Abstract
Human skull morphology results from complex processes that involve the coordinated growth and interaction of its skeletal components to keep a functional and structural balance. Previous histological works have studied the growth of different craniofacial regions and their relationship to functional spaces in humans up to 14 years old. Nevertheless, how the growth dynamics of the facial skeleton and the mandible are related and how this relationship changes through the late ontogeny remain poorly understood. To approach these two questions, we have compared the bone modelling activities of the craniofacial skeleton from a sample of subadult and adult humans. In this study, we have established for the first time the bone modelling pattern of the face and the mandible from adult humans. Our analyses reveal a patchy distribution of the bone modelling fields (overemphasized by the presence of surface islands with no histological information) reflecting the complex growth dynamics associated to the individual morphology. Subadult and adult specimens show important differences in the bone modelling patterns of the anterior region of the facial skeleton and the posterior region of the mandible. These differences indicate developmental changes in the growth directions of the whole craniofacial complex, from a predominantly downward growth in subadults that turns to a forward growth observed in the adult craniofacial skeleton. We hypothesize that these ontogenetic changes would respond to the physiological and physical requirements to enlarge the oral and nasal cavities once maturation of the brain and the closure of the cranial sutures have taken place during craniofacial development.
Keywords: bone formation and resorption bone histology, facial skeleton, Homo sapiens, mandible, modelling pattern, morphology, ontogeny
Introduction
The skull is an anatomically complex system, which has been a focal point for studies in vertebrate biology for more than two centuries. It presents unique opportunities to examine the role of the multiple, intricate developmental processes involved in the craniofacial morphology and in the evolutionary origin of the hominid cranium (de Beer, 1937; Enlow, 1975; Atchley & Hall, 1991; Lieberman, 2011). Understanding the development of the skull can be achieved through the study of the growth dynamics of their skeletal elements considering the Moss functional matrices theory (Moss & Young, 1960; Moss & Rankow, 1968; Moss & Salentijn, 1969; Moss, 1997c,1997d) and the Enlow counterpart principle (Enlow et al. 1969; Enlow & Hans, 1996). According to this theoretical framework, the human craniofacial skeleton results from the interactions of their different components that are influenced by both internal (hormonal and genetic factors; e.g. Moss & Young, 1960; Enlow & Hans, 1996; Tomoyasu et al. 2009; Lieberman, 2011) and external stimuli (soft tissue growth, dental maturation, biomechanical factors; e.g. Moss, 1997a,1997b,1997c,1997d; Atchley & Hall, 1991; Enlow & Hans, 1996; Lieberman et al. 2002; Klingenberg et al. 2003; Lieberman, 2011; Gröning et al. 2013). The growth of the skeletal elements involves changes in their size and shape as well as their relative position within the craniofacial system in order to maintain the proper bone alignment, function and proportionate growth (e.g. O'Higgins et al. 1991; Enlow & Hans, 1996; McCollum, 2008; Lieberman, 2011). During the human development, the skeletal elements from the neurocranium, viscerocranium and mandible are intimately associated to the functional spaces (cranial, orbital, nasal and oral cavities) and the soft tissues in which they are embedded (e.g. brain, muscles, connective tissues) (Moss & Young, 1960; Enlow & Hans, 1996; see also Lieberman, 2011 and cites there in).
The skull grows through two simultaneous and interrelated processes: growth modelling and growth displacements of the skeletal elements. Growth modelling consists in the coordinated activity of two cellular groups, osteoblasts forming bone on one surface and osteoclasts removing bone in the opposite surface (Enlow, 1962; Bloom & Fawcett, 1994; Enlow & Hans, 1996). This mechanism results in the increase in size of the bone and the subsequent movement in the direction of the forming bone surfaces, also termed cortical drift (Enlow, 1962, 1963; Enlow & Harris, 1964). As a consequence of the bone modelling growth, the skeletal components are displaced within the craniofacial system with coordinated and passive movements – the primary and secondary displacements – as well as rotations (for a detailed description of these movements see Björk, 1969; Moss & Young, 1960; Björk & Skieller, 1972, 1976; Enlow & Hans, 1996).
In the last century, Enlow showed that the activity of osteoblasts and osteoclasts is recorded in the bone surface (last-formed bony lamellae) as fields of growth activity (Enlow, 1963; Enlow & Hans, 1996). The distribution of these growth fields – the bone modelling pattern – is species-specific and its interpretation following the craniofacial biology principles provides data on the growth dynamics of the craniofacial skeletal components during human ontogeny (e.g. Enlow & Harris, 1964; Mauser et al. 1975; Kurihara et al. 1980; Enlow & Hans, 1996; McCollum, 2008). According to these studies, the prenatal craniofacial system shows a general growth as indicated by the bone deposition surfaces in all skeletal elements (Mauser et al. 1975; Enlow & Hans, 1996; Radlanski & Klarkowski, 2001).Bone resorption activity is first reported in the mandibular corpus and ramus around the 8.5th–9th prenatal weeks (Mauser et al. 1975; Enlow & Hans, 1996; Radlanski & Klarkowski, 2001), indicating a lateral growth of the mandibular corpus and a posterior relocation of the ramus (Mauser et al. 1975; Enlow & Hans, 1996). In the postnatal period, the human facial skeleton is depository until 3 months of age, when bone resorption surfaces appear in the nasoalveolar clivus (Kurihara et al. 1980; Enlow & Hans, 1996; McCollum, 2008). From 2 to 14 years old, resorbing activity spreads out over the nasomaxillary region. The extension and the location of resorbing fields change throughout ontogeny, indicating changes in the growth dynamics associated to downward growth of the human face (Kurihara et al. 1980; McCollum, 2008). Postnatal changes in the bone modelling activity are also observed in the human mandible (Enlow & Harris, 1964; Kurihara et al. 1980; Hans et al. 1995). At 2 years old, bone resorption fields appear for the first time in the alveolar region of the buccal symphyseal region. From this age to 14 years old, resorption extends towards the basal area of the symphyseal region and/or through the anterior area of the mandibular corpus (Kurihara et al. 1980). During this postnatal period, the mandibular ramus shows a complex modelling pattern indicating a posterior growth of the mandible and its anterior displacement (Enlow & Harris, 1964; Enlow & Hans, 1996). Previous studies have analysed facial skeleton growth up to 14 years old but the bone modelling activities during the adulthood period remain almost unstudied. The adult craniofacial skeleton and mandible show morphological changes related to their horizontal increase in the size of the maxilla and the mandible and their increase in the height of the anterior face (e.g. Behrents, 1985; Bishara et al. 1985; Forsberg et al. 1991; Bondevik, 1995; Enlow & Hans, 1996; Doual et al. 1997; West & McNamara, 1999; Akgül & Toygar, 2002; Albert et al. 2007; Williams & Slice, 2010; Tsiopas et al. 2013).
In the present study, we have analysed the postnatal growth dynamics of the craniofacial skeleton comparing subadult and adult specimens. We have observed that subadult and adult specimens show different bone modelling patterns. Adults present an increase of bone formation surfaces in the maxilla and mandible that explains the horizontal and vertical changes observed in the adult craniofacial skeleton. In addition, we have explored how modelling activities of the facial skeleton and mandible regions are related during the ontogeny. As mentioned above, the skeletal components growing within the craniofacial complex system interact with each other keeping a functional and structural balance, whereas they increase in size during development (Moss & Young, 1960; Enlow & Hans, 1996). Correspondences between different anatomical parts of the skull have been demonstrated by morphometric analyses (see Bastir et al. 2006). However, previous studies on the craniofacial growth through the analysis of modelling activities have focused on particular facial or mandibular regions, except for Enlow's reference work on craniofacial morphology in individuals up to 14 years old (Enlow & Hans, 1996). In the present study, we work on the assumption that ontogenetical changes of the bone modelling also reflect the relationships between the facial and mandible skeleton to maintain the functional and physiological balance of the craniofacial system. Results obtained in this work will allow us to infer how these relationships could be involved in the morphology of the human skull.
Materials and methods
The sample analysed in this study comprises 12 human skulls of known age and sex divided into two subgroups: six specimens in the subadult group and six specimens in the adult group (Table 1). All specimens belong to the Anthropological Collection of the University of Coimbra (Portugal). Individuals with malformations, traumatic injuries or alveolar bone resorption caused by tooth loss during life were excluded.
Table 1.
