Abstract
Mineralisation of the tendon tissue has been described in various models of injury, ageing and disease. Often resulting in painful and debilitating conditions, the processes underlying this mechanism are poorly understood. To elucidate the progression from healthy tendon to mineralised tendon, an appropriate model is required. In this study, we describe the spontaneous and non-pathological ossification and calcification of tendons of the hindlimb of the domestic chicken (Gallus gallus domesticus). The appearance of the ossified avian tendon has been described previously, although there have been no studies investigating the developmental processes and underlying mechanisms leading to the ossified avian tendon. The tissue and cells from three tendons – the ossifying extensor and flexor digitorum longus tendons and the non-ossifying Achilles tendon – were analysed for markers of ageing and mineralisation using histology, immunohistochemistry, cytochemistry and molecular analysis. Histologically, the adult tissue showed a loss of healthy tendon crimp morphology as well as markers of calcium deposits and mineralisation. The tissue showed a lowered expression of collagens inherent to the tendon extracellular matrix and presented proteins expressed by bone. The cells from the ossified tendons showed a chondrogenic and osteogenic phenotype as well as tenogenic phenotype and expressed the same markers of ossification and calcification as the tissue. A molecular analysis of the gene expression of the cells confirmed these results. Tendon ossification within the ossified avian tendon seems to be the result of an endochondral process driven by its cells, although the roles of the different cell populations have yet to be elucidated. Understanding the role of the tenocyte within this tissue and the process behind tendon ossification may help us prevent or treat ossification that occurs in injured, ageing or diseased tendon.
Keywords: ageing, chicken, mineralisation, ossification, tendon
Introduction
Incidences of tendon pathology have risen in the past two decades, as shown in various studies across Europe (Leppilahti et al. 1996; Moller et al. 1996; Levi, 1997; Houshian et al. 1998; Maffulli et al. 1999). This change in incidence seems to correlate with higher sporting participation in the general population (Benjamin & Ralphs, 2000). In our ever-ageing population, an understanding of our musculoskeletal unit is of utmost importance. Despite the surprisingly high tensile strength of tendon, some capable of undergoing over 4000 N of force (Sandelin et al. 1988), tendons are particularly vulnerable to damage both with age and following injury. Tendon mineralisation can cause pain and creates weakness, making it an important target of musculoskeletal research (Leppilahti et al. 1996; Moller et al. 1996; Levi, 1997; Houshian et al. 1998; Maffulli et al. 1999; O'Brien et al. 2012).
Tendon is a key component of the musculoskeletal unit – transmitting forces from muscle to bone to bring about movement, and despite some apparent neglect has, in recent years, become a focus of research interest. Tendon fibroblasts, known as tenocytes, are the main cells found in tendon tissue. These spindle-shaped cells with an elongated cytoplasm are arranged longitudinally between collagen fibrils, making contact with each other via gap junctions and adherens junctions (McNeilly et al. 1996; Benjamin & Ralphs, 2000; Benjamin et al. 2000; Stanley et al. 2007). High collagen content gives the tendon tissue its characteristic crimp morphology: this morphology allows the fibres to stretch beyond their resting length. Collagen fibres can also slide along each other, adding to this stretching capability (Sandelin et al. 1988; Screen, 2008).
In some cases of tendon degeneration and disease, bone-like material has been found within the damaged tissue (Järvinen et al. 1997). The deposit of mineral in tendons or its alteration towards a bony or cartilaginous structure has the consequence of transforming the tissue into a more rigid structure which cannot perform the wide variety of movements needed for the transfer of tension and force from muscle to bone. It is unclear how this mineralisation process occurs and whether some tendons are preferentially more affected than others. In addition and in relation to reparative processes it is of upmost importance to know whether the mineralisation can be reversed. The mineralisation process of tendons in some species occurs naturally, for example in the lower limbs of certain birds (Abdalla, 1979). Some dinosaurs have also been shown to have had mineralised tendons (Adams & Organ, 2005; Organ & Adams, 2005; Klein et al. 2012). The tendons in these species have naturally evolved to become mineralised (Hutchinson, 2002; Summers & Koob, 2002) but it is not known why this might have been an evolutionary asset. Nevertheless, such a phenomenon provides a possible model system to investigate. Tendon problems contribute to a large proportion of the number of days off a year, with rotator cuff mineralisation contributing between 2.7 and 22% of the cases (Leppilahti et al. 1996; Moller et al. 1996; Levi, 1997; Houshian et al. 1998; Maffulli et al. 1999; Oliva et al. 2011b), among several other tendons affected by mineralisation (Kannus & Jozsa, 1991; Benjamin & Ralphs, 2000; Richards et al. 2008).
