Abstract
Background
Arthralgia is a common and debilitating side effect experienced by breast cancer patients receiving aromatase inhibitors (AIs) and often results in premature drug discontinuation.
Methods
We conducted a randomized controlled trial of electro-acupuncture (EA) as compared to waitlist control (WLC) and sham acupuncture (SA) in postmenopausal women with breast cancer who self-reported arthralgia attributable to AIs. Acupuncturists performed ten EA/SA treatments over eight weeks using a manualized protocol with 2 Hz electro-stimulation delivered by a TENS unit. Acupuncturists administered SA using Streitberger (non-penetrating) needles at non-traditional acupuncture points without electro-stimulation. The primary endpoint was pain severity by Brief Pain Inventory (BPI) between EA and WLC at Week 8; durability of response at Week 12 and comparison of EA to SA were secondary aims.
Findings
Of the 67 randomly assigned patients, mean reduction in pain severity was greater in the EA group than in the WLC group at Week 8 (−2.2 vs. −0.2, p=0.0004) and at Week 12 (−2.4 vs. −0.2, p<0.0001). Pain-related interference measured by BPI also improved in the EA group compared to the WLC group at both Week 8 (−2.0 vs. 0.2, p=0.0006) and Week 12 (−2.1 vs. −0.1, p=0.0034). SA produced a magnitude of change in pain severity and pain-related interference at Week 8 (−2.3, −1.5 respectively) and Week 12 (−1.7, −1.3 respectively) similar to that of EA. Participants in both EA and SA groups reported few minor adverse events.
Interpretations
Compared to usual care, EA produced clinically important and durable improvement in arthralgia related to AIs in breast cancer patients, and SA had a similar effect. Both EA and SA were safe.
Keywords: Acupuncture, breast neoplasm, clinical trial, Aromatase inhibitors/*adverse effects, musculoskeletal, joint pain
INTRODUCTION
Arthralgia, or joint pain, is a debilitating side effect of aromatase inhibitors (AIs) among postmenopausal women with hormone receptor positive breast cancer taking these drugs.(1) Nearly half of AI-users in the clinical setting report arthralgia attributable to AIs.(2) Arthralgia ranks as the top symptom associated with AIs discussed in online breast cancer-specific message boards(3) and often results in poor adherence, or discontinuation.(4) Premature discontinuation negatively impacts disease free and overall breast cancer survival.(5)
There is emerging evidence(6) and acceptance(7) for acupuncture, a practice in Traditional Chinese Medicine, as a component of pain management. Many breast cancer patients desire the integration of acupuncture into their conventional cancer care(8) and 60% of National Cancer Institute designated comprehensive cancer centers in the U.S. recommend acupuncture as an approach for patient symptom management.(9) Despite growing interest from patients and cancer centers, rigorous research is needed to guide its evidence-based integration into cancer care to improve patient outcomes.
A few studies have suggested that acupuncture may be safe and effective for managing AI-related arthralgia(10–12). However, lack of controls, small sample sizes, and high drop-out levels in intervention arms limit the interpretation of these results. Additionally, lack of comparison with usual care makes it difficult to evaluate the clinical relevance of the overall effect of acupuncture for this condition. To more definitively test the clinical effect of acupuncture, we conducted a Phase-II randomized controlled trial (RCT) to evaluate the short term effects and safety of electro-acupuncture (EA) for AI-related arthralgia compared to usual care. We chose EA because animal research has demonstrated its clear physiological effect on the endogenous opioid system (enkaphalin, beta-endorphin, and endomorphin) and pain reduction.(13) Our primary hypothesis was that patients receiving EA would have a greater reduction in arthralgia and improved function at Week 8 compared to the Waitlist Control (WLC) “usual care” group. As secondary aims, we evaluated the durability of response with a repeated measure at Week 12 and evaluated the magnitude of response to sham acupuncture (SA) to inform the design of a future phase III trial.
METHODS
STUDY PARTICIPANTS
We conducted a three arm RCT (EA, SA, and WLC) from September 2009 through May 2012 at the Abramson Cancer Center of the Hospital of the University of Pennsylvania, a tertiary care academic medical center in Philadelphia. The institutional review board of the University of Pennsylvania approved the study protocol. Eligible patients were women with a history of early stage breast cancer (stages I–III) who were currently receiving an aromatase inhibitor (Anastrozole, Letrozole, or Exemestane), had joint pain that they attributed to their AI for at least three months, reported a worst pain rating of at least four or greater on an 11 point (0–10) numerical rating scale in the preceding week, reported at least 15 days with pain in the preceding 30 days, and signed the informed consent. We excluded individuals who had metastatic (stage IV) breast cancer or who had a history of a bleeding disorder.
STUDY DESIGN
Participants were randomly assigned to treatment groups using computer-generated numbers sealed in opaque envelopes. The research coordinator first opened the envelope to inform the subject whether she was randomized to the acupuncture or WLC group. Changing block sizes of 3 or 6 were used to ensure a 2 to 1 acupuncture vs. WLC allocation. Subsequently for the acupuncture group, the treating acupuncturist opened a second envelope using computer-generated numbers at the first acupuncture visit to determine if the subject was to receive EA or SA. All participants were educated on joint pain, staying physically active, and continuing with current medical treatments (including prescription and over-the-counter pain medications) as usual. Patients in the WLC were told that they could receive 10 real acupuncture treatments after follow-up. To minimize potential reporting bias, WLC patients were informed that if their arthralgia improved during the waiting period, they could still receive acupuncture for other reasons (e.g. relaxation).