List of the Homo sapiens specimens from the Anthropological Collection of the University of Coimbra (Portugal) analysed with reflected light microscopy
| Specimen | Age (years) | Age group | Sex |
|---|---|---|---|
| 101 | 12 | Subadult | Female |
| 218 | 10 | Subadult | Female |
| 284 | 17 | Subadult | Female |
| 100 | 7 | Subadult | Male |
| 100A | 11 | Subadult | Male |
| 126 | 8 | Subadult | Male |
| 52 | 38 | Adult | Female |
| 144 | 29 | Adult | Female |
| 342 | 28 | Adult | Female |
| 46 | 38 | Adult | Male |
| 92 | 27 | Adult | Male |
| 98 | 24 | Adult | Male |
Obtaining the bone modelling pattern requires microscope analysis of the bone surface to identify bone formation and resorption fields. We used a non-destructive methodology that involves the replication of the bone surface and the microscope analysis of these replicas (Martinez-Maza et al. 2010; see also Bromage, 1989). The best preserved half part of both facial skeleton and mandible was employed in the analyses. Specimens were first cleaned with 60% alcohol applied with a smooth hair brush to eliminate any particles adhering to the microrelief of the bone. Secondly, the negative impressions of the periosteal bone from the facial skeleton and the mandible were made using a low-viscosity silicone (Exaflex injection type 3 low viscosity; DVD Dental, SA, Spain). Negative impressions were made independently from anatomical regions of the facial skeleton (glabella, superciliar arch, nasal bones, nasomaxillary region, zygomatic bone) and the mandible (buccal and lingual side of the symphysis, mandibular corpus and ramus) to fit the microscope's size limitations and to facilitate the manipulation during observation. Once silicone was cured, the negative cast was removed from the bone surface and delimited with a retaining wall elaborated with a silicone Optosil P plus and Optosil Xantopren (DVD Dental SA, Spain). Finally, positive replicas of each anatomical region were generated using polyurethane resin Feropur (Feroca, SA, Spain). Replicas were then coated with gold (sputter coater SC510 BioRad) prior to observation under a reflected light microscope (Olympus BX51TRF microscope equipped with an Olympus DP11 digital camera) using a 20× objective (Martinez-Maza et al. 2010). To facilitate the localization of the remodelling microfeatures of the bone surface, a grid of 5 × 5 mm squares was drawn on the surface of the gold-coated replica using a sharp permanent pen. Each square was referred to by a coordinate (x,y) starting on the lower left square (1,1). This grid and the outline of the anatomical region were drawn on paper to record the data from the microscope.
Identification of bone modelling fields: forming and resorbing surfaces
The microscope analysis of the replicas from the periosteal bone surfaces allowed us to identify and map the fields of growth modelling activities following the criteria provided by Martinez-Maza et al. (2010; see also Boyde, 1972; Bromage, 1989). The microscopy analysis of the replicas from the periosteal bone surfaces allowed us to identify and map the fields of growth modelling activities following the criteria provided by previous authors (Boyde, 1972; Bromage, 1989; Martinez-Maza et al. 2010). We could distinguish two main kinds of bone surfaces, forming surfaces and resorbing surfaces, which are associated to the activity of osteoblasts and osteoclasts respectively. Moreover, previous works indicated that bone surface features also make it possible to differentiate active from resting modelling fields (Boyde, 1972; Jones & Boyde, 1976; Marks et al. 1996).
During growth, osteoblasts synthesize and mineralize the collagenous and non-collagenous bone proteins that constitute the organic matrix or osteoid. This active phase of the osteoblast activity is related to bone surfaces leftacterized by delineated collagen fibre bundles with a preferred orientation. When inactive, osteoblasts become flat (so-called lining-cells), the mineralizing front advances, and the ground substance covers and masks the collagen fiber bundles. The resulting resting forming surface is observed as a smooth and bright surface (Boyde, 1972; Jones & Boyde, 1976; Marks et al. 1996; Radlanski et al. 2003).
Features of the resorbing surfaces can also indicate the active or the resting phase of the osteoclasts but differences between these two surfaces are less clear than in the forming surfaces. Osteoclasts adhere to the bone surface and remove the mineralized bone tissue, forming a concavity called Howship's lacunae. The (active) resorbing surfaces show lacunae of variable size and shape, delineated by sharp walls. However, the resting resorbing surfaces show shallower and less sharp-edged concavities (Boyde, 1972; Jones & Boyde, 1976).
The identification of resting surfaces is complicated when studying skeletonized remains because similar features could result from taphonomical processes which damage the bone surface, as is observed in fossil bones (Bromage, 1984, 1987; Rosas & Martinez-Maza, 2010; Martinez-Maza et al. 2011). All specimens studied in the present work present slightly damaged bone surfaces with recognizable histological features except in the more damaged surfaces. Our sample also present manipulation marks such as trampling, tool marks, fissures or writing marks. Consequently, we have decided not to distinguish between resting and active states of the bone modelling activity in our analysis. We have distinguished two bone modelling surfaces, (i) bone-forming surfaces leftacterized by mineralized collagen fibre bundles produced by osteoblasts (Fig. 1, left; Boyde, 1972; Bromage, 1989; Martinez-Maza et al. 2006, 2010) and (ii) bone-resorbing surfaces leftacterized by Howship's lacunae produced by the osteoclasts (Fig. 1, right; Boyde, 1972; Bromage, 1989; Martinez-Maza et al. 2010). From these data, we have drawn maps showing the distribution of the modelling fields for each individual (Figs 2 and 3). Following previous works, we have established generalized modelling patterns for the subadult and the adult groups through the identification of intraspecific similarities in the bone modelling field distribution of each anatomical region of the facial skeleton and mandible (Fig. 4; Enlow & Hans, 1996; Bromage, 1989; Rosas & Martinez-Maza, 2010; Martinez-Maza et al. 2011).
Figure 1.
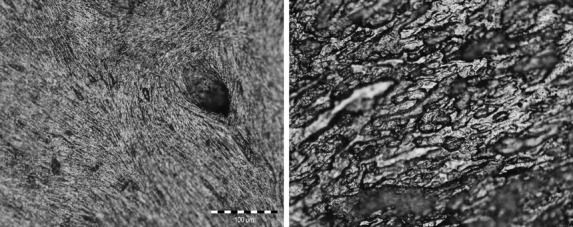
Bone formation (left) and resorption (right) surfaces identified in the sample of Homo sapiens analysed in this study. Image on left shows a bone formation surface from the buccal side of the mandibular corpus region (specimen 126), which is leftacterized by collagen fiber bundles. Image on right shows a bone resorption surface from the maxilla (specimen 218) leftacterized by Howship's lacunae. Scale bar: 100 μm.
Figure 2.
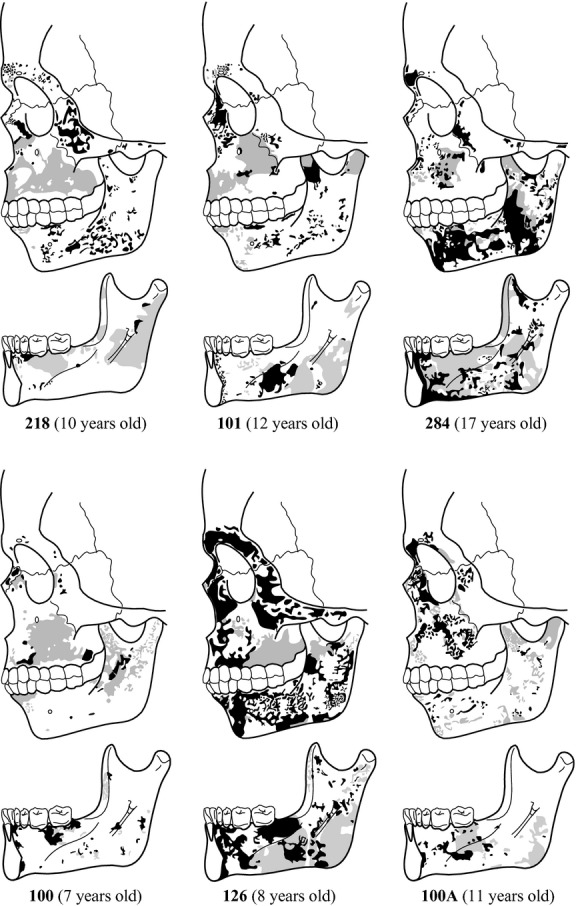
Schematic bone modelling patterns from the specimens of the subadult group. Black colour: bone formation surfaces; grey colour: bone resorption surfaces; white areas: damaged bone surfaces with no histological data.
Figure 3.