Two types of mineralisation can occur within the tendon: calcification (deposit of mineral on the tendon) and ossification (laying down of new bone material). The process which human tendons undergo in injury and disease has only been studied in a case-by-case manner without a clear common conclusion, with the processes occurring either separately or together in different instances (Fisher & Woods, 1970; Lotke, 1970; Fink & Corn, 1982; Sandelin et al. 1988; Hatori et al. 1994, 2002; Joshi et al. 1994; Yu et al. 1994; Goyal & Vadhva, 1997; Aksoy & Surat, 1998; Parton et al. 1998; Mady & Vajda, 2000). A wide range of case studies have reported ectopic ossification and calcification post injury, particularly in the Achilles tendon and the anterior cruciate ligament (ACL; Brown et al. 1986; Rooney et al. 1993; Leppilahti et al. 1996; Moller et al. 1996; Levi, 1997; Houshian et al. 1998; Maffulli et al. 1999; Erdogan et al. 2004; Kraus et al. 2004; Liden et al. 2006; O'Brien et al. 2012).
Why do these calcium deposits form in human tendon and what are the cell sources? A variety of different theories have arisen from these studies. Ossification/calcification might be an extension from the development of the enthesis (the tendon-to-bone junction), linking it to entesopathies (Benjamin & Ralphs, 1996, 2000; McNeilly et al. 1996; Benjamin et al. 2000; Stanley et al. 2007) with the ossification being either endochondral or intramembranous in nature. Some studies have focused on outlining genetic factors in these diseases (Sandelin et al. 1988; Oliva et al. 2011a). Candidates include biglycan and aggrecan, which could have roles in ectopic chrondro-osteogenesis (Järvinen et al. 1997; Young et al. 2002; Lui et al. 2011), chondro-osteogenetic factors such as various bone morphogenetic proteins (BMPs; Abdalla, 1979; Lui et al. 2009a) but also osteopontin and other associated factors (Nakase et al. 2000; Adams & Organ, 2005; Organ & Adams, 2005; Oliva et al. 2011a; Klein et al. 2012). Intratendinous accumulation of calcium deposits is common in tendon pathology (Kannus & Jozsa, 1991; Hutchinson, 2002; Summers & Koob, 2002). There is evidence that the formation of these calcific deposits is due to a cell-mediated process (Rui et al. 2011a). Some mineralised deposits in Achilles and patella tendons occur by a process resembling endochondral ossification, with bone formation and remodelling mediated by populations of osteoblasts and osteoclasts (Fenwick et al. 2002). There have been suggestions of incorrect differentiation of tendon cells within the tissue resulting in ectopic tissue formation (Archer et al. 1993).
Different theories and observations have been hypothesised as to what cellular processes lead to this differentiation. A chondrocytic environment has been observed around calcium deposits within tendons, including chondrocytes and multinucleated giant cells (Uhthoff & Loehr, 1997; Nakase et al. 2000; Fenwick et al. 2002). The cells in tendon are capable, in the right environment, of forming fibrocartilage, a precursor to bone formation. Could they not also be capable of bone formation?
Most recently, studies have been focusing on tendon-derived stem-like cells (TDSCs), with the finding that stem cells are present within tendon tissue (Bi et al. 2007; Clegg et al. 2007; Ruzzini et al. 2013) and have the potential to differentiate into various different cell types including those of osteogenic and chondrogenic lineages. It has been hypothesised that this is due to the erroneous differentiation of TDSCs as a result of an alteration of the mechanical and biological environment (Rui et al. 2011a,2011b,2011c). Recent data have suggested that TDSCs could be at the source of abnormal ossification and calcifying processes in diseases such as fibrodysplasia ossificans progressiva and calcifying tendinopathy (reviewed in Lui & Chan, 2011).
To further investigate the nature of tendon mineralisation we propose using as a model the adult chicken, in which a non-pathological mineralisation process occurs in the tendon of the hindlimb. This mineralisation has been reported previously but the process underlying it has not been investigated (Ranvier, 1875; Engel & Zerlotti, 1967; Abdalla, 1979; Landis & Silver, 2002; Spiesz et al. 2012). This study aims to characterise the tissue and cells from the adult ossified tendon and compare these with the embryonic non-ossified avian tendon. A clear understanding of the histology of the tissue, its immunological profile as well as characterisation of the molecular and immunological profile of the cells within it may lead towards a better understanding of the process behind this non-pathological mineralisation.
Material and methods
Tissue preparation
Tendons were removed from chickens aged between 20 and 24 months, killed by cervical dislocation. Limbs were thoroughly rinsed in disinfectant, followed by 70% IMS (industrial methylated spirit) and the extensor digitorum longus tendon (EDL), flexor digitorum longus tendon (FDL) and Achilles tendon (AT) carefully dissected out under sterile conditions. As the EDL and FDL tendons contain both ossified and non-ossified regions, samples from the centre of both these regions were taken.