ELECTRO-ACUPUNCTURE
Two licensed non-physician acupuncturists with 8 and 20 years of experience, respectively, administered interventions twice a week for two weeks, then weekly for six more weeks, for a total of ten treatments over eight weeks. Informed by our prior feasibility trial,(11) we developed a manualized protocol (See Appendix) based on the Traditional Chinese Medicine theory that regards joint pain as part of the Bi Syndrome(14, 15). The acupuncturist chose at least four local points around the joint with the most pain. Additionally, at least four distant points were used to address constitutional symptoms such as depression/anxiety and fatigue that are commonly seen in conjunction with pain. The needles (30 mm or 40mm and 0.25 mm gauge, (Seirin-America Inc., Weymouth, MA) were inserted until “De Qi” (sensation of soreness, tingling, etc.) was reported by patients.(16) Two pairs of electrodes were connected at the needles adjacent to the painful joint(s) with two hertz electro-stimulation provided by a TENS unit. The decision to use low frequency electro-stimulation was based on our experience with the prior trial (11) and basic research suggesting electro-acupuncture at a low frequency can stimulate the brain to release specific endogenous opioid peptides (13). The needles were left in place for 30 minutes with brief manipulation at the beginning and the end of therapy.
SHAM ACUPUNCTURE
The treatment frequency and duration were the same for SA except: 1) We used Streitberger needles, which acted like a stage dagger with the shaft of the needle retracting into the handle, creating a shortened appearance to lead patients to believe that needles were inserted into the skin;(17) 2) The acupuncturist selected between 8 and 12 non-acupuncture, non-trigger points at least 5 cm from the joint where pain was perceived to be maximal; 3) Instead of eliciting “De Qi,” the needles were minimally manipulated to avoid eliciting sensations other than the initial contact with skin; and 4) Instead of adding a small electric current to the needles, the dial of the TENS unit was turned on to a different channel, so that the subject could observe the light blinking without receiving the electricity.
OUTCOME MEASURES AND FOLLOW-UP
The primary outcome was the change in pain severity score as measured by the Brief Pain Inventory (BPI) at the end of Week 8 of intervention compared to that at Baseline between the EA and the WLC groups. The BPI is a patient-reported outcome of pain with demonstrated reliability, validity, and responsiveness to change among patients with cancer.(18) The numerical rating scale ranges from 0–10, with 10 indicating the greatest severity or pain-related interference. The pain-related interference domain of the BPI was used as a secondary outcome. To test the durability of the intervention, the BPI was repeated at Week 12, four weeks following completion of the intervention.
The patient’s Global Impression of Change was measured at Week 8 to define clinical importance from the patient’s perspective.(19) We also evaluated other secondary outcomes at Baseline and Week 8 including: Western Ontario and McMasters Universities Osteoarthritis (WOMAC) Index to measure lower extremity pain, stiffness, and function limitations(20) and the Quick Disability of Arm, Shoulder, Hand (DASH) scale to measure upper extremity functional limitation.(21) Trained staff blinded to treatment groups also performed the Physical Performance Test (PPT).(22) The 9-item PPT is an objective measure of physical function that includes assessments of both lower and upper extremity function, as well as balance and endurance.
MASKING
In this trial, the PI, study investigators, patients, study staff, and statistician were all blinded to the treatment assignments between EA and SA with the exception of the acupuncturists. Throughout the study, research staff monitored adverse events using a standard adverse-event (AE) case report form at each visit. Individuals in the WLC group were contacted at the same frequency by phone by the research staff. Blinding between EA and SA was evaluated by the credibility rating at Week 8(23). In addition, patients were asked to guess whether they received EA, SA, or were unsure.
STATISTICAL ANALYSIS
We based our sample size calculation on the comparison between EA and WLC of BPI pain severity at Week 8. Per preliminary data,(11) the baseline pain severity score had a mean of 5.3 +/− standard deviation (SD) of 1.5, and we assumed EA would improve the score by 1.6 (30%) as compared to the WLC to be clinically meaningful.(24) In order to have 80% power to detect this difference using a two-sided significance level of 0.05, we needed 18 subjects in each of the WLC and EA groups. Assuming a 20% dropout rate, we needed to recruit 23 subjects per arm to fall within the precision noted in the sample size calculation. By design, our trial was not powered to detect significant differences between EA and SA.
ANOVA or Chi-square test was used to compare baseline variables among groups. Because our primary and secondary outcome measures were repeated measures over time, we assessed differences of changes from Baseline to Week 8 and Week 12 using mixed-effect models.(25) Time and group were treated as categorical variables and a random intercept term was included in the mixed-effect model.(26) Tests of intention-to-treat differences between intervention arms with respect to the change were based on time-intervention interactions in the mixed-effect models. Results are presented as between-group differences with 95% confidence intervals. All statistical tests were two-sided. Statistical significance was set at the < 0.05 level.