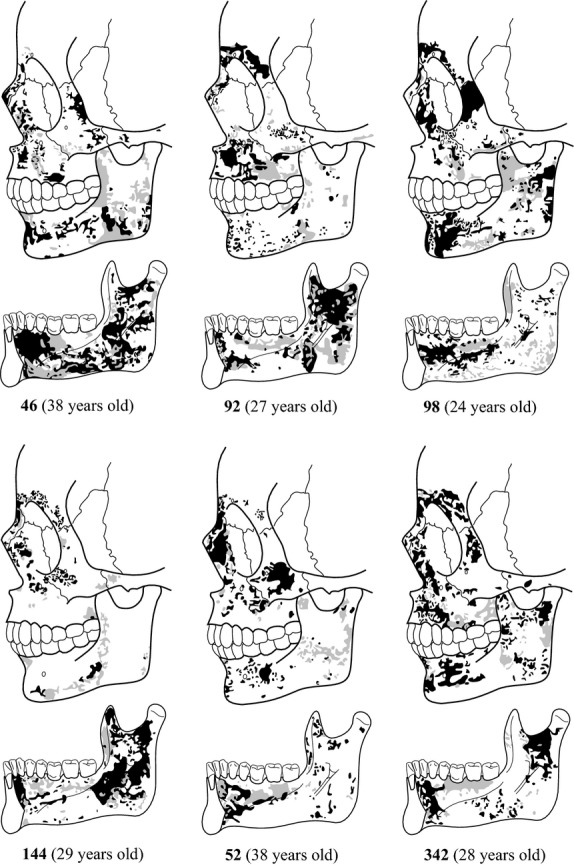
Schematic bone modelling patterns from the specimens of the adult group. Black colour: bone formation surfaces; grey colour: bone resorption surfaces; white areas: damaged bone surfaces with no histological data.
Figure 4.
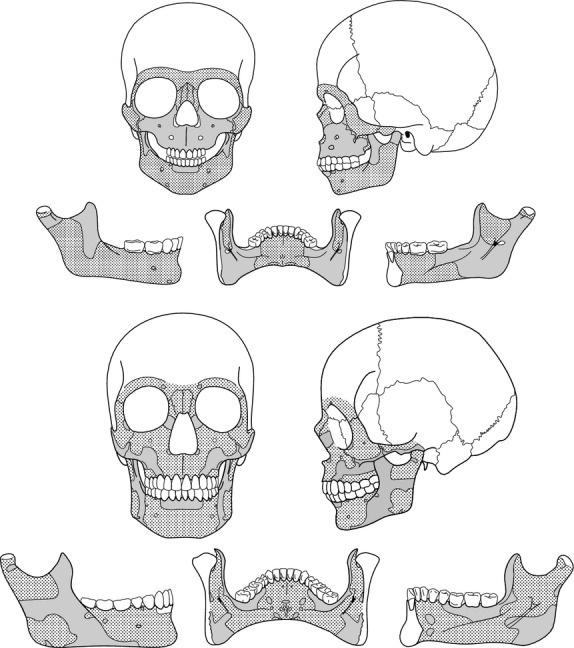
Generalized bone modelling patterns from subadult and adult humans. Stippling areas represent bone deposition and grey areas represent bone resorption.
From these data, modelling patterns for each individual were drawn (Figs 2 and 3). Following previous works, generalized modelling patterns for the subadult and the adult groups were established through the identification of intraspecific similarities in the bone modelling field distribution of each anatomical region of the facial skeleton and mandible (Fig. 4; Enlow & Hans, 1996; Bromage, 1989; Rosas & Martinez-Maza, 2010; Martinez-Maza et al. 2011).
Results
Schematic bone modelling maps of subadult and adult specimens analysed in this study are represented in Figs 2 and 3. Individual patterns show bone modelling fields with variable size and shape, irregular boundaries, and patchy distribution, which is emphasized by the presence of areas without histological areas. Even though different specimens show damaged surfaces lacking information, histological data recorded from the facial and mandibular regions allowed elaboration of generalized bone modelling patterns for adults and subadults (Fig. 4). A detailed description of the modelling fields identified in the facial skeleton and the mandible is provided. Finally, we compare the generalized bone modelling patterns of subadult and adult groups.
Facial skeleton: subadult specimens
The upper region of the facial skeleton (glabella and superciliar arch) is mainly depository. Small resorption fields are only found in the superciliary arch–glabella contact area close to the frontonasal suture in individuals 101 and 218, and in the inferior area of the superciliary arch of specimen 100A. In the nasal bones, depository surfaces are present in all specimens but 126 and 100A, which present resorptive fields close to the pyriform aperture. The nasomaxillary region shows high variability in the distribution of modelling fields respect to other facial regions. This region displays predominantly resorptive surfaces in the maxillary bone and depository surfaces in the nasal or frontal processes in individuals 218 and 101. This last specimen also presents small bone formation fields in the canine fossa region close to the infraorbital foramen and in the lateral margins of the nasal aperture. Specimen 100 shows bone resorption in both the nasomaxillary bone and the nasal process, while tiny depository surfaces are found close to the frontonasal suture and two fields in the alveolar region of the maxilla. Specimens 284 and 126 show similar patterns leftacterized by bone formation fields in the nasal process, in the lateral margins of the nasal aperture, in the zygomaticomaxillary suture, and small depository fields in the canine fossa area. In contrast, specimen 100A shows mainly depository surfaces both in the nasal process and in the maxillary bone, while resorptive surfaces are found close to the lacrimal area, in the lateral margins of the nasal aperture, in the canine fossa area, and in the zygomaticomaxillary suture. The zygomatic bone in all specimens displays primarily depository surfaces but three specimens show bone resorption activity in the orbital margin of the frontal process either close to the glabella (specimen 100) or extending from the zygomaticomaxillary suture to the level of the infraorbital foramen (specimens 126 and 100A).
Facial skeleton: adult specimens
The bone modelling map of the upper facial region is leftacterized by bone formation surfaces. Both the glabella and the superciliar arch regions are entirely depository in specimen 52, whereas specimens 92, 98, 144 and 342 show bone resorption fields in the glabella and in the area between the glabella and superciliar arch, and even in the frontonasal suture (individuals 92 and 144). The remaining specimen (46) shows eroded bone surfaces in most of the glabella and superciliar arch regions but small resorption fields are identified in the glabella–superciliar arch region, and tiny bone formation fields can be identified in the frontomaxillary suture and in the upper region of the superciliar arch. The nasal bones are leftacterized by bone formation surfaces. This region is entirely depository in specimens 46 and 342, while in specimens 144 and 52 small resorptive fields are observed close to the pyriform aperture and in the frontonasal suture area (specimen 144). In contrast, specimens 92 and 98 show predominantly bone resorption fields of variable size. The nasal process of the nasomaxillary bone is also leftacterized by bone formation surfaces occupying the whole area in specimens 98 and 342, whereas specimens 46, 92, 52 and 144 show small resorption fields in the area between the frontal process and the maxillary body, and distributed from the orbitary lateral margin to the lateral margin of the nasal aperture. Specimen 144 also displays resorptive surfaces along the lateral orbital margin. The studied specimens display a highly similar distribution of the growth fields in the maxilla. This facial region is predominantly depository, with bone resorption fields extending from the infraorbital foramen to the canine alveolus (46, 92, 98, 342 and 52). Small resorbing surfaces are also observed close to the nasal process in specimens 46, 52, 144 and 92, in the zygomatic nasomaxillary suture (specimens 46 and 92), and in the lateral-inferior margin of the nasal aperture (specimens 144 and 52). The zygomatic bone shows some variability, being mainly depository in specimens 46, 98, 52 and 342, while in specimens 92 and 144 bone formation is reduced to the infraorbital foramen area. Resorption fields are observed in the zygomatic maxillary suture in individuals 46, 92 and 98, also along the inferior margin of the bone zygomatic to the temporal zygomatic suture in 92 and 98, and in the area extending from the zygomatic maxillary suture to the infraorbital foramen level in specimen 46. The specimen 92 displays bone resorption activity along the lateral orbital margin to frontozygomatic suture. In this suture, a resorption field is also observed in the specimen 144.
Mandible: subadult specimens
Among subadults, bone modelling activity is preserved in the mandibles of specimens 284 and 126, whereas specimens 101, 100A, 126 and 100 present a combination of eroded surfaces and modelling fields of variable size and distributed along different mandibular regions. In the symphyseal region, specimens 284 and 126 display predominantly bone formation fields from the alveolar process to the inferior symphyseal border, whereas the specimen 100A shows small depository fields in the mental fossae at the level of the central incisors. All specimens show bone resorption fields in the alveolar process of the buccal side. Small resorptive fields are also observed above the mental protuberance and at the level of the canine in specimens 101 and 100A, and in the mental fossae in individuals 100, 126 and 218. The lingual side of the symphyseal region is leftacterized by depository fields distributed both in the alveolar process and in the basal component in specimens 284, 126 and 101, whereas depository fields of variable size are observed in the lingual alveolar process of specimens 218 and 100, in the sublingual fovea of 100 and 100A, and in the inferior border of specimen 100. Resorptive fields are restricted to the alveolar process of specimen 218, the sublingual fovea of specimens 101 and 284, and the inferior border of specimen 284.