For embryonic samples, fertilised White Leghorn chicken eggs (Henry Stewart & Co. Ltd, Lincolnshire, UK) were incubated at 38 °C in a humidified incubator until day 19 (stage 45; Hamburger & Hamilton #joa12078-bib-01001951). The embryos were killed by cervical dislocation and the extensor digitorum longus tendon (EDL), flexor digitorum longus tendon (FDL) and Achilles tendon (AT) carefully dissected from the foot and shank regions and cleaned of any adherent tissue.
Histological staining
All specimens were processed for routine histological examination. Paraffin sections for each sample were cut in the sagittal plane at a thickness of 4 μm, collected onto glass slides and air dried for 24 h. Samples were stained with haematoxylin and eosin (HE), Alcian Blue, Alizarin Red S and von Kossa. Briefly, sections were de-waxed in xylene and then rehydrated through a series of graded alcohols. Sections were stained according to the criteria of each staining method/protocol and subsequently dehydrated through a series of graded alcohols and xylene before mounting with DPX mountant and subsequent viewing.
Cell culture
Adult primary cell culture
FDL, EDL and AT were carefully dissected from adult hindlimbs; ossified and non-ossified regions of each tendon were separated and cleaned in Dulbecco's phosphate-buffered saline (DPBS) supplemented with 25 μg mL−1 amphotericin B. Samples were then placed into complete Dulbecco's modifed Eagle medium (DMEM) supplemented with 1% insulin-transferrin-selenium (ITS; Fisher), 0.5% gentamycin (PAA) and 0.5% amphotericin B (PAA) overnight. Tendon samples were then dissected into small fragments, washed in DPBS and incubated in 10 mL supplemented complete DMEM per 1 g tissue containing 1 mg mL−1 Pronase (Sigma) for 1 h at 37 °C, 5% CO2 with agitation. The tissue was incubated with 20 mL supplemented complete DMEM per 1 g tissue containing 0.25 mg mL−1 Collagenase type XI (Sigma) and 0.55 mg mL−1 Dispase (Sigma) for 3 h at 37 °C, 5% CO2 with agitation. Following each incubation period, media was collected and cells filtered through a 70-μm-pore cell strainer and collected by centrifugation at 320 g for 5 min. Supernatant was removed and cell pellets washed in supplemented DPBS, centrifuged and resuspended in supplemented complete DMEM. Cells were grown on tissue culture-treated plastic and expanded up to passage 2.
Embryological primary cell cultures
Tendons from eight embryonic limbs were dissected and harvested into complete DMEM (Gibco) supplemented with 10% fetal calf serum (PAA), 1% sodium pyruvate (PAA), 25 mm HEPES (PAA) and antibiotics (1% penicillin/streptomycin; PAA), The tissue was dissected into small fragments and digested in a solution of 0.05% trypsin/EDTA (Gibco) at 37 °C for 30 min. Following trypsin inactivation, cells were harvested by centrifugation at 210 g for 10 min. The cell pellets were resuspended in complete DMEM and seeded onto tissue culture plastic flasks (Nunc).
Growth curves
Cells frozen down at P2 for each cell type were defrosted and allowed to grow on T25 cell culture flasks until confluent. Cells were passaged to P3 into T75 cell culture flasks. Once confluent, cells were trypsinised, counted and seeded at a density of 30 000 cells per well into 24-well plates. Cells were then counted daily. For each cell type, the cells were counted over a period of either 6 or 12 days, in triplicate.
Immunofluorescent labelling
Tissue
Cryosections (7 μm) were cut in the sagittal plane and mounted on poly-l-lysine coated glass slides (Thermo Scientific). Cryosections were rehydrated in PBS plus 0.1% (v/v) Tween-20® (PBST; Fisher Scientific) and blocked with PBS containing 5% (v/v) goat serum (Fisher Scientific) for 30 min. Immunolocalisation was performed using standard indirect immunofluorescence with monoclonal mouse antibodies (see Supporting Information Table S1). The secondary antibody AlexaFluor488 conjugated goat anti-mouse IgG (Invitrogen, Paisley, UK) was used at a final concentration of 15 μg mL−1 diluted in 5% (v/v) goat serum. Cell nuclei were counterstained with propidium iodide solution (1 μg mL−1; Sigma-Aldrich, Dorset, UK) and mounted in a hard-set mounting medium (Vectashield; Vector Laboratories, Burligame, CA, USA).