RESULTS
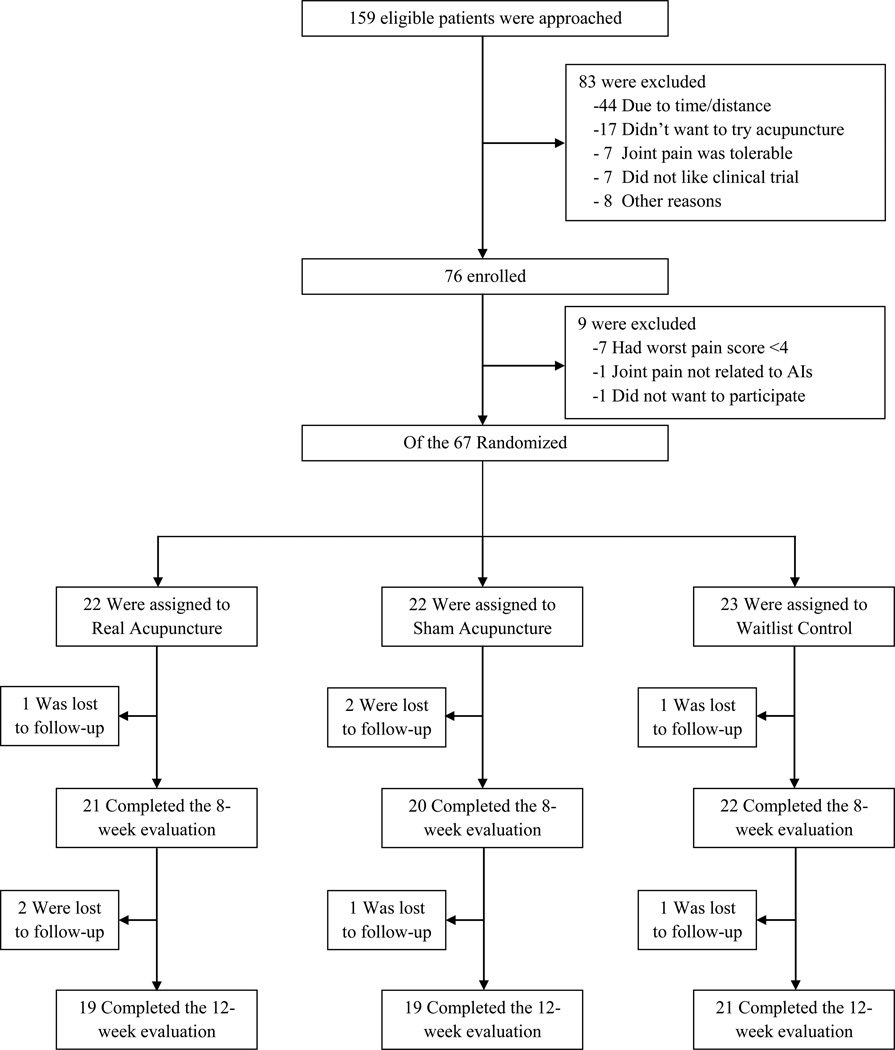
Between September 2009 and May 2012, we screened 159 patients. Of the 76 patients who qualified for baseline evaluation, nine were further excluded (seven had patient-reported pain level lower than inclusion criteria, one had severe pain unrelated to AIs, and another did not want to participate), and the 67 eligible participants were randomly assigned to EA, SA, or WLC. Among participants, 21 (95.4%) in the EA group and 20 (90.5%) in the SA group received all ten treatments. Four (6%) and eight (12%) patients were lost to follow-up before Week 8 and 12, respectively (Fig. 1).
Figure 1. Screening, Randomization and Completion of 8-Week and 12-Week Evaluations.
BASELINE CHARACTERISTICS OF THE PATIENTS
Table 1 shows baseline data for the 67 participants. The mean age of the women enrolled was 59.7 years, (range 41 to 76), and 48 (71.6%) were White, while 16 (23.9%) were Black. Forty-four patients (66%) were receiving anastrozole at the time of randomization, and, on average, participants had been on an AI for 25.9 (range 3 to 56) months. The mean BPI pain severity score was 4.9 and pain-related interference score was 3.7. Baseline characteristics were well balanced and not significantly different among the three groups.
Table 1.
Baseline Characteristics of the Study Participants.1
| Variables | EA (N=22) |
SA (N=22) |
WLC (N=23) |
|---|---|---|---|
| Age (years) | 57.5±10.1 | 60.9±6.5 | 60.6±8.2 |
| Race - # of subjects (%)2 | |||
| White | 13 (59) | 17 (77) | 18 (78) |
| Non-white | 9 (41) | 5 (23) | 5 (22) |
| Employment - # of subjects (%) | |||
| Employed | 14 (64) | 12 (55) | 12 (52) |
| Not employed | 8 (36) | 10 (45) | 11 (48) |
| Education - # of subjects (%) | |||
| High school or less | 2 (9) | 3 (14) | 5 (22) |
| College or above | 20 (91) | 19 (86) | 18 (78) |
| Body Mass Index (lb/inches)3 | 28.5±4.7 | 30.0±5.1 | 30.1±7.6 |
| Menopause - # of subjects (%) | |||
| Natural | 6 (27) | 13 (59) | 11 (50) |
| Surgically induced | 9 (41) | 6 (27) | 7 (32) |
| Chemically induced | 7 (32) | 3 (14) | 4 (18) |
| Years since menopause | 11.5±11.6 | 13.5±7.6 | 13.6±10.9 |
| Stage - # of subjects (%) | |||
| Stage I | 11 (50) | 11 (50) | 11 (48) |
| Stage II | 8 (36) | 7 (32) | 7 (30) |
| Stage III | 3 (14) | 4 (18) | 5 (22) |
| Aromatase Inhibitors - # of subjects (%) | |||
| Anastrozole (Arimidex) | 13 (59) | 16 (73) | 15 (65) |
| Letrozole (Femara) | 4 (18) | 4 (18) | 4 (17) |
| Exemestane (Aromasin) | 5 (23) | 2 (9) | 4 (17) |
| Duration of AI (months) | 26.9±17.3 | 19.5±16.9 | 31.1±22.1 |
| Duration of Joint Pain (months) | 58.9±88.1 | 43.4±48.3 | 62.9±84.0 |
| Adjuvant Chemotherapy - # of subjects (%) | 10 (45) | 12 (54) | 16 (69) |
| Adjuvant Taxane - # of subjects (%) | 9 (41) | 11 (50) | 13 (56) |
| Primary Joint Treated - # of subjects (%) | |||
| Lower extremity | 8 (36) | 15 (68) | 12 (52) |
| Back/Hip | 6 (27) | 3 (14) | 3 (13) |
| Upper extremity | 8 (36) | 4 (18) | 8 (35) |
| BPI4 | |||
| Severity | 5.1±1.8 | 4.7±1.7 | 4.9±1.3 |
| Interference | 3.8±2.6 | 3.4±2.3 | 3.9±1.7 |
| WOMAC5 | |||
| Pain | 186.6±117.3 | 207.4±85.8 | 206.8±82.8 |
| Stiffness | 105.7±39.3 | 99.4±43.4 | 101.1±47.8 |
| Function | 603.7±339.7 | 616.3±324.1 | 636.6±338.4 |
| Normalized | 125.8±54.6 | 127.2±55.6 | 129.5±56.0 |
| Quick DASH6 | 36.0±19.7 | 29.3±17.5 | 35.8±16.3 |
| PPT7 | 29.1±3.6 | 28.9±4.6 | 28.3±5.1 |
Abbreviations: EA, Electroacupuncture; SA, Sham acupuncture; WLC, Waitlist control
Plus-minus values are means ± SD unless otherwise noted.
