerance.
Acknowledgments
Sources of Funding
This study was supported by grants from the NHLBI: U01HL084904 (for the data coordinating center), and U01HL084861, U01HL084875, U01HL084877, U01HL084889, U01HL084890, U01HL084891, U01HL084899, U01HL084907, and U01HL084931 (for the clinical centers). This work is also supported by the National Center for Advancing Translational Sciences (NCATS): UL1TR000454; and the National Institute on Minority Health and Health Disparities (NIMHD): U54 MD007588. R. Zakeri is supported by the Mayo Clinic Center for Clinical and Translational Science (Grant UL1TR000135) from the National Center for Advancing Translational Science). R. Zakeri and S.F. Mohammed are Heart Failure Network Clinical Research Skills Development Fellows, and support for mentoring R. Zakeri and S.F. Mohammed is provided by U01HL084907 and U10HL110262. Support for S.F. Mohammed was provided by T32 HL007111.
Footnotes
Disclosures
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The framingham heart study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 2.Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, Puu M, Yusuf S, Pfeffer MA, Investigators C. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: Results from the candesartan in heart failure-assessment of reduction in mortality and morbidity (charm) program. Journal of the American College of Cardiology. 2006;47:1997–2004. doi: 10.1016/j.jacc.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 3.Pardaens K, Van Cleemput J, Vanhaecke J, Fagard RH. Atrial fibrillation is associated with a lower exercise capacity in male chronic heart failure patients. Heart. 1997;78:564–568. doi: 10.1136/hrt.78.6.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shelton RJ, Clark AL, Goode K, Rigby AS, Houghton T, Kaye GC, Cleland JG. A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure: (cafe-ii study) Heart. 2009;95:924–930. doi: 10.1136/hrt.2008.158931. [DOI] [PubMed] [Google Scholar]
- 5.Hsu LF, Jais P, Sanders P, Garrigue S, Hocini M, Sacher F, Takahashi Y, Rotter M, Pasquie JL, Scavee C, Bordachar P, Clementy J, Haissaguerre M. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373–2383. doi: 10.1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
- 6.Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman-Haley SL, McDonagh TA, Underwood SR, Markides V, Wong T. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61:1894–1903. doi: 10.1016/j.jacc.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 7.Vasan RS, Levy D. Defining diastolic heart failure: A call for standardized diagnostic criteria. Circulation. 2000;101:2118–2121. doi: 10.1161/01.cir.101.17.2118. [DOI] [PubMed] [Google Scholar]
- 8.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, Lewinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 10.Hornsten TR, Bruce RA. Effects of atrial fibrillation on exercise performance in patients with cardiac disease. Circulation. 1968;37:543–548. doi: 10.1161/01.cir.37.4.543. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher GF, Balady G, Froelicher VF, Hartley LH, Haskell WL, Pollock ML. Exercise standards. A statement for healthcare professionals from the american heart association. Writing group. Circulation. 1995;91:580–615. doi: 10.1161/01.cir.91.2.580. [DOI] [PubMed] [Google Scholar]
- 12.Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, Deswal A, Hernandez AF, Lee KL, Braunwald E. Phosphdiesterase-5 inhibition to improve clinical status and exercise capacity in diastolic heart failure (relax) trial: Rationale and design. Circ Heart Fail. 2012;5:653–659. doi: 10.1161/CIRCHEARTFAILURE.112.969071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: A report from the american society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the european association of echocardiography, a branch of the european society of cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129:S49–55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 15.Arena R, Myers J, Abella J, Pinkstaff S, Brubaker P, Moore B, Kitzman D, Peberdy MA, Bensimhon D, Chase P, Forman D, West E, Guazzi M. Determining the preferred percent-predicted equation for peak oxygen consumption in patients with heart failure. Circ Heart Fail. 2009;2:113–120. doi: 10.1161/CIRCHEARTFAILURE.108.834168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 17.Cohen-Solal A, Tabet JY, Logeart D, Bourgoin P, Tokmakova M, Dahan M. A non-invasively determined surrogate of cardiac power (‘circulatory power’) at peak exercise is a powerful prognostic factor in chronic heart failure. Eur Heart J. 2002;23:806–814. doi: 10.1053/euhj.2001.2966. [DOI] [PubMed] [Google Scholar]