Cells
Cells were cultured on coverslips in 24-well plates at a seeding density of 3.0 × 106 cm−2. Prior to antibody labelling, media was removed and the coverslips rinsed gently with PBST. Cells were fixed in chilled 4% (w/v) methanol-free paraformaldehyde (PFA) for 3–5 min, and subsequently rinsed with PBST. Cells were permeabilised with 0.1% (v/v) Triton X solution for 5 min and rinsed with PBST, then incubated for 15 min with 5% NGS (normal goat serum) blocking solution. Primary antibodies were added (see Table S1) and incubated at room temperature for 30 min. Following rinsing in PBST, AlexaFluor488 conjugated goat anti-mouse IgG (15 μg mL−1) was added and cells were incubated for a period of 30 min at room temperature. Coverslips were subsequently rinsed in PBST before adding propidium iodide solution (1 μg mL−1) to stain cell nuclei. Cells were then incubated for 20 min at room temperature. Coverslips were then rinsed thoroughly with PBST and mounted on slides using Vectashield mounting medium. Slides were left to dry at 4 °C, protected from light.
Morphology labelling
Cells were seeded and fixed onto coverslips as above, and permeabilised with 0.1% Triton X-100. Following rinsing with PBST, cells were stained for filamentous actin (F-actin using phalloidin (1/40) for 20 min. Cells were rinsed in PBST and counterstained with DAPI (4′,6-diamidino-2-phenylindole – 1/1000) for 5 min. Once rinsed, cells were mounted in Vectashield.
Quantitative microscopy
Tissue
Following histological preparation, images of each tendon of interest were taken (Leica DM5000B microscope) using las imaging software. A minimum of 10 images were produced for each tendon sample. Cells from each image were counted using imagej software. A total area of 8.5 mm2 for the embryonic sections and 38 mm2 for the adult sections was analysed. Cell nuclei were counted inside this field using an imagej counting macro. Cell counts were converted into average cells mm−2. These numbers were also correlated to the average length and width of each tendon of interest to analyse the average cell count in a tendon slice.
Cells
Following immunocytochemical preparation, images of cells were taken using a Leica DM5000B microscope and las imaging software. All images were visualised on the same equipment and using the same exposure settings to ensure consistency of immunolabelling. Separate RFP (Red Fluorescent Protein) and GFP (Green Fluorescent Protein) images were taken for each image. Biological and experimental triplicates were performed, yielding nine images for each cell type/antibody set. To quantify the immunolabelling in each image, the total pixelated area for each GFP image was calculated using the imagej ‘Measurements’ tool and correlated to the number of cells of each RFP image (calculated using the imagej ‘Analyse Particles’ tool).
Molecular analysis
RNA isolation and cDNA synthesis
Total cellular RNA was isolated by TRI Reagent Lysis Buffer (Sigma-Aldrich) according to the manufacturer's protocol. Total RNA was quantified by spectrophotometry using a Nanodrop ND-1000 and decontaminated using a Precision DNAse kit (Primer Design). Briefly, 1 μL of 10× Precision DNAse enzyme and 8 μL 10× Precision DNAse reaction buffer were added to 80 μL mRNA and incubated at 30 °C for 10 min. The enzyme was deactivated by heating at 55 °C for 5 min. mRNA was reverse-transcribed (1 μg per reaction) into complementary DNA (cDNA) using a Precision nanoScript cDNA Reverse Transcription kit (Primer Design). A total reaction volume of 9 μL, containing RNA and RNase-free water, was added to 1 μL Oligo-dT primers and heated at 65 °C for 5 min before returning to ice. The mix was then added to 2 μL 10× reaction buffer, 1 μL dNTP mix (10 mm each), 2 μL 10× DTT (100 mm) and 1 μL nanoScript enzyme to obtain a final volume of 20 μL. The synthesis of cDNA from RNA was performed according to the manufacturer's recommendations: 20 min at 55 °C followed by 15 min at 75 °C on a thermal cycler (Eppendorf Mastercycler EP Gradient S, Eppendorf North America). cDNA integrity was tested by PCR with reference genes GAPDH and β-actin using AmpliTaq Gold 360 Master Mix (AB) following the manufacturer's protocol. Primers were designed for reference genes and genes of interest (see Supporting Information Table S2). cDNA 1 μL was used as a template for each PCR reaction.
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
Quantitative PCR was performed using TaqMan Brilliant II qPCR Low ROX Master Mix (Agilent Technologies) and TaqMan Gene Expression Assays (AB) according to the recommended protocol (see Supporting Information Table S3). The PCR cycling consisted of an initial set-up of 2 min at 50 °C followed by 10 min at 95 °C and 40 cycles of amplification of the template DNA with primer denaturing at 95 °C for 15 s and primer annealing at 60 °C for 1 min using Stratagene MX3005P.