Race was reported by the subjects.
The body-mass index is calculated using weight in lbs. times 703 divided by height in inches squared.
The Brief Pain Inventory (BPI) was used to assess the severity of pain and the degree to which pain interferes with common dimensions of feeling and function. Both BPI severity and interference scores range from 0–10, with higher scores indicating more pain and interference.
The Western Ontario and McMaster Universities (WOMAC) Osteoarthritis index assesses clinically-important symptoms in the areas of pain, stiffness, and physical function in lower extremities. It has 24 questions and 3 dimensions. The range of possible subscale scores for the 3 dimensions is as follows: pain=0–500, stiffness=0–200, physical function=0–1700, with higher scores indicating more pain, stiffness, and functional difficulty. A normalized score was calculated by normalizing each of the subscale scores on a 0–100 scale and then summate to provide a single value. The normalized score ranges from 0–300.
QuickDASH measures physical function and symptoms of the upper limb. Scores range from 0–100, with higher scores indicating more severe symptoms.
The Physical Performance Test (PPT) measures the physical function of the subjects. Scores range from 0–36, with higher scores indicating better physical function.
STUDY OUTCOMES
Table 2 shows changes in all primary (Figure 2) and secondary outcomes (Figure 3) at Week 8 and Week 12 compared to Baseline among three treatment groups.
Table 2.
Changes in Primary and Secondary Outcomes1
| Variables | Mean Change from Baseline (95% CI) | Between Group Difference (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| EA (N=22) |
SA (N=22) |
WLC (N=23) |
EA vs. WLC | P-Value2 | SA vs. WLC | P-Value2 | |
| Pain | |||||||
| severity3 | |||||||
| Week 4 | −1.7 | −1.5 | −0.4 | −1.3 | x0.014 | −1.1 | 0.016 |
| (−2.3 to −1.0) | (−2.3 to −0.7) | (−1.2 to 0.5) | (−2.3 to −0.3) | (−2.2 to −0.0) | |||
| Week 8 | −2.2 | −2.3 | −0.2 | −1.9 | 0.0004 | −2.0 | <0.0001 |
| (−3.2 to −1.2) | (−3.2 to −1.3) | (−0.9 to 0.5) | (−3.1 to −0.8) | (−3.1 to −0.9) | |||
| Week 12 | −2.4 | −1.7 | −0.2 | −2.3 | <0.0001 | −1.5 | 0.0036 |
| (−3.3to −1.5) | (−2.6 to −0.8) | (−0.8 to 0.5) | (−3.4 to −1.1) | (−2.7 to −0.4) | |||
| Pain-related interference3 | |||||||
| Week 4 | −1.3 | −1.1 | −0.1 | −1.3 | 0.043 | −1.1 | 0.06 |
| (−2.2 to −0.4) | (−1.9 to −0.3) | (−0.9 to 0.8) | (−2.5 to −0.03) | (−2.2 to −0.1) | |||
| Week 8 | −2.0 | −1.5 | 0.2 | −2.2 | 0.0006 | −1.7 | 0.0037 |
| (−3.2 to −0.8) | (−2.3 to −0.7) | (−0.6 to 0.9) | (−3.5 to −0.8) | (−2.7 to −0.6) | |||
| Week 12 | −2.1 | −1.3 | −0.1 | −2.0 | 0.0034 | −1.2 | 0.049 |
| (−3.2 to −1.1) | (−2.2 to −0.4) | (−1.2 to 1.0) | (−3.5 to −0.6) | (−2.6 to 0.2) | |||
| WOMAC4 | |||||||
| Pain | |||||||
| Week 4 | −51.5 | −35.2 | 4.1 | −55.6 | 0.020 | −39.3 | 0.064 |
| (−83.7 to −19.3) | (−72.4 to 2.0) | (−19.6 to 27.8) | (−94.6 to −16.5) | (−81.4 to 2.9) | |||
| Week 8 | −78.9 | −78.0 | 0.0 | −78.9 | 0.0009 | −78.0 | 0.001 |
| (−118.7 to −39.0) | (−119.6 to −36.3) | (−26.3 to 26.3) | (−124.7 to −33.0) | (−124.2 to −31.7) | |||
| Stiffness | |||||||
| Week 4 | −11.9 | −16.4 | 6.3 | −18.2 | 0.124 | −22.7 | 0.037 |