Subadult mandibular corpus is leftacterized by depository surfaces in the buccal side and resorbing surfaces in the lingual side. However, some resorbing fields are found in the buccal side in the alveolar process at the level of the second premolar in specimens 100A and 284, and in the basal component in the anterior region of the corpus of specimen 101, close to the mandibular foramen in specimens 101 and 126, in the posterior region of the corpus in the oblique line area of specimens 284 and 100A, and in the inferior region as a stripe of small resorptive fields extending from the symphyseal region to the ramus of specimens 100A. Specimen 100 shows a high degree of erosion, but preserves resorption surfaces in the alveolar process at the level of the incisors and the canine and close to the anterior border of the ramus. At the lingual side, the sublingual fossa is leftacterized by bone resorption fields in the premolar and molar area in specimens 218, 101 and 284 and in the molar region of specimens 100 and 100A. Conversely, all specimens display depository surfaces in the anterior area of the sublingual fossa at the level of the lateral incisors and the canines, from the alveolar process to the mylohyoid line. The submandibular fossa is also leftacterized by depository fields in specimens 101, 284, 100 and 100A, while erosion precluded obtaining histological data from specimen 218. The lingual side of specimen 126 shows a particular modelling pattern leftacterized by bone formation surfaces in the sublingual fossa, whereas the submandibular fossa is predominantly resorptive, with depository fields at the level of the first premolar and second molar.
In the mandibular ramus, the buccal side is predominantly depository in specimens 218, 101, 284 and 126, whereas in specimens 100 and 100A this region is leftacterized by bone resorption surfaces. The bone formation activity in the specimens 218, 101, 284 and 126 is distributed as large (284 and 126) or small (218 and 101) fields throughout the buccal side of the ramus. Among them, specimens 101, 284 and 126 display resorbing fields in the anterior border of the ramus, the coronoid process and the condyle neck. Also, in specimen 284, bone resorption fields extend as a diagonal stripe from the coronoid to the angle of the ramus. The remaining two specimens, 100 and 100A, are leftacterized by resorbing surfaces, although bone formation is observed in the area between the coronoid process and the condylar neck, and, in specimen 100A, close to the angle of the mandible. In the lingual side of the ramus, bone resorption activity predominates. Resorption fields appear in three areas: between the anterior border and the endocoronoid crest (specimens 218, 284, 100 and 126), along the posterior region from the condyle neck to the angle of the ramus (specimens 218, 101 and 126), and in the area associated to the pterygoideus internus from where resorption fields extend to the mandibular corpus in all specimens except in specimen 100. Depository surfaces are observed close to the mandibular foramen between the condyle and the coronoid in specimens 218, 101, 284 and 126, and in the corpus-ramus contact area of specimens 101, 284 and 126. Three specimens, 284, 100 and 126, also display small depository fields below the mandibular foramen and in the mylohyoid groove.
Mandible: adult specimens
The symphyseal region shows resorptive fields in the alveolar component of the buccal side of specimens 46, 92 and 144, whereas specimens 52, 98 and 342 display predominantly bone formation fields. The basal component of this region is always depository, although specimen 342 also presents small resorbing fields at the mental fossa. Similarly, the lingual side is leftacterized by depository surfaces in the alveolar process, but specimens 46, 52, 98 and 144 also show small resorptive fields. The lingual basal component of the symphysis mainly displays bone formation fields with resorptive fields in the mental spine and in the digastric fossa regions. In specimens 52, 92, 98 and 144, resorptive activity is also seen in the sublingual fossa.
The mandibular corpus is predominantly depository. In its alveolar component, bone resorption activity is only found at the level of the canine of specimen 46, the premolar of 92, and the molar regions of specimens 92 and 98. On the other hand, the basal component of the corpus displays small resorptive fields close to the mental foramen area in specimens 46 and 342, in the contact region between the mandibular corpus and the ramus in specimens 46, 92, 98 and 144, and as a large stripe of resorptive fields along the inferior region of the corpus from the premolar area to the ramus in specimens 98 and 144. In the lingual side of the corpus, specimens 46, 52 and 342 display depository surfaces in the anterior region of the sublingual fossa extending from the symphyseal region to the premolar region. Small depository surfaces are also identified in the molar region close to the mylohyoid line of all specimens but 342, and in the alveolar component of specimens 92, 98 and 144. The premolar–molar region of the sublingual fossa of all individuals is leftacterized by bone resorption fields. The submandibular fossa displays depository surfaces along the mylohyoid line area and throughout the molar area in specimens 46, 98 and 342. Small depository surfaces are also identified in the anterior part of the submandibular fossa in specimens 46, 92, 98, and 342. A large field of bone resorption activity is observed in the anterior area of this fossa at the level premolar level in specimen 46, whereas small fields are identified in the first molar level in 144 and 342 and in the area extending from the symphyseal region to the ramus in specimen 98.
The adult mandibular ramus shows bone resorption surfaces in the buccal side of specimens 46, 52, 92, and 144, whereas specimens 98 and 342 are leftacterized by depository surfaces. On the one hand, resorbing activity is identified in the coronoid area in specimens 46, 92, 98, 144 and 52, in the condyle neck in 46, 92, 98, and 52, along the area running parallel to the posterior border in 46, 92, 52 and 342, in the angle of the ramus in 144 and 52, and close to the inferior border in 46, 98, 144 and 52. On the other hand, depository surfaces are observed in the coronoid area in specimens 98 and 342, in the mandibular notch area in 92, 98 and 342, in the condylar neck in 98, 52 and 342, along the area running parallel to the posterior border in specimen 98, and in the gonial region of specimen 342. The lingual side of the adult ramus displays predominantly bone formation activity. Bone resorption activity is located in the area between the anterior border and the endocoronoid crest in all specimens, in the neck of the condyle of specimens 46, 92 and 98, in the area parallel to the posterior border of specimens 46, 92, 98 and 144, and small resorptive fields in the mandibular notch area in 46, 92, 52 and 342. All specimens also display resorbing surfaces in the area associated to the pterigoideus internus and in the corpus–ramus contact area.
The generalized bone modelling patterns for subadult and adult groups (Fig. 4) are obtained through the identification of intraspecific similarities in the bone modelling field distribution of each anatomical region from the facial skeleton and mandible (Bromage, 1989; Enlow & Hans, 1996; Rosas & Martinez-Maza, 2010; Martinez-Maza et al. 2011). The subadult face generalized pattern shows bone formation fields in the upper (glabella and superciliar arch) and middle face (nasal bones and frontal apophysis of the maxillary bone), whereas bone resorption fields extend throughout the lower face (maxillary bone) and the frontal process of the zygomatic bone. In the subadult mandible, the buccal side is leftacterized by depository surfaces but resorption fields are identified in the alveolar component of the symphyseal region, the coronoid region and the condyle neck. In the lingual side, the anterior region of the mandible and the mandibular notch area are depository whereas the molar region in the submandibular and the sublingual fossae is resorptive.
In adults, the generalized pattern of the facial skeleton is predominantly depository but bone resorption activity, comparing with subadult pattern, is reduced to small fields in the glabella, in the frontal apophysis of the maxillary bone and in the frontal apophysis of the zygomatic bone (orbital margin). The resorbing activity of the lower face extends from the canine fossa and along the inferior border of the zygomatic bone. The adult mandible shows in the buccal side resorption activity in the alveolar component of the symphyseal region, along the inferior region of the corpus, and a large field in the corpus-ramus contact area that extends from the inferior margin to the coronoid region. The condylar neck and the mandibular angle region show small resorptive fields. Unlike subadult specimens, the lingual side is leftacterized by bone formation surfaces. The symphyseal region shows small resorptive fields in the digastric fossa and mental spine region. In the lingual mandibular corpus, the resorption activity is located in the molar region of the submandibular fossa and extends along the coronoid region. A large resorptive field is identified in the gonial region and close to the condyle neck.