Statistical analysis
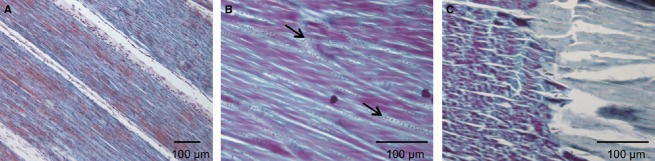
Relative quantities present in each sample were assessed using the 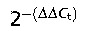 method; the values obtained for each gene were normalised on a reference gene (β-actin). Using an appropriate statistical analysis package (graphpad prism), analysis of variance (anova) with Bonferroni post hoc test or independent samples t-test was performed on data sets where appropriate using a significance of P < 0.05. Statistically significant differences in gene expression values detected between the adult ossified, adult non-ossified and embryonic tendon cells were analysed by paired Student's t-test. Significance was predetermined at P < 0.05.
method; the values obtained for each gene were normalised on a reference gene (β-actin). Using an appropriate statistical analysis package (graphpad prism), analysis of variance (anova) with Bonferroni post hoc test or independent samples t-test was performed on data sets where appropriate using a significance of P < 0.05. Statistically significant differences in gene expression values detected between the adult ossified, adult non-ossified and embryonic tendon cells were analysed by paired Student's t-test. Significance was predetermined at P < 0.05.
Results
Characterisation of the ossified avian tendon tissue
Trichrome staining of prepared paraffin wax sections of each individual adult tendon was performed to assess morphological differences and localisation of the ossification, if present. Figure 1 shows the morphology of the embryonic and adult tendon in the FDL. Figure 1A shows the morphology of embryonic tendon and Figure 1B demonstrates the appearance of the adult tendon. The tendon tissue appears degenerated and less cellular than that of the embryonic FDL (Fig. 1B) and also appears interspersed by cartilage and fibrocartilage tissue (black arrowheads). Figure 1C shows the appearance of the junction between the ossified and the non-ossified regions of the adult tendon: a clear boundary is observable where a degenerated tissue is visible at the ossification centre.
Figure 1.
Trichrome staining of embryonic and adult FDL tendon. (A) Appearance of embryonic tendon tissue. (B) Appearance of the adult tendon: the tissue appears less condensed than the embryonic tissue, cells appear sparser and areas of chondrocytic cells and fibrocartilage are visible (black arrows). (C) Appearance of the junction between the ossified and non-ossified regions of adult tendon and demonstrates the clear demarcation of the ossification centre.
The adult ossified tendon appears to have an ossification centre
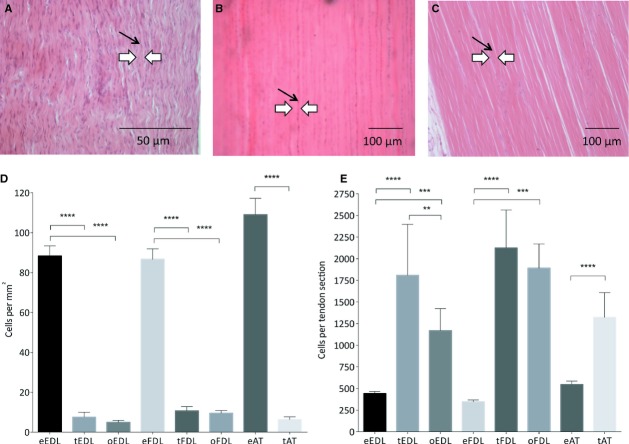
To assess the differences between embryonic and adult tendons, paraffin wax sections of each tendon of interest were prepared from both embryonic and adult tendons. An overall morphological analysis of all three embryonic tendons showed similar patterns. All showed the distinct crimp morphology inherent to tendon tissue (black arrows denote the tenocytes and white arrows the collagen fibres surrounding them) and a high number of cells when stained with a haematoxylin and eosin (H&E) stain (here illustrated by the embryonic EDL tendon – Fig. 2A).
Figure 2.