| (−31.8 to 8.0) | (−35.5 to 2.6) | (−8.4 to 20.9) | (−42.3 to 6.0) | (−45.8 to 0.37) | |||
| Week 8 | −35.7 | −28.3 | 6.8 | −42.5 | 0.0014 | −35.1 | 0.0086 |
| (−53.2 to −18.2) | (−51.7 to −4.9) | (−14.9 to 28.5) | (−69.7 to −15.4) | (−66.0 to −4.2) | |||
| Function | |||||||
| Week 4 | −130.1 | −129.2 | −1.8 | −128.3 | 0.089 | −127.4 | 0.062 |
| (−249.3 to −10.8) | (−226.4 to −32.0) | (−108.3 to 104.7) | (−283.6 to 27.1) | (−266.9 to 12.0) | |||
| Week 8 | −255.6 | −250.4 | 7.4 | −262.9 | 0.0005 | −257.8 | 0.0004 |
| (−396.2 to −114.9) | (−387.4 to −113.4) | (−81.4 to 96.2) | (−422.6 to −103.3) | (−412.9 to −102.5) | |||
| Normalized | |||||||
| Week 4 | −23.9 | −22.4 | 3.8 | −27.8 | 0.029 | −26.2 | 0.022 |
| (−41.9 to −5.9) | (−42.7 to −2.0) | (−11.2 to 18.8) | (−50.6 to −4.9) | (−50.4 to −1.9) | |||
| Week 8 | −48.7 | −44.4 | 3.8 | −52.6 | <0.0001 | −48.2 | 0.0003 |
| (−69.4 to −28.0) | (−70.1 to −18.6) | (−14.5 to 22.2) | (−79.3 to −25.8) | (−78.2 to −18.2) | |||
| Quick Dash5 | |||||||
| Week 4 | −6.1 | −4.9 | −0.8 | −5.3 | 0.204 | −4.1 | 0.193 |
| (−12.5 to 0.3) | (−11.1 to 1.3) | (−6.3 to 4.7) | (−13.5 to 2.8) | (−12.0 to 3.9) | |||
| Week 8 | −12.5 | −11.6 | −0.7 | −11.8 | 0.005 | −10.9 | 0.006 |
| (−19.9 to −5.0) | (−18.1 to −5.1) | (−6.2 to 4.7) | (−20.8 to −2.7) | (−19.0 to −2.7) | |||
| PPT6 | |||||||
| Week 8 | 2.0 | 1.4 | 0.2 | 1.8 | 0.061 | 1.2 | 0.164 |
| (0.5 to 3.5) | (0.6 to 2.3) | (−0.9 to 1.4) | (−0.1 to 3.6) | (−0.2 to 2.7) | |||
Abbreviations: EA, Electroacupuncture; SA, Sham acupuncture; WLC, Waitlist control
All values are means, with 95% confidence intervals (CI).
P-values were calculated using the mixed-effects model.
The Brief Pain Inventory (BPI) was used to assess the severity of pain and the degree to which pain interferes with common dimensions of feeling and function. Both BPI severity and interference scores range from 0–10, with higher scores indicating more pain and interference.
The Western Ontario and McMaster Universities (WOMAC) Osteoarthritis index assesses clinically-important symptoms in the areas of pain, stiffness, and physical function in lower extremities. It has 24 questions and 3 dimensions. The range of possible subscale scores for the 3 dimensions is as follows: pain=0–500, stiffness=0–200, physical function=0–1700, with higher scores indicating more pain, stiffness, and functional difficulty. A normalized score was calculated by normalizing each subscale score on a 0–100 scale and then summate to provide a single value. The normalized scores range from 0–300.
QuickDASH measures physical function and symptoms of the upper limb. Scores range from 0–100, with higher scores indicating more severe symptoms.
The Physical Performance Test (PPT) measures the physical function of the subjects. Scores range from 0–36, with higher scores indicating better physical function.
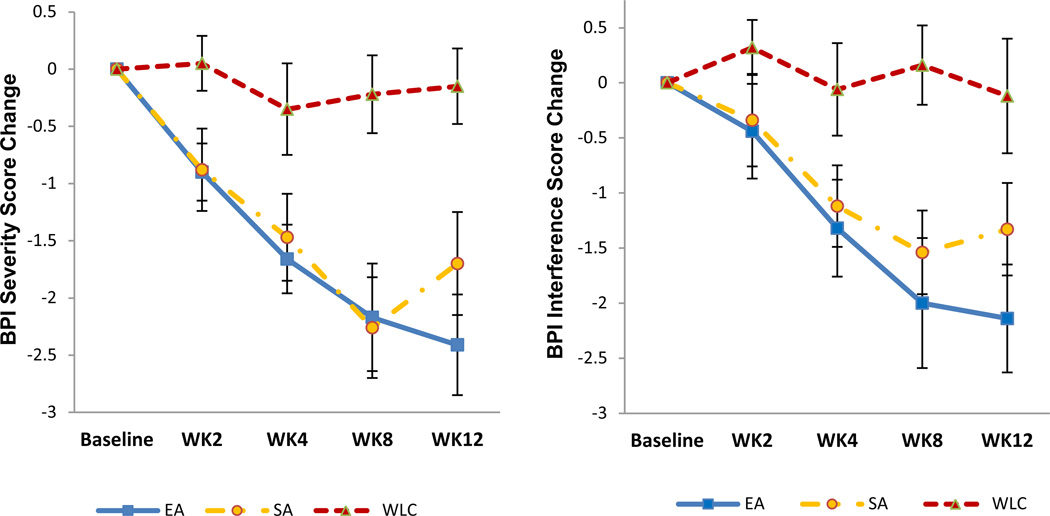
Figure 2. Mean Change in Pain Severity and Pain-related Interference at 8 and 12 Weeks from Baseline, According to Treatment Group.
Change in Brief Pain Inventory (BPI) severity and interference scores from baseline are shown for electro-acupuncture (EA), sham acupuncture (SA), and waitlist control (WLC) groups.