- 18.Arena R, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, Guazzi M. Development of a ventilatory classification system in patients with heart failure. Circulation. 2007;115:2410–2417. doi: 10.1161/CIRCULATIONAHA.107.686576. [DOI] [PubMed] [Google Scholar]
- 19.Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl. 1960;49:1–92. [PubMed] [Google Scholar]
- 20.Brawner CA, Ehrman JK, Schairer JR, Cao JJ, Keteyian SJ. Predicting maximum heart rate among patients with coronary heart disease receiving beta-adrenergic blockade therapy. Am Heart J. 2004;148:910–914. doi: 10.1016/j.ahj.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: The case of stress echocardiography. Circulation. 2001;104:1911–1916. [PubMed] [Google Scholar]
- 22.Agostoni P, Emdin M, Corra U, Veglia F, Magri D, Tedesco CC, Berton E, Passino C, Bertella E, Re F, Mezzani A, Belardinelli R, Colombo C, La Gioia R, Vicenzi M, Giannoni A, Scrutinio D, Giannuzzi P, Tondo C, Di Lenarda A, Sinagra G, Piepoli MF, Guazzi M. Permanent atrial fibrillation affects exercise capacity in chronic heart failure patients. Eur Heart J. 2008;29:2367–2372. doi: 10.1093/eurheartj/ehn361. [DOI] [PubMed] [Google Scholar]
- 23.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2D–8D. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 24.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, Connolly SJ, Dubuc M, Ducharme A, Guerra PG, Hohnloser SH, Lambert J, Le Heuzey JY, O’Hara G, Pedersen OD, Rouleau JL, Singh BN, Stevenson LW, Stevenson WG, Thibault B, Waldo AL. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 25.Rienstra M, Damman K, Mulder BA, Van Gelder IC, McMurray J, Van Veldhuisen DJ. Beta-blockers and outcomes in heart failure and atrial fibrillation. A meta-analysis. JACC: Heart Fail. 2013;1:21–28. doi: 10.1016/j.jchf.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Badheka AO, Rathod A, Kizilbash MA, Bhardwaj A, Ali O, Afonso L, Jacob S. Comparison of mortality and morbidity in patients with atrial fibrillation and heart failure with preserved versus decreased left ventricular ejection fraction. Am J Cardiol. 2011;108:1283–1288. doi: 10.1016/j.amjcard.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 27.Linssen GC, Rienstra M, Jaarsma T, Voors AA, van Gelder IC, Hillege HL, van Veldhuisen DJ. Clinical and prognostic effects of atrial fibrillation in heart failure patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail. 2011;13:1111–1120. doi: 10.1093/eurjhf/hfr066. [DOI] [PubMed] [Google Scholar]
- 28.Fung JW, Sanderson JE, Yip GW, Zhang Q, Yu CM. Impact of atrial fibrillation in heart failure with normal ejection fraction: A clinical and echocardiographic study. J Card Fail. 2007;13:649–655. doi: 10.1016/j.cardfail.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Rusinaru D, Leborgne L, Peltier M, Tribouilloy C. Effect of atrial fibrillation on long-term survival in patients hospitalised for heart failure with preserved ejection fraction. Eur J Heart Fail. 2008;10:566–572. doi: 10.1016/j.ejheart.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 30.McKelvie RS, Komajda M, McMurray J, Zile M, Ptaszynska A, Donovan M, Carson P, Massie BM. Baseline plasma nt-probnp and clinical characteristics: Results from the irbesartan in heart failure with preserved ejection fraction trial. J Card Fail. 2010;16:128–134. doi: 10.1016/j.cardfail.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Riley M, Elborn JS, McKane WR, Bell N, Stanford CF, Nicholls DP. Resting energy expenditure in chronic cardiac failure. Clin Sci (Lond) 1991;80:633–639. doi: 10.1042/cs0800633. [DOI] [PubMed] [Google Scholar]
- 32.Obisesan TO, Toth MJ, Donaldson K, Gottlieb SS, Fisher ML, Vaitekevicius P, Poehlman ET. Energy expenditure and symptom severity in men with heart failure. Am J Cardiol. 1996;77:1250–1252. doi: 10.1016/s0002-9149(96)00176-2. [DOI] [PubMed] [Google Scholar]