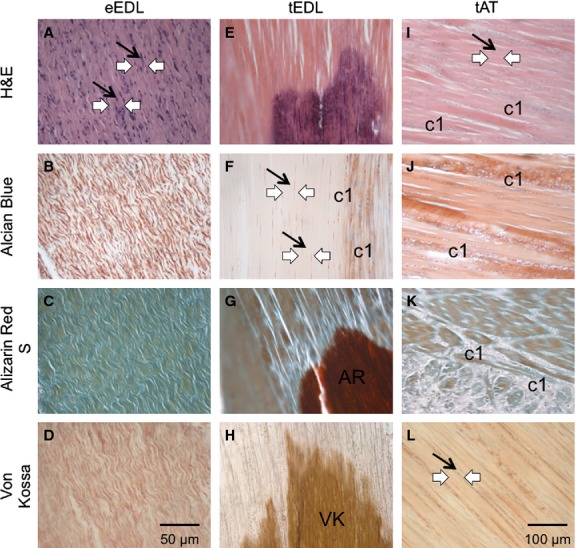
Comparative analysis of EDL tendon structure from embryonic and adult chicken. (A–D) Characteristic crimp morphology of embryonic tendon tissue, here in the EDL tendon. The tenocytes are located longitudinally (black arrows) between the collagen fibres of the extracellular matrix (white arrows). (E-H) Histological profile of the adult tendon tissue, here in the EDL tendon. Characteristic crimp morphology is lost (white arrows) and the tenocytes are sparser and longer (black arrows). (F) Staining for cartilage specific proteoglycans reveals more rounded, chondrocytic cells (c1) in the tissue. (G) Calcium deposits are revealed by Alizarin Red S staining (AR). (H) Mineralisation of the tissue is revealed by Von Kossa staining (VK). (E, G, H) Highlight the separation of the adult tendon tissue into two distinct areas: mineralised tendon and non-mineralised tendon, creating a mineralisation centre reminiscent of an endochondral ossification model. (I,J) Histological profile of the adult tendon tissue in the non-mineralising Achilles tendon. The tissue shows similar ageing patterns, loss of crimp morphology (white arrows) and more sparse, longer tenocytes (black arrows) and the presence of chondrocyte-like cells but does not show any other signs of mineralisation.
In all three adult tissues, certain characteristics of old age were seen with the H&E staining, including loss of crimp morphology and reduction of cells. The EDL and FDL adult tendons revealed similar changes in the tissues: the adult tendons looked degenerated and damaged, with rather long and thin cells (here illustrated by the adult EDL tendon – Fig. 2E). In addition to the ageing of the tissues (less crimp and fewer cells), they both showed characteristics of ossification and calcification. The Alcian blue staining revealed a large amount of blue cartilage cells within the tissues, spread around and in between the tenocytes (Fig. 2F), whereas the embryonic tissue did not possess any cartilage cells and there were no apparent signs of Alcian blue-positive cells (Fig. 2B). Both Alizarin Red and von Kossa staining methods revealed strong calcium deposits and mineralisation at the centre of the EDL and FDL tendons (Fig. 2G,H). In these cases, the stain was particularly strong at the centre of the tissues. These were not detected in the embryonic tissue, as shown by a lack of positive staining from Alizarin Red (Fig. 2C) and von Kossa (Fig. 2D). It appears the ossification of the adult tendon is isolated to a mid-portion of the tendon length, whereas the rest of the tissue remains tendon-like.
In contrast, the Achilles tendon had a different pattern of change. The chosen range of stains revealed the appearance of cartilage cells within the adult tissue, interspersed between and around the tendon cells (Fig. 2I,J). The base of the Achilles tendon is composed of naturally occurring fibrocartilage even at a young age, due to the compression around the bone and cartilage area of the knee. However, the spread of cartilage cells and thus of fibrocartilaginous tissue in the adult tissue was found throughout the tendon, even up to the myotendinous junction with the gastrocnemius muscle. Although the Achilles tendon did show the same signs of ageing as the other tendons, with a similar loss of cells and loosening of the crimp morphology of the tissue, neither ossification nor mineralisation was present within the tissue. Both Alizarin Red and von Kossa staining showed no more than background levels of staining and fibrocartilage tissue (Fig. 3K,L).
Figure 3.
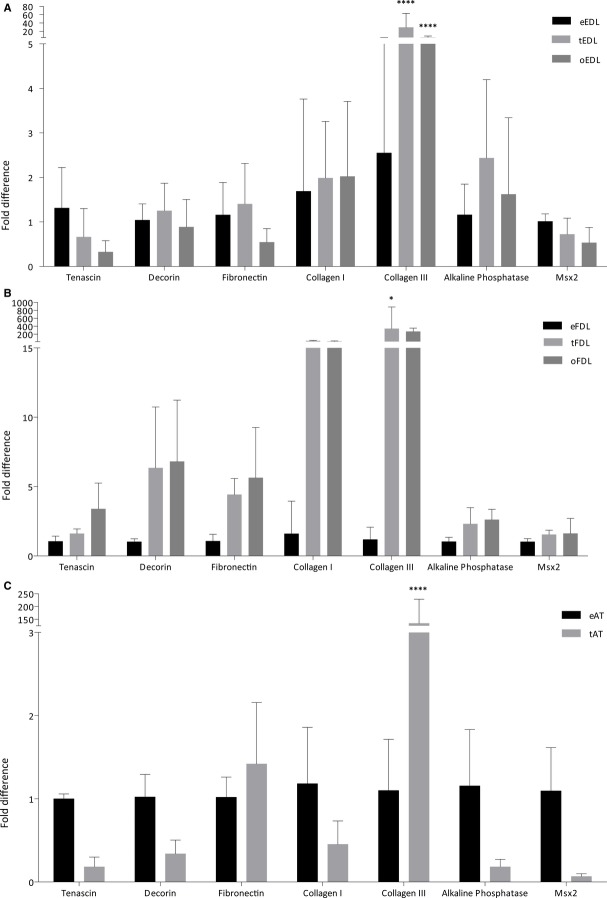
Cellularity analysis (n = 10 images). (A-C) Histological appearance of the EDL tendon from embryonic (A) to non-ossified (B) and ossified (C) adult tendon. Characteristic crimp morphology is lost (white arrows) and the tenocytes are sparser and more elongated (black arrows). (D) Cellularity analysis of cells per mm2 for each tendon from embryonic (e) to non-ossified (t) and ossified (o) adult tendon. (E) Cellularity analysis correlated to the wholemount size of the tendon generating a number of total cells per tendon slice. Although the number of tenocytes per mm2 is significantly reduced in adult ossified and non-ossified tendon, the data suggest the total number of tenocytes per tendon is significantly higher in the adult ossified and non-ossified tendon.