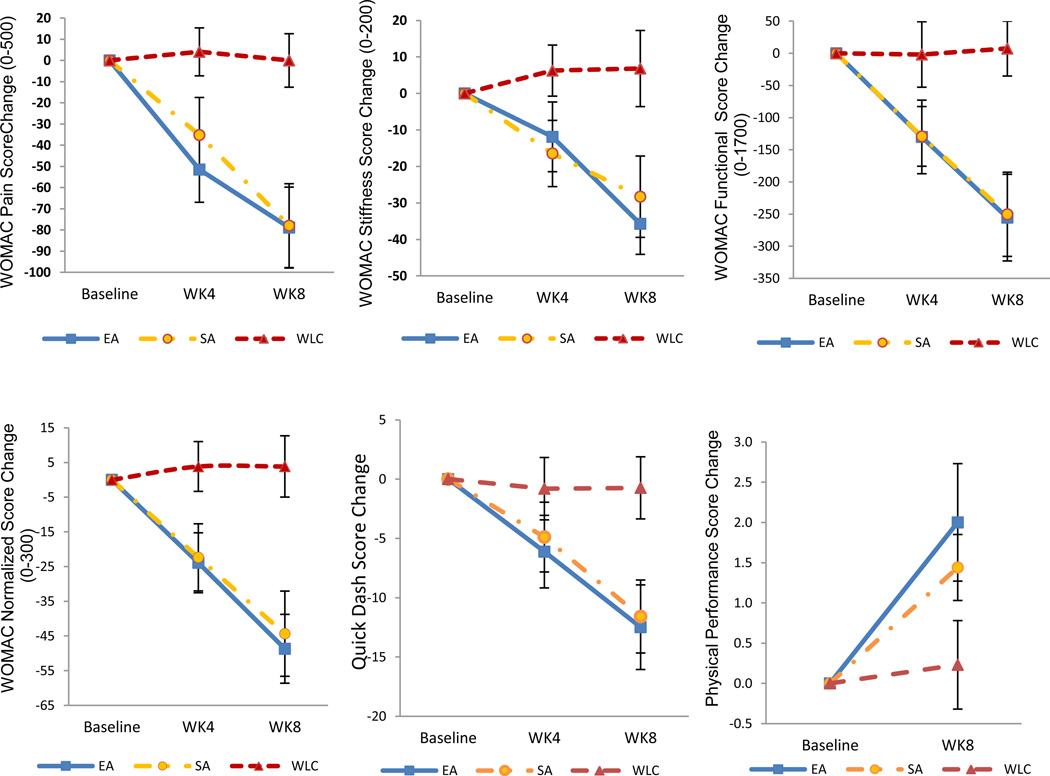
Figure 3. Mean Changes in Secondary Outcomes at 8 and 12 Weeks from Baseline, According to Treatment Group.
Changes in outcome scores from baseline are shown for electro-acupuncture (EA), sham acupuncture (SA), and waitlist control (WLC) groups.
At Week 8, the EA group had a statistically significant greater decrease in the BPI severity score than did the WLC group −2.2 points (95% confidence interval [CI], −3.2 to −1.2) vs. −0.2 points (95% CI, −0.9 to 0.5), and the mean between-group difference was −1.9 points (95% CI, −3.1 to −0.8, p=0.0004, Cohen’s d = 0.76). The EA group also had statistically significant greater reduction in pain-related interference score than the WLC group −2.0 points (95% CI, −3.2 to −0.8) vs. 0.2 points (95% CI, −0.6 to 0.9), and the mean between-group difference was −2.2 points (95% CI, −3.5 to −0.8, p=0.0006), Cohen’s d = 1.04. Compared to Baseline, EA produced a 43.1% reduction in pain severity and a 52.6% reduction in pain-related interference over the Week 8 intervention period. Based on Patient Global Impression of Change, more patients in the EA than WLC group reported arthralgia being “much improved” or “very much improved” (57.1% vs. 4.6%, p<0.001).
Compared to the WLC, the EA group also had a statistically significant greater improvement in lower extremity outcomes as measured by the WOMAC index: −78.9 points (95% CI, −124.7 to −33.0, p=0.0009, Cohen’s d = 0.99) for pain; −42.5 points (95% CI, −69.7 to −15.4, p=0.0014, Cohen’s d = 0.94) for stiffness, and −262.9 points (95% CI, −422.6 to −103.3, p=0.0005, Cohen’s d = 1.00) for function. The EA group also had statistically significant greater improvement in the upper extremity disability score as measured by the Quick-DASH (−11.8 points, 95% CI, −20.8 to −2.7, p=0.005, Cohen’s d = 0.58). The EA group had non-significant improvement in the observed PPT score as compared to WLC (1.8 points, 95% CI, −0.1 to 3.6, p=0.061, Cohen’s d = 0.61).
At Week 12, four weeks beyond the end of treatment, the EA group continued to have significant reduction in both pain severity (p<0.0001) and pain-related interference (p=0.0034) compared to the WLC group.
The SA group also showed a statistically significant greater decrease in the BPI severity score compared to the WLC group at both Week 8 (p<0.001) and Week 12 (p=0.0036). EA and SA showed no statistical difference in all study outcomes at Week 8. At Week 12, EA had non-significant improvement as compared to SA in BPI severity (−0.66, p=0.22) and BPI interference (−0.80, p=0.34).
ADVERSE EFFECTS
Despite needle placement in the same arm as breast cancer surgery, no case of infection, no reports of development or worsening of lymphedema occurred in either EA or SA groups. Eighteen related adverse events (AEs) were reported by eight subjects in the EA or SA groups during 398 intervention episodes. These AEs were mild in severity and spontaneously resolved without additional medical interventions. The EA group had more adverse events reported than the SA group (16 vs. 4). A major category of AEs reported in the EA group was related to the “De Qi” sensation (N=6 such as tingling, numbness during the acupuncture process). Both EA and SA groups had similar rates of pain at the needling site (5 and 4 respectively).
ASSESSMENT OF BLINDING BETWEEN EA AND SA
At the end of active intervention, individuals in both EA and SA considered the interventions credible (4.3 vs. 4.0, p=0.54). The proportion of individuals who guessed that they received EA vs. Not Sure vs. SA were 57.9%, 26.3%, 15.8% for EA group and 27.8%, 33.3%, 38.9% for SA group, p=0.15.
DISCUSSION
This randomized controlled trial met its primary endpoint, demonstrating that EA produced statistically significant and clinically important improvements in pain severity, pain-related interference, and functional outcomes in both upper and lower extremities when compared to WLC usual care. The effects were observed at Week 8 when intervention completed, and persisted at the Week 12 follow-up visit. SA also produced a similar magnitude of change in pain-related outcomes that were significantly better than the WLC. Both EA and SA were safe, without significant adverse events.