- 33.Alings M, Smit MD, Moes ML, Crijns HJ, Tijssen JG, Brugemann J, Hillege HL, Lane DA, Lip GY, Smeets JR, Tieleman RG, Tukkie R, Willems FF, Vermond RA, Van Veldhuisen DJ, Van Gelder IC. Routine versus aggressive upstream rhythm control for prevention of early atrial fibrillation in heart failure: Background, aims and design of the race 3 study. Neth Heart J. 2013;21:354–363. doi: 10.1007/s12471-013-0428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guazzi M, Belletti S, Tumminello G, Fiorentini C, Guazzi MD. Exercise hyperventilation, dyspnea sensation, and ergoreflex activation in lone atrial fibrillation. Am J Physiol Heart Circ Physiol. 2004;287:H2899–2905. doi: 10.1152/ajpheart.00455.2004. [DOI] [PubMed] [Google Scholar]
- 35.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeder MT, Thompson BR, Htun N, Kaye DM. Hemodynamic determinants of the abnormal cardiopulmonary exercise response in heart failure with preserved left ventricular ejection fraction. J Card Fail. 2012;18:702–710. doi: 10.1016/j.cardfail.2012.06.530. [DOI] [PubMed] [Google Scholar]
- 37.Hagens VE, Crijns HJ, Van Veldhuisen DJ, Van Den Berg MP, Rienstra M, Ranchor AV, Bosker HA, Kamp O, Tijssen JG, Veeger NJ, Van Gelder IC. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: Results from the rate control versus electrical cardioversion (race) study. Am Heart J. 2005;149:1106–1111. doi: 10.1016/j.ahj.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 38.Clark DM, Plumb VJ, Epstein AE, Kay GN. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol. 1997;30:1039–1045. doi: 10.1016/s0735-1097(97)00254-4. [DOI] [PubMed] [Google Scholar]
- 39.Kristensson BE, Arnman K, Ryden L. The haemodynamic importance of atrioventricular synchrony and rate increase at rest and during exercise. Eur Heart J. 1985;6:773–778. doi: 10.1093/oxfordjournals.eurheartj.a061940. [DOI] [PubMed] [Google Scholar]
- 40.Guazzi M, Belletti S, Bianco E, Lenatti L, Guazzi MD. Endothelial dysfunction and exercise performance in lone atrial fibrillation or associated with hypertension or diabetes: Different results with cardioversion. Am J Physiol Heart Circ Physiol. 2006;291:H921–928. doi: 10.1152/ajpheart.00986.2005. [DOI] [PubMed] [Google Scholar]
- 41.Gosselink AT, Smit AJ, Crijns HJ, Hillege HH, Lie KI. Alteration of peripheral vasodilatory reserve capacity after cardioversion of atrial fibrillation. Eur Heart J. 1996;17:926–934. doi: 10.1093/oxfordjournals.eurheartj.a014975. [DOI] [PubMed] [Google Scholar]
- 42.Chua TP, Ponikowski P, Harrington D, Anker SD, Webb-Peploe K, Clark AL, Poole-Wilson PA, Coats AJ. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol. 1997;29:1585–1590. doi: 10.1016/s0735-1097(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 43.Ariansen I, Edvardsen E, Borchsenius F, Abdelnoor M, Tveit A, Gjesdal K. Lung function and dyspnea in patients with permanent atrial fibrillation. Eur J Intern Med. 2011;22:466–470. doi: 10.1016/j.ejim.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Lundstrom T, Karlsson O. Improved ventilatory response to exercise after cardioversion of chronic atrial fibrillation to sinus rhythm. Chest. 1992;102:1017–1022. doi: 10.1378/chest.102.4.1017. [DOI] [PubMed] [Google Scholar]
- 45.Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Hillege HL, Bergsma-Kadijk JA, Cornel JH, Kamp O, Tukkie R, Bosker HA, Van Veldhuisen DJ, Van den Berg MP. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 46.Mulder BA, Van Veldhuisen DJ, Crijns HJ, Tijssen JG, Hillege HL, Alings M, Rienstra M, Groenveld HF, Van den Berg MP, Van Gelder IC, Lenient vs. Lenient vs. strict rate control in patients with atrial fibrillation and heart failure: a post-hoc analysis of the RACE II study. Eur J Heart Fail. 2013;15:1311–8. doi: 10.1093/eurjhf/hft093. [DOI] [PubMed] [Google Scholar]
- 47.Yancy CW, Jessup M, Bozkurt B, Masoudi FA, Butler J, McBride PE, Casey DE, Jr., McMurray JJ, Drazner MH, Mitchell JE, Fonarow GC, Peterson PN, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 accf/aha guideline for the management of heart failure: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]