The adult ossified tendon is degenerated
As mentioned above, differences can be seen between the embryonic and adult tendons, in this case in the EDL tendon (Fig. 3): characteristic crimp morphology is lost in both the tendon and ossified portions of the adult samples (white arrows) and the tenocytes are sparser and longer (black arrows). Cellularity analysis of cells per mm2 for each tendon from embryonic (e) to non-ossified (t) and ossified (o) adult tendon revealed the number of cells per mm2 was significantly lower in both the ossified and non-ossified adult tendon compared with the embryonic tendon. The number of cells per mm2 was also significantly lower in the ossified tendon than the non-ossified tendon (Fig. 3D). When normalised to the whole mount size of the tendons, the total number of cells in a tendon was significantly higher in both the ossified and non-ossified adult tendon compared with the embryonic tendon. The number of cells in the non-ossified tendon remained higher than in the ossified tendon (Fig. 3E).
A changing immunological profile in the adult ossified tendon
Figure 4 shows the immunological profile of the ossifying tendon (here the FDL tendon), in the embryonic and in the adult ossified tendon (both ossified and non-ossified regions).
Figure 4.
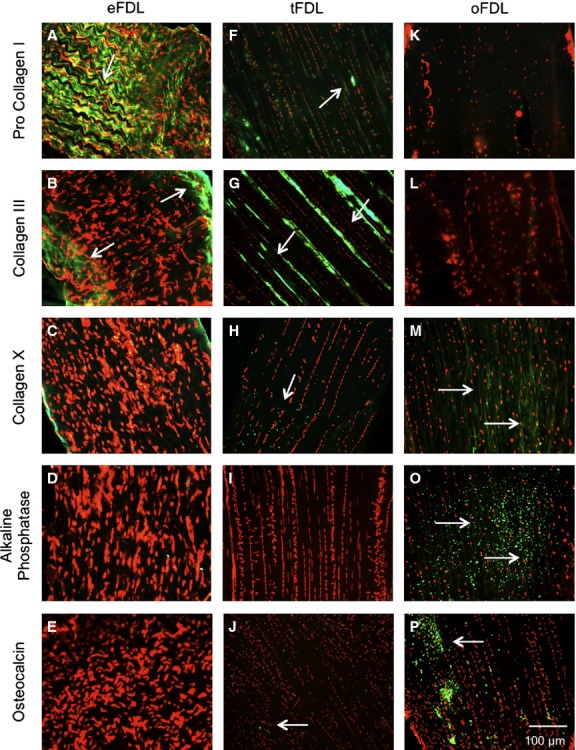
Immunological profile of the embryological and adult FDL tendon. (A-E) Expression profile characteristics of embryonic tendon: a high expression of procollagen I (A) and collagen III (B), both part of the tendon extracellular matrix and no expression of collagen X (C), alkaline phosphatase (D) and osteocalcin (E), all markers for endochondral ossification and bone. (F–J) Expression profile of the non-ossified adult tendon: the expression of tendon markers is considerably lowered (F,G), whereas the expression of bone and ossification markers is slightly increased (H-J). (K-P) Expression profile of the ossified adult tendon: the expression of tendon markers is noticeably lowered (K,L) and bone and ossification markers have an observably strong expression (M-P).
All labelling for key tendon proteins (tenascin, decorin and fibronectin) was highly visible in the embryonic tendon and showed a significant decrease in expression in the adult tendon (data not shown). Pro collagen I showed a very high expression level in the embryonic tendon (Fig. 4A) and a considerably decreased expression in the adult non-ossified (Fig. 4F) and adult ossified (Fig. 4K) tendon. Collagen III showed strong visual labelling in the embryonic tendon (Fig. 4B); however, it was less visible and showed a different distribution in adult non-ossified tendon (Fig. 4G) and a lack of expression in adult ossified tendon (Fig. 4L).