To our knowledge, our study is the largest RCT of acupuncture for AI-related arthralgia to date ***(N=67) compared to two prior studies with sample sizes of 38 (10) and 47 (12). More importantly, our study is the only study to date that utilized a “usual care” waitlist control group. The Cohen’s ds between EA and usual care for pain severity and interference indicated moderately large effect sizes that are clinically relevant. Additionally, compared to the usual care WLC group, 50% of participants receiving EA reported joint pain “much improved” or better (Number to Treat = 2). Further, our study is the only study to date that had a four week follow-up “no-treatment period” which demonstrated that the effect of EA appears to be durable. This is clinically important because it suggests that acupuncture may be effective without requiring continuous use throughout the five year AI treatment period. Until more definitive and long-term studies are conducted, these results provide a context for both patients and clinicians to decide whether acupuncture is an option for them to manage AI-related arthralgia.
We included a sham acupuncture arm in our study as an additional control group to determine whether the therapeutic effect of acupuncture required the components of location, “De Qi,” and electrical stimulation. We found that SA produced similar effects to EA in the short term, consistent with the trial conducted by Bao et al. (12) but different from the results of Crew et al. (10). While the effect of real acupuncture was similar in magnitude among all three studies, only the Crew study failed to find a therapeutic effect of SA. Sham acupuncture produced almost no change in pain severity scores in Crew’s study (10) while in both our study and that by Bao (12), the sham control appeared to have produced comparable effects to EA at the endpoints of interest. Our findings are also consistent with acupuncture research performed in non-cancer related musculoskeletal pain that shows sham acupuncture has therapeutic effects in pain reduction. (6) Substantial controversy exists regarding whether sham acupuncture is, in fact, an active intervention rather than “placebo,” since tactile stimulation with sham device can result in actual physiologic change that is not inert (27). Recent functional brain imaging studies also suggest that sham acupuncture can produce changes in brain regions that are an integral part of the pain pathways (28).
While the exact mechanism of action of acupuncture for AI-related arthralgia is unknown, estrogen deprivation has been temporally associated with this condition.(29) It is hypothesized that estrogen deprivation may decrease the generation of endogenous opioids, thereby leading to a lowered pain threshold.(30) A specific component of EA analgesia is mediated through endogenous opioid release in animal research.(13) Using C-carfentanil positron emission tomography imaging, Harris et al. found that real acupuncture increased mu-opioid receptor (MOR) binding potential in key areas of brain pain central processing (e.g., cingulate, caudate, and amygdale), while SA produced slightly decreased MOR binding potential, suggesting divergent mechanistic pathways for real and sham acupuncture.(31) Since both forms of acupuncture produced similar and clinically meaningful pain reduction, future research incorporating functional brain imaging or pain sensitivity testing may increase our understanding of the potential mechanism of AI-related arthralgia and acupuncture effect.
It is important to acknowledge several limitations of the current study. While our study was powered to detect a difference between EA and WLC, it was not powered to detect a statistically significant difference between EA and SA. Additionally, our follow-up period may have been too short to see a difference between EA and SA, as we began to observe a potential separation of effect between the two groups at Week 12. Finally, as discussed before, our sham control may not function as a physiologically inert placebo. Future studies should therefore incorporate controls that address attention but do not produce physiological changes associated with tactile stimulation, such as those produced by needles, in order to better evaluate the efficacy of acupuncture.
In conclusion, arthralgia remains a major component of symptom burden in breast cancer survivors receiving aromatase inhibitors and leads many women to stop AIs prematurely (4). Currently, no treatment has been found to be definitively effective for this condition, and many oncologists discontinue AIs in order for their patients to decrease joint pain and regain a sense of quality of life. Our findings add to the small but growing body of literature suggesting that acupuncture may have clinical benefit for reducing AI-attributable pain and pain-related interference in function.
Supplementary Material
Acknowledgements
We thank our acupuncturists, Lorna Lee and Adam Schreiber, for delivering the interventions. We thank Christina Seluzicki, Rana Leed, Courtney Penn, Shawn Fernandes, Xiaoyan Han, and Qing S. Li for their dedication to clinical trial coordination, data collection and management, and statistical programming. We thank Brian Strom MD MPH for valuable feedback on the manuscript. Sincere thanks also go to the patients, oncologists, nurse practitioners, and clinical staff for their support of this study.
Funding Source
This study is supported by grants from the National Institutes of Health / National Center for Complementary and Alternative Medicine (NCCAM) R21 AT004695. Dr. Mao is a recipient of the NCCAM K23 AT004112 award. The funding agencies had no role in the design or conduct of the study. Dr. Mao has full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration: NCT01013337
Author Contributions
All authors participated in study design, writing of and approving the final manuscript. JJM obtained and funding and managed trial and data collection process. SSX performed data analysis and data interpretation. JTF, CTS, MAB, DB, and AD provided expertise during study design, data interpretation and revision of the manuscript.
Conflicts of Interests
Dr. Mao has consulted for Pfizer on issues unrelated to aromatase inhibitors. Dr. Farrar has consulted for Pfizer and AstaZeneca on issues that are unrelated to aromatase inhibitors. The other co-authors had no conflict of interest to declare.