Discussion
In the present study, we have examined the postnatal growth dynamics of the human facial skeleton and mandible through the analysis of the bone modelling activity (Fig. 5). The bone modelling patterns obtained in this work reflect the distribution of forming and resorbing fields but without distinguishing between active or resting fields. A bone modelling map incorporating the distribution of the growth fields as well as the state (active or resting) and the rate of the cellular activity would be extremely valuable to understand the bone growth dynamics. Further research is needed to develop non-destructive methods to obtain this information. In spite of this lack of information, the interpretation of the bone modelling maps obtained in this work allowed us to approach the main, general, hypothetical growth dynamics that leftacterize the subadult and adult human facial skeleton and mandible. The bone modelling patterns from all specimens show a general downward-forward growth vector. However, there are ontogenetic differences in the distribution of growth fields that demonstrate postnatal changes in the bone growth dynamics. Integration of the modelling data from the different anatomical elements informs us about the general growth dynamics of the whole skull and its relationships with ontogenetic postnatal changes of the craniofacial system.
Figure 5.
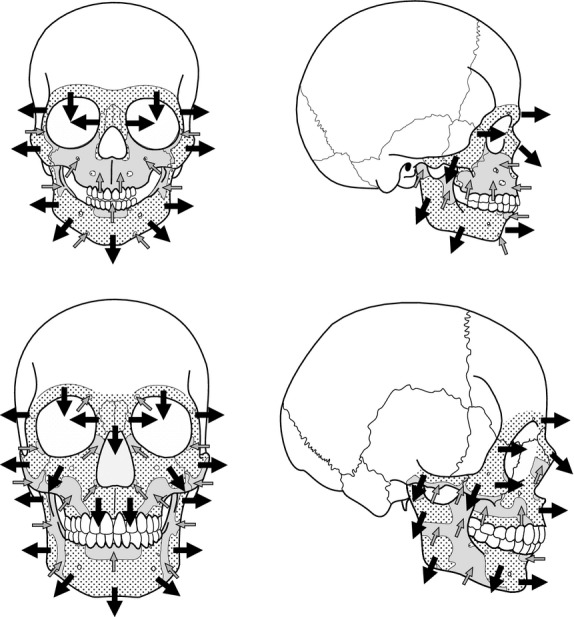
Figure shows the growth vectors inferred from the generalized bone modelling patterns from subadult and adult humans. Black arrows show the direction of growth by bone formation and grey arrows the direction of growth by bone resorption.
Bone growth dynamics in the subadult face and mandible
The modelling pattern of the face and the mandible from the subadult specimens established here is similar to the pattern described by Enlow & Hans (1996). The facial skeleton is leftacterized by depository surfaces in the upper (supraorbital region) and middle face (orbital and nasal regions) and bone resorption fields in the lower face (nasomaxillary region). According to this map, the upper and the middle face grow in a lateral and forward direction, whereas the zygomatic region grows laterally and is relocated posteriorly, in agreement with the resorbing surfaces present at the orbital margins. The lower face shows a large bone resorption field related to the formation of the canine fossa – a depression on the external surface of the maxillary bone region specific to Homo sapiens (Enlow & Hans, 1996). As reported by Enlow & Hans (1996), resorption in the nasomaxillary region occurs simultaneously with bone formation in the posterior region of the face (specifically in the craniofacial sutures) and in the nasal cavity floor and palate. Consequently, the lower face shows a downward or vertical growth related to the formation of the canine fossa and the increase in height of the nasal cavity (Kurihara et al. 1980; Enlow & Hans, 1996; McCollum & Ward, 1997).
The mandible pattern is leftacterized by depository surfaces in the symphyseal region and the anterior corpus, whereas the posterior region of the corpus and the ramus show a complex pattern of resorption and formation fields. According to these data, the mandible shows a forward growth associated to the deposition in the symphysis and the corpus, as well as to the lengthening of the posterior region of the corpus. At the same time, the ramus and the molar region of the corpus show a mainly lateral growth, together with a downward growth of the ramus and a posterior relocation of the condyle and coronoid region. It is worth mentioning that the symphyseal region presents a human-specific resorption field in the alveolar component of the buccal side related to the dental movements, the mental growth, and ultimately the development of the human chin (Enlow & Hans, 1996).
As a whole, the bone modelling pattern indicates that the subadult facial skeleton and mandible grow following a downward-forward vector. As we mentioned before, this general pattern agrees with the results of Enlow & Hans (1996), albeit with some differences. In opposition to Enlow & Hans (1996), the lingual side of the corpus shows resorption fields in the sublingual fossa and in the region anterior to the mandibular foramen, and formation in the submandibular fossa. Our modelling map also suggests a more marked lateral growth of the molar region in the mandibular corpus and ramus than in Enlow & Hans (1996) model. Other differences regarding the extension of the resorbing surfaces in the buccal side of the ramus could be considered artefacts due to the variability of the distribution of modelling fields observed in the human mandible (Enlow & Harris, 1964; Kurihara et al. 1980; Hans et al. 1995).
Our analyses also reveal variability in the distribution of the modelling fields mainly observed in the anterior lower face and in the mandibular ramus. In the anterior face, variability in the distribution and the extension of the resorption fields of the anterior face agrees with the modelling data provided by Kurihara et al. (1980) for humans up to 14 years old, but disagrees with the mainly depository anterior lower face observed by McCollum (2008). As mentioned by Kurihara et al. (1980) and later by McCollum (2008), variability in the modelling maps from this facial region could be due to morphological variations associated to different geographical origin. Variability in the mandible ramus involves the extension and location of the resorption fields in the coronoid and the condyle neck, due to lateral adjustments while growing upward and relocating posteriorly. Besides these two main areas, some variability is also observed in the buccal symphyseal region previously reported by Kurihara et al. (1980). In the mandibular corpus, individual 126 shows in the submandibular fossa a modelling pattern that resembles that established by Enlow & Hans (1996) but opposite to the general pattern obtained in the present work.
Bone growth dynamics in the adult face and mandible
In the present study, we have established for the first time, to our knowledge, the bone modelling pattern of the face and the mandible from adult humans. The facial pattern is mainly depository with resorption surfaces restricted to the nasal region, the canine fossae, and the inferior margin of the zygomatic region. Resorption at the nasal region could be associated to the increase in height of the nasal aperture (McCollum, 2008) and the forward projection of this region (Bastir, 2008). The resorption fields in the area of the canine fossae are likely related to the development of this specific feature of Homo sapiens. In the zygomatic region, resorption surfaces would reflect the complex growth dynamics associated to the mechanical strains of the masticatory force (Herring, 1993; Lieberman et al. 2000, see references therein). The increase of the bone formation surfaces that leftacterized the adult modelling map leads us to hypothesize that the adult face shows downward and forward growth directions with the main emphasis in the horizontal direction, in agreement with morphometric data obtained in previous work (Bastir, 2008). However, more data, particularly on the modelling processes at the nasomaxillary complex, are needed to fully understand the downwards movements of the lower face during adulthood.
In the adult mandible, the symphysis and the anterior region of the corpus are mainly depository, with resorbing surfaces restricted to the alveolar component of the buccal side of the symphysis. This modelling field distribution is similar to that observed in subadult specimens and reflects the growth dynamics associated to dental movements and also to the development of this particular feature of Homo sapiens (Enlow & Hans, 1996). Resorption fields are also present in the lingual side of the symphysis. These fields are located in sites related to the attachments of the gastricus, genioglossus, geniohyoideus, and the anterior part of the mylohyoideus muscles. The pattern of the posterior region of the corpus and the ramus differs greatly from the subadult patterns established in the present and in previous works (Kurihara et al. 1980; Enlow & Hans, 1996). The buccal side shows resorption fields covering the posterior region of the corpus and the anterior region of the ramus, whereas the resorption fields of the lingual side are located in the submandibular fossa of the corpus, and in the coronoid region and the lower half (gonial area) of the ramus. This map indicates a forward growth direction of the symphysis and a lateral growth of the molar region of the corpus, whereas the anterior region of the ramus grows in a posterior and medial direction. The posterior region of the ramus experiences complex growth dynamics leftacterized by a lateral growth of the gonial area, a medial growth of the mandibular notch area, and a lateral and medial growth of the condyle area. These growth directions indicate that the lower part of the ramus is taking or maintaining a vertical position while the upper area increases in width and grows backwards.
The modelling pattern of the facial skeleton and the mandible varies less in adult than in subadult specimens. Variability is observed in the extension of the resorption fields of the anterior nasomaxillary region and the mandibular ramus, as observed in subadults. Following the previous interpretations for subadult specimens (Kurihara et al. 1980; Enlow & Hans, 1996; McCollum, 2008), we hypothesize that these variations could respond to individual differences in morphological leftacteristics.
Postnatal changes in the growth dynamics of the human face
According to the data obtained in the present study, the facial skeleton and mandible from subadult and adult specimens show a general downward and forward growth, in agreement with Enlow & Hans (1996). Both groups also show a marked spatial gradient of the variability in the modelling field distribution from the anterior region of the maxilla with high variability to the almost constant upper facial regions in the proximity of the neurocranium. However, bone modelling patterns differ between both age groups. Subadult specimens show a marked downward growth direction, whereas adults are leftacterized by a forward direction. The interpretation of these ontogenetic changes within the craniofacial biology context allows us to approach how different skeletal components interact to maintain the functional and structural balance, but increase in size during the postnatal development (Moss & Young, 1960; Enlow & Hans, 1996).
During the subadult stage, the facial skeleton experiences a downward growth and a forward displacement, while the maxilla increases in length. This growth and displacement of the facial block is accompanied by an upward maxillary rotation (airorhynchy) due to the higher bone growth in the craniofacial sutures that attach the midface to the basicranium than in the anterior region of the maxilla (Enlow and Bang, 1965; Björk & Skieller, 1976; Bromage, 1989; Enlow & Hans, 1996; McCollum & Ward, 1997). Rotation of the premaxilla would be balanced by a downward rotation through compensatory resorption activity in the external surfaces of the anterior region of the maxilla (Björk, 1969; Björk & Skieller, 1976, 1983; see also Bromage, 1989; McCollum & Ward, 1997 and references therein). The resulting downward growth vector contributes to the leftacteristic facial retraction of Homo sapiens (Bromage, 1989; Enlow & Hans, 1996). Simultaneously, the mandible is displaced forward and downward to compensate the displacements of the maxilla and to maintain the occlusal plane (Enlow & Hans, 1996). The forward displacement of the face becomes balanced through the growth of the posterior region of the mandibular corpus, whereas the vertical growth is compensated by the increase in height of the ramus and, particularly, the condyle (Enlow & Hans, 1996). During this displacement, the mandibular corpus increases in width in the anterior region, whereas the molar region and the ramus show a lateral drift. The lateral drift and the vertical growth of the ramus have been related to the growth of the basicranium to keep the mandible in contact with the neurocranium through the temporomandibular joint.
With adulthood, the modelling pattern changes, reflecting the biological changes that occurred during the craniofacial development. The most important differences are observed in the anterior region of the face, where the resorbing surfaces that occupy the subadult nasomaxillary region become restricted to the canine fossa in adults. This increase of the bone formation fields in adult specimens suggests a mainly forward growth of the whole facial skeleton with an increase in the height of the nasal region. The mandible responds to these changes and shows bone modelling differences located mainly in the ramus. Complex growth dynamics in this mandibular region show a lateral and medial growth that maintains the vertical position and the contact with the neurocranium through the temporomandibular joint (Enlow & Hans, 1996). As well, the ramus increases in a way highly similar to the nasomaxillary region, whereas the whole mandible grows with the main forward direction accompanying the anterior region of the face growth.
To understand the biological meaning of these modelling changes, we need to consider several parallel developmental events that occur in the craniofacial system. The growth and development of each craniofacial bone should be analysed as part of the integrated complex system influenced by multifactorial processes involving genetic and epigenetic factors (Moss & Young, 1960; Atchley & Hall, 1991; Enlow & Hans, 1996; Lieberman et al. 2002). Early in the ontogeny, the craniofacial growth is leftacterized by the increase in the size of the brain, the growth of both the cranial base and the neurocranium, and the flexion of the cranial base. During the rapid expansion of the brain, the neurocranium enlarges mainly from deposition within the cranial sutures, as well as expanding through modelling activity, with bone deposition in the ectocranial surface and bone resorption in the endocranial surface (Duterloo & Enlow, 1970; Enlow & Hans, 1996). The basicranium also increases in length and breadth through the modelling mechanism and the sutural bone formation (Enlow & Hans, 1996: Opperman, 2000). These cranial dynamics, and particularly the cranial base flexure, influence the size and projection of the facial skeleton that shows the main downward growth as inferred from the bone modelling data obtained in the present and previous studies (e.g. Lieberman et al. 2000; Bastir et al. 2010). The mandible also shows a downward growth direction that increases in height as it grows forward in order to maintain the occlusal plane with the maxilla. As well, the lateral growth of the ramus inferred in the present work indicates how the ramus grows to maintain contact with the cranial base through the temporomandibular joint.
Critical changes occur in the human skull at around 14 years of age and new relationships emerge among craniofacial components. The brain, having reached 95% of the total size by age 6 years, reaches its final brain size at an average age of 14.5 years in males and 11.5 years in females (Giedd et al. 1999, 2006; Lenroot & Giedd, 2006, 2010). In the second decade of the postnatal development, the growth of the cranial base ceases and most occipital bone sutures fuse, whereas the sphenoid bone sutures fuses in the first decade (Madeline & Elster, 1995,b; Lingawi, 2012). The calvarial sutures also fuse completely at 20–30 years old (Vijay Kumar et al. 2012). However, most facial sutures remain patent until late adulthood, e.g. the frontomaxillary, nasomaxillary and zygomaticomaxillary, which start to fuse until the 7th or 8th decade of life (Rice, 2008). Interestingly, the facial skeleton continues growing into adulthood, whereas the neurocranium and cranial base by that time have already stopped growing (Bastir et al. 2006; Edwards et al. 2007; Holton & Franciscus, 2008). Reduction of the resorption fields in the adult anterior face could be indicating that this continue growth of the face occurs with a mainly forward direction. Thus, the retracted facial skeleton – a defining leftacteristic of Homo sapiens – is established in the subadult period (e.g. Moss & Young, 1960; Lieberman et al. 2002; Holton et al. 2010). The mandible also responds to these developmental changes, as reflected in its modelling pattern observed mainly in the ramus. As the neurocranium and basicranium stop growing, the distance between the mandible fossae becomes established and the condyles would adapt to this distance, changing the growth of the condyles to maintain the contact with the neurocranium through the temporomandibular joint (Enlow & Hans, 1996). The increase in height of the ramus also responds to the vertical growth of the nasomaxillary region in order to maintain the occlusal plane.
The particular features of the modelling pattern of the adult face and mandible could be related to the increase of the volume of the oro-naso-pharyngeal cavities. This hypothesis would agree with the relationship established between the growth and development of the craniofacial complex and the nasal respiratory function (Moss & Young, 1960; Moss, 1962; Klein, 1986; Hall, 2005a; Chinn et al. 2006; Rosas et al. 2006; Weinstein, 2008; Gungora & Turkkahramanb, 2009). Moreover, it has been proposed that the bone growth of the facial skeleton and particularly the nasomaxillary complex and the mandible are related via physiological factors to the general body size or the metabolic requirements (Hall, 2005b; Rosas et al. 2006; Bastir, 2008 and references therein). In agreement, we hypothesize that, once the growth of the neurocranium and cranial base ceases, the forward growth direction of the adult human face indicates an increase of airway dimensions – the oro-naso-pharyngeal cavities – to maintain the integration between airway, relative body and lung size due to the augmented energy requirements (Henry & Rees, 1991; Rosas & Bastir, 2002; Rosas et al. 2006).
In conclusion, our results demonstrate postnatal changes in the hypothetical growth dynamics of the facial skeleton and the mandible from a mainly downward direction in subadults to a mainly forward direction in adults. We hypothesize that these changes are related to biological events occurring in the craniofacial system, such as cessation of the brain growth, fusion of the craniofacial sutures, growth of the cranium and cranial base, as well as flexion of the cranial base. During adulthood, a new relationship among skeletal elements of the skull emerges and growth dynamics change, stressing the forward growth of the face. We hypothesize that the adult growth of the face is related to the increase of the airway dimensions (the nasal and oral cavities) to cope with the physiological demands.
Acknowledgments
We thank Eugenia Cunha for access to the anthropological collection of Homo sapiens held in the Universidade de Coimbra, Portugal. This research is founded by the Spanish Ministry of Economy and Competitiveness (Projects CGL2009-09013 and CGL2012-36682).
References
- Akgül A, Toygar T. Natural craniofacial changes in the third decade of life: A longitudinal study. Am J Orthod Dentofacial Orthop. 2002;122:512–522. doi: 10.1067/mod.2002.128861. [DOI] [PubMed] [Google Scholar]
- Albert AM, Ricanek K, Patterson E. A review of the literature on the aging adult skull and face: implications for forensic science research and applications. Forensic Sci Int. 2007;172:1–9. doi: 10.1016/j.forsciint.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Atchley WR, Hall B. A model for development and evolution of complex morphological structures. Biol Rev. 1991;66:101–157. doi: 10.1111/j.1469-185x.1991.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Bastir M. A systems-model for the morphological analysis of integration and modularity in human craniofacial evolution. J Anthropol Sci. 2008;86:37–58. [PubMed] [Google Scholar]
- Bastir M, Rosas A, O'Higgins P. Craniofacial levels and the morphological maturation of the human skull. J Anat. 2006;209:637–654. doi: 10.1111/j.1469-7580.2006.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastir M, Rosas A, Stringer C. Effects of brain and facial size on basicranial form in human and primate evolution. J Hum Evol. 2010;58:424–431. doi: 10.1016/j.jhevol.2010.03.001. [DOI] [PubMed] [Google Scholar]
- de Beer GR. The Development of the Vertebrate Skull. London: Oxford University Press; 1937. [Google Scholar]
- Behrents RG. Growth in the Aging Craniofacial Skeleton: Craniofacial Growth Series, Monograph 17. Ann Arbor: University of Michigan; 1985. [Google Scholar]
- Bishara SE, Hession TJ, Peterson LC. Longitudinal soft-tissue profile changes: a study of three analyses. Am J Orthod. 1985;88:209–223. doi: 10.1016/s0002-9416(85)90216-7. [DOI] [PubMed] [Google Scholar]
- Björk A. Prediction of mandibular growth rotation. Am J Orthod. 1969;55:586–599. doi: 10.1016/0002-9416(69)90036-0. [DOI] [PubMed] [Google Scholar]
- Björk A, Skieller V. Facial development and tooth eruption. An implant study at the age of puberty. Am J Orth. 1972;55:339–383. doi: 10.1016/s0002-9416(72)90277-1. [DOI] [PubMed] [Google Scholar]
- Björk A, Skieller V. Factors Affecting the Growth of the Midface. Craniofacial Growth Series, Vol. 6. (ed. McNamara FA Jr), pp. Ann Arbor: University of Michigan; 1976. Postnatal growth and development of the maxillary complex; pp. 61–99. [Google Scholar]
- Björk A, Skieller V. Normal and abnormal growth of the mandible. A synthesis of longitudinal cephalometric implant studies over a period of 25 years. Eur J Orthod. 1983;5:1–46. doi: 10.1093/ejo/5.1.1. [DOI] [PubMed] [Google Scholar]
- Bloom W, Fawcett DW. A Textbook of Histology. 12th edn. New York: Chapman and Hall; 1994. [Google Scholar]
- Bondevik O. Growth changes in the cranial base and the face: a longitudinal cephalometric study of linear and angular changes in adult Norwegians. Eur J Orthod. 1995;17:525–532. doi: 10.1093/ejo/17.6.525. [DOI] [PubMed] [Google Scholar]
- Boyde A. Scanning electron microscope studies of bone. In: Bourne GH, editor. The Biochemistry and Physiology of Bone. 2nd edn. Vol. 1. New York: Academic Press; 1972. pp. 259–310. [Google Scholar]
- Bromage TG. Interpretation of scanning electron microscopic images of abraded forming bone surfaces. Am J Phys Anthropol. 1984;64:161–178. doi: 10.1002/ajpa.1330640210. [DOI] [PubMed] [Google Scholar]
- Bromage TG. The scanning electron microscopy/replica technique and recent applications to the study of fossil bone. Scan Microsc. 1987;1:607–613. [PubMed] [Google Scholar]
- Bromage TG. Ontogeny of the early hominid face. J Hum Evol. 1989;18:751–773. [Google Scholar]
- Chinn DJ, Cotes J, Martin A. Modelling the lung function of Caucasians during adolescence as a basis for reference values. Ann Hum Biol. 2006;33:64–77. doi: 10.1080/03014460500442797. [DOI] [PubMed] [Google Scholar]
- Doual JM, Ferri J, Laude M. The influence of senescence on craniofacial and cervical morphology in humans. Surg Radiol Anat. 1997;19:175–183. doi: 10.1007/BF01627970. [DOI] [PubMed] [Google Scholar]
- Duterloo HS, Enlow DH. A comparative study of cranial growth in Homo and Macaca. Am J Anat. 1970;127:357–367. doi: 10.1002/aja.1001270403. [DOI] [PubMed] [Google Scholar]
- Edwards CB, Marshall S, Qian F. Longitudinal study of facial skeletal growth completion in 3 dimensions. Am J Orthod Dentofacial Orthop. 2007;132:762–768. doi: 10.1016/j.ajodo.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Enlow DH. A study of the postnatal growth and remodelling of bone. Am J Anat. 1962;110:79–101. doi: 10.1002/aja.1001100202. [DOI] [PubMed] [Google Scholar]
- Enlow DH. Principles of Bone Remodelling. Springfield: Leftles C Thomas; 1963. [Google Scholar]
- Enlow DH. The Human Face: An Account of the Postnatal Growth and Development of the Craniofacial Skeleton. New York: Harpers and Row; 1975. [Google Scholar]
- Enlow DH, Bang S. Postnatal growth and remodeling of the human maxilla. Am J Orthodontics. 1965;51:446–464. doi: 10.1016/0002-9416(65)90242-3. [DOI] [PubMed] [Google Scholar]
- Enlow DH, Hans MG. Essentials of Facial Growth. Philadelphia: WB Saunders Company; 1996. [Google Scholar]
- Enlow DH, Harris DB. A study of the postnatal growth of the human mandible. Am J Orthod. 1964;50:25–50. [Google Scholar]
- Enlow DH, Moyers RE, Hunter WS. A procedure for the analysis of intrinsic facial form and growth. Am J Orthod. 1969;56:6–23. doi: 10.1016/0002-9416(69)90254-1. [DOI] [PubMed] [Google Scholar]
- Forsberg C, Eliasson S, Westergren H. Face height and tooth eruption in adults – a 20-year follow-up investigation. Eur J Orthod. 1991;13:249–254. doi: 10.1093/ejo/13.4.249. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal JD, Jeffries NO. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Gröning F, Fagan M, O'Higgins P. Comparing the distribution of strains with the distribution of bone tissue in a human mandible: a finite element study. Anat Rec. 2013;296:9–18. doi: 10.1002/ar.22597. [DOI] [PubMed] [Google Scholar]
- Gungora AY, Turkkahramanb H. Effects of airway problems on maxillary growth: a review. Eur J Dent. 2009;3:250–254. [PMC free article] [PubMed] [Google Scholar]
- Hall BK. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. London: Elsevier Academic Press; 2005a. [Google Scholar]
- Hall RL. Energetics of nose and mouth breathing, body size, body composition, and nose volume in young adult males and females. Am J Hum Biol. 2005b;17:321–330. doi: 10.1002/ajhb.20122. [DOI] [PubMed] [Google Scholar]
- Hans MG, Enlow DH, Noachtar R. Age-related differences in mandibular ramus growth: a histologic study. Angle Orthod. 1995;65:335–340. doi: 10.1043/0003-3219(1995)065<0335:ADIMRG>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Henry CJK, Rees DG. New predictive equations for the estimation of basal metabolic rate in tropical peoples. Eur J Clin Nutrit. 1991;45:177–185. [PubMed] [Google Scholar]
- Herring SW. Epigenetic and functional influences on skull growth. In: Hanken J, Hall BK, editors. The Skull. Vol. 1. Chicago: University of Chicago Press; 1993. pp. 153–206. [Google Scholar]
- Holton NE, Franciscus RG. The paradox of a wide nasal aperture in cold-adapted Neandertals: a causal assessment. J Hum Evol. 2008;55:942–951. doi: 10.1016/j.jhevol.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Holton NE, Franciscus RG, Nieves MA. Sutural growth restriction and modern human facial evolution: an experimental study in a pig model. J Anat. 2010;216:48–61. doi: 10.1111/j.1469-7580.2009.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SJ, Boyde A. Is there a relationship between osteoblasts and collagen orientation in bone? Isr J Med Sci. 1976;12:98–107. [PubMed] [Google Scholar]
- Klein JC. Nasal respiratory function and craniofacial growth. Arch Otolaryngol Head Neck Surg. 1986;112:843–849. doi: 10.1001/archotol.1986.03780080043009. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP, Mebus K, Auffray JC. Developmental integration in a complex morphological structure: how distinct are the modules in the mouse mandible? Evol Dev. 2003;5:522–531. doi: 10.1046/j.1525-142x.2003.03057.x. [DOI] [PubMed] [Google Scholar]
- Kurihara S, Enlow DH, Rangel RD. Remodelling reversals in anterior parts of the human mandible and maxilla. Angle Orthod. 1980;50:98–106. doi: 10.1043/0003-3219(1980)050<0098:RRIAPO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Coch D, Fischer KW, Dawson G. Human Behavior, Learning, and the Developing Brain: Typical Development. New York: The Guilford Press; 2010. The structural development of the human brain as measured longitudinally with magnetic resonance imaging; pp. 50–73. Chap. 3. [Google Scholar]
- Lieberman DE. The Evolution of the Human Head. Cambridge, MA: Belknap (Harvard University) Press; 2011. [Google Scholar]
- Lieberman DE, Pearson OM, Mowbray KM. Basicranial influence on overall cranial shape. J Hum Evol. 2000;38:291–315. doi: 10.1006/jhev.1999.0335. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, McBratney BM, Krovitz G. The evolution and development of cranial form in Homo sapiens. Proc Natl Acad Sci U S A. 2002;99:1134–1139. doi: 10.1073/pnas.022440799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingawi SS. Determination of the chronological age of skull base suture closure using computed tomography. J Bas App Sci. 2012;8:247–252. [Google Scholar]
- Madeline LA, Elster AD. Suture closure in the human chondrocranium: CT assessment. Radiology. 1995;196:747–756. doi: 10.1148/radiology.196.3.7644639. a) [DOI] [PubMed] [Google Scholar]
- Madeline LA, Elster AD. Postnatal development of the central skull base: normal variants. Radiology. 1995;196:757–763. doi: 10.1148/radiology.196.3.7644640. b) [DOI] [PubMed] [Google Scholar]
- Marks SC, Cielinski MJ, Sundquist KT. Bone surface morphology reflects local skeletal metabolism. Microsc Res Tech. 1996;33:121–127. doi: 10.1002/(SICI)1097-0029(19960201)33:2<121::AID-JEMT3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Martinez-Maza C, Rosas A, Garcia-Vargas S. Bone paleohistology and human evolution. J Anthropol Sci. 2006;84:77–81. [Google Scholar]
- Martinez-Maza C, Rosas A, Nieto-Diaz M. Identification of bone formation and resorption surfaces by reflected light microscopy. Am J Phys Anthropol. 2010;143:313–320. doi: 10.1002/ajpa.21352. [DOI] [PubMed] [Google Scholar]
- Martinez-Maza C, Rosas A, Estalrrich A. Bone remodelling in Neanderthal mandibles from the El Sidrón site (Asturias, Spain) Biol Lett. 2011;7:593–596. doi: 10.1098/rsbl.2010.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauser C, Enlow DH, Overman DO. McNamara JA. Determinants of Mandibular Form and Growth: Craniofacial Growth Series, Monograph 5. Ann Arbor: University of Michigan; 1975. Growth and remodelling of the human fetal face and cranium; pp. 243–275. [Google Scholar]
- McCollum MA. Nasomaxillary remodelling and facial form in robust Australopithecus: a reassessment. J Hum Evol. 2008;54:2–14. doi: 10.1016/j.jhevol.2007.05.013. [DOI] [PubMed] [Google Scholar]
- McCollum MA, Ward SC. Subnasoalveolar anatomy and hominoid phylogeny: evidence from comparative ontogeny. Am J Phys Anthropol. 1997;102:377–405. doi: 10.1002/(SICI)1096-8644(199703)102:3<377::AID-AJPA7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Moss ML. Kraus B, Reidel R. Vistas in orthodontics. Philadelphia: Lea and Febiger; 1962. The functional matrix; pp. 85–98. [Google Scholar]
- Moss ML. The functional matrix hypothesis revisited. 1. The role of mechanotransduction. Am J Orthod Dentofacial Orthop. 1997a;112:8–11. doi: 10.1016/s0889-5406(97)70267-1. [DOI] [PubMed] [Google Scholar]
- Moss ML. The functional matrix hypothesis revisited. 2. The role of an osseus connected cellular network. Am J Orthod Dentofacial Orthop. 1997b;112:221–226. doi: 10.1016/s0889-5406(97)70249-x. [DOI] [PubMed] [Google Scholar]
- Moss ML. The functional matrix hypothesis revisited. 3. The genomic thesis. Am J Orthod Dentofacial Orthop. 1997c;112:338–342. doi: 10.1016/S0889-5406(97)70265-8. [DOI] [PubMed] [Google Scholar]
- Moss ML. The functional matrix hypothesis revisited. 4. The epigenetic antithesis and the resolving synthesis. Am J Orthod Dentofacial Orthop. 1997d;112:410–417. doi: 10.1016/s0889-5406(97)70049-0. [DOI] [PubMed] [Google Scholar]
- Moss ML, Rankow RM. The role of the functional matrix in mandibular growth. Angle Orthod. 1968;38:95–103. doi: 10.1043/0003-3219(1968)038<0095:TROTFM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Moss ML, Salentijn L. The primary role of functional matrices in facial growth. Am J Orthod. 1969;55:566–577. doi: 10.1016/0002-9416(69)90034-7. [DOI] [PubMed] [Google Scholar]
- Moss ML, Young RW. A functional approach to craniology. Am J Phys Anthropol. 1960;18:281–292. doi: 10.1002/ajpa.1330180406. [DOI] [PubMed] [Google Scholar]
- O'Higgins P, Bromage TG, Johnson DR. A study of facial growth in the sooty mangabey Cercocebus atys. Folia Primatol. 1991;56:86–94. doi: 10.1159/000156532. [DOI] [PubMed] [Google Scholar]
- Opperman LA. Cranial sutures as intramembranous bone growth sites. Dev Dyn. 2000;219:472–485. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1073>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Radlanski RJ, Klarkowski MC. Bone remodelling of the human mandible. 3D reconstructions, morphometry and bone remodelling pattern, sizes 12–117 mm CRL. Anat Embryol. 2001;207:221–232. doi: 10.1007/s00429-003-0343-4. [DOI] [PubMed] [Google Scholar]
- Radlanski RJ, Renz H, Klarkowski MC. Prenatal development of the human mandible. 3D reconstructions, morphometry and bone remodeling pattern, sizes 12–117 mm CRL. Anat Embryol. 2003;207:221–232. doi: 10.1007/s00429-003-0343-4. [DOI] [PubMed] [Google Scholar]
- Rice DP. Craniofacial Sutures: Development, Disease, and Treatment. Basel: Karger; 2008. Clinical features of syndromic craniosynostosis; pp. 91–106. [Google Scholar]
- Rosas A, Bastir M. Thin-plate spline analysis of allometry and sexual dimorphism in the human craniofacial complex. Am J Phys Anthropol. 2002;117:236–245. doi: 10.1002/ajpa.10023. [DOI] [PubMed] [Google Scholar]
- Rosas A, Martinez-Maza C. Bone remodelling of the Homo heidelbergensis mandible. The Atapuerca-SH sample. J Hum Evol. 2010;58:127–137. doi: 10.1016/j.jhevol.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Rosas A, Bastir M, Martinez-Maza C. Inquiries into Neanderthal craniofacial development and evolution, ‘accretion’ vs ‘organismic’ models. In: Harvati K, Harrison T, editors. Neanderthals Revisited: New Approaches and Perspectives. New York: Springer; 2006. pp. 38–69. [Google Scholar]
- Tomoyasu Y, Yamaguchi T, Tajima A. Further evidence for an association between mandibular height and the growth hormone receptor gene in a Japanese population. Am J Orthod Dentofacial Orthop. 2009;136:536–541. doi: 10.1016/j.ajodo.2007.10.054. [DOI] [PubMed] [Google Scholar]
- Tsiopas N, Nilner M, Bondemark L. A 40 years follow-up of dental arch dimensions and incisor irregularity in adults. Eur J Orthod. 2013;35:230–235. doi: 10.1093/ejo/cjr121. [DOI] [PubMed] [Google Scholar]
- Vijay Kumar AG, Agarwal SS, Bastia Fusion of skull vault sutures in relation to age. A cross sectional post-mortem study done in 3rd, 4th and 5th decades of life. J Forensic Res. 2012;3:173. [Google Scholar]
- Weinstein K. Thoracic morphology in Near Eastern Neandertals and early modern humans compared with recent modern humans from high and low altitudes. J Hum Evol. 2008;54:287–295. doi: 10.1016/j.jhevol.2007.08.010. [DOI] [PubMed] [Google Scholar]
- West K, McNamara J. Changes in the craniofacial complex from adolescence to midadulthood: a cephalometric study. Am J Orthod Dentofacial Orthop. 1999;115:521–532. doi: 10.1016/s0889-5406(99)70274-x. [DOI] [PubMed] [Google Scholar]
- Williams SE, Slice DE. Regional shape change in adult facial bone curvature with age. Am J Phys Anthropol. 2010;143:437–447. doi: 10.1002/ajpa.21332. [DOI] [PubMed] [Google Scholar]