Collagen II was not expressed in the embryonic or the adult non-ossified tendon but showed some low level expression in the adult ossified tendon (data not shown). Collagen X was not expressed in the embryonic tendon (Fig. 4C) but was expressed at low levels in the adult non-ossified (Fig. 4H) and adult ossified (Fig. 4M) tendon. The expression of bone markers alkaline phosphatase, msx2 and osteocalcin were not expressed in the embryonic tendon (Fig. 4D,E) but were positive in both non-ossified (Fig. 4I,J) and ossified adult tendon (Fig. 4O,P). Table 1 summarises the differences in immunohistochemical labelling between the embryonic and adult tendons.
Table 1.
Summary of immunohistochemistry
| Antibody | Tissue type |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| eEDL | tEDL | oEDL | eFDL | tFDL | oFDL | eAT | tAT | ||
| Tenascin | ++ | ++ | + | ++ | ++ | + | ++ | + | |
| Decorin | ++ | ++ | + | ++ | ++ | + | ++ | + | |
| Procollagen I | ++ | ++ | − | ++ | − | − | ++ | + | |
| Collagen III | ++ | + | − | ++ | + | − | ++ | + | |
| Fibronectin | ++ | + | ++ | ++ | + | + | ++ | + | |
| Collagen II | − | − | − | − | − | − | − | − | |
| Collagen X | − | + | ++ | − | + | ++ | − | + | |
| Msx2 | − | + | ++ | − | + | ++ | − | + | |
| Osteocalcin | − | + | ++ | − | + | ++ | − | + | |
| Alkaline phosphatase | − | + | ++ | − | + | ++ | − | + | |
e, embryonic; t, non-ossified adult tendon; o, ossified adult tendon.
Characterisation of the cells from the ossified avian tendon
A heterogeneous population
Cells from the ossified and non-ossified regions of the tendons were harvested and cultured leading to five different populations: non-ossified EDL (tEDL), ossified EDL (oEDL), non-ossified FDL (tFDL), ossified FDL (oFDL) and non-ossified AT (tAT).
It is apparent in Figure 5 that the cells from the different regions of the tendons are morphologically different to the cells from embryonic tendons. The non-ossified regions of the adult tendon of both the EDL (Fig. 5A) and FDL (Fig. 5B) as well as the ossified region (Fig. 5C) appear to contain a heterogeneous population of cells: cells of a spindle-shaped nature are visible (black arrowheads – Fig. 5E), other cells with a more polygonal (white arrowheads – Fig. 5F) or cuboidal (thick black arrowheads – Fig. 5G) are also visible.
Figure 5.
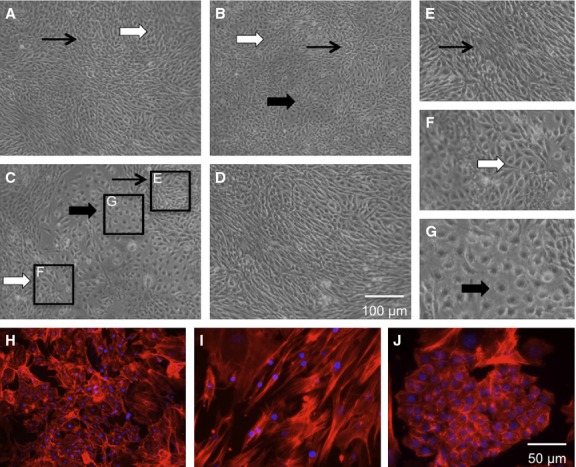
Morphological appearance of cells cultured from adult tendons. (A,B) Cells from the non-ossified region of both EDL (A) and FDL (B) adult tendon. (C) Cells from the ossified region of the adult FDL. All three show the presence of spindle-shaped cells (black arrows), cuboidal-shaped cells (white arrows) and polygonal-shaped cells (thick black arrows), although the cuboidal and polygonal cells are present more in (C). (D) Appearance of cells from the embryonic FDL, mainly composed of spindle-shaped tenocytes. (E-G) Close-ups of the different-shaped cells from the ossified adult EDL tendon (C). (H-J) Morphological appearance of the cells from the adult ossified tendon when stained with an actin marker (phalloidin) to show the cell shapes. (I,J) Highlights the morphological appearance of embryonic tendon cells (I) and bone cells (J) under the same conditions.
The mineralisation process does not affect cell growth
Growth curves were performed on cells from both the ossified and non-ossified regions of the adult tendons and on cells from the embryonic tendons (Fig. 6). The growth rates of the EDL adult population show little variation from that of the embryonic population, although statistical analysis of the oEDL growth rate showed it to be significantly different to the eEDL. The growth rates of both adult FDL populations show no significant variation from the embryonic