Reference
- 1.Burstein HJ, Winer EP. Aromatase inhibitors and arthralgias: a new frontier in symptom management for breast cancer survivors. J Clin Oncol. 2007 Sep 1;25(25):3797–3799. doi: 10.1200/JCO.2007.11.9529. [DOI] [PubMed] [Google Scholar]
- 2.Mao JJ, Stricker C, Bruner D, Xie S, Bowman MA, Farrar JT, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009 Aug 15;115(16):3631–3639. doi: 10.1002/cncr.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao JJ, Chung A, Benton A, Hill S, Ungar L, Leonard C, et al. Online discussion of drug side effects and discontinuation among breast cancer survivors. Pharmacoepidemiology and Drug Safety. 2013 doi: 10.1002/pds.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012 Mar 20;30(9):936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011 Apr;126(2):529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vickers AJ, Cronin AM, Maschino AC, Lewith G, Macpherson H, Foster NE, et al. Acupuncture for Chronic Pain: Individual Patient Data Meta-analysis. Arch Intern Med. 2012 Sep;10:1–10. doi: 10.1001/archinternmed.2012.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Houghton M, Seefeld L, Malarick C, Mao J. A survey of selected physician views on acupuncture in pain management. Pain Med. 2010 Apr;11(4):530–534. doi: 10.1111/j.1526-4637.2010.00815.x. [DOI] [PubMed] [Google Scholar]
- 8.Bonner-Millar L, Casarett D, Vapiwala N, DeMichele A, Stricker C, Velder L, et al. Integrating complementary therapies into an academic cancer center: The perspective of breast cancer patients. Journal of Society for Integrative Oncology. 2010;8(3):106–113. [Google Scholar]
- 9.Brauer JA, El Sehamy A, Metz JM, Mao JJ. Complementary and alternative medicine and supportive care at leading cancer centers: a systematic analysis of websites. J Altern Complement Med. 2010 Feb;16(2):183–186. doi: 10.1089/acm.2009.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crew KD, Capodice JL, Greenlee H, Brafman L, Fuentes D, Awad D, et al. Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J Clin Oncol. 2010 Mar 1;28(7):1154–1160. doi: 10.1200/JCO.2009.23.4708. [DOI] [PubMed] [Google Scholar]
- 11.Mao JJ, Bruner DW, Stricker C, Farrar JT, Xie SX, Bowman MA, et al. Feasibility trial of electroacupuncture for aromatase inhibitor--related arthralgia in breast cancer survivors. Integr Cancer Ther. 2009 Jun;8(2):123–129. doi: 10.1177/1534735409332903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao T, Cai L, Giles JT, Gould J, Tarpinian K, Betts K, et al. A dual-center randomized controlled double blind trial assessing the effect of acupuncture in reducing musculoskeletal symptoms in breast cancer patients taking aromatase inhibitors. Breast Cancer Res Treat. 2013 Feb;138(1):167–174. doi: 10.1007/s10549-013-2427-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han JS. Acupuncture and endorphins. Neurosci Lett. 2004 May 6;361(1–3):258–261. doi: 10.1016/j.neulet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Cheng X, editor. Chinese acupuncture and moxibustion. Beijing, China: Foreign Languages Press; 1987. [Google Scholar]
- 15.Anonymous. Essentials of Chinese Acupuncture. Beijing, P.R. China: Foreign Languages Press; 1993. [Google Scholar]
- 16.Mao JJ, Farrar JT, Armstrong K, Donahue A, Ngo J, Bowman MA. De qi: Chinese acupuncture patients' experiences and beliefs regarding acupuncture needling sensation--an exploratory survey. Acupunct Med. 2007 Dec;25(4):158–165. doi: 10.1136/aim.25.4.158. [DOI] [PubMed] [Google Scholar]
- 17.Streitberger K, Kleinhenz J. Introducing a placebo needle into acupuncture research. Lancet. 1998 Aug 1;352(9125):364–365. doi: 10.1016/S0140-6736(97)10471-8. [DOI] [PubMed] [Google Scholar]
- 18.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994 Mar;23(2):129–138. [PubMed] [Google Scholar]
- 19.Sloan JA, Aaronson N, Cappelleri JC, Fairclough DL, Varricchio C. Clinical Significance Consensus Meeting G. Assessing the clinical significance of single items relative to summated scores. 2002 May;:479–487. [PubMed] [Google Scholar]
- 20.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988 Dec;15(12):1833–1840. [PubMed] [Google Scholar]
- 21.Gummesson C, Ward MM, Atroshi I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord. 2006;7:44. doi: 10.1186/1471-2474-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc. 1990 Oct;38(10):1105–1112. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 23.Vincent C. Credibility assessment in trials of acupuncture. Complementary Medicine Research. 1990;4:8–11. [Google Scholar]
- 24.Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2000 Dec 1;88(3):287–294. doi: 10.1016/S0304-3959(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 25.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982 Dec;38(4):963–974. [PubMed] [Google Scholar]
- 26.Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 27.Langevin HM, Hammerschlag R, Lao L, Napadow V, Schnyer RN, Sherman KJ. Controversies in acupuncture research: selection of controls and outcome measures in acupuncture clinical trials. J Altern Complement Med. 2006 Dec;12(10):943–953. doi: 10.1089/acm.2006.12.943. [DOI] [PubMed] [Google Scholar]
- 28.Huang W, Pach D, Napadow V, Park K, Long X, Neumann J, et al. Characterizing acupuncture stimuli using brain imaging with FMRI--a systematic review and meta-analysis of the literature. PLoS One. 2012;7(4):e32960. doi: 10.1371/journal.pone.0032960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao JJ, Su HI, Feng R, Donelson ML, Aplenc R, Rebbeck TR, et al. Association of functional polymorphisms in CYP19A1 with aromatase inhibitor associated arthralgia in breast cancer survivors. Breast Cancer Res. 2011 Jan 20;13(1):R8. doi: 10.1186/bcr2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felson DT, Cummings SR. Aromatase inhibitors and the syndrome of arthralgias with estrogen deprivation. Arthritis Rheum. 2005 Sep;52(9):2594–2598. doi: 10.1002/art.21364. [DOI] [PubMed] [Google Scholar]
- 31.Harris RE, Zubieta JK, Scott DJ, Napadow V, Gracely RH, Clauw DJ. Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on mu-opioid receptors (MORs) Neuroimage. 2009 Sep;47(3):1077–1085. doi: 10.1016/j.neuroimage.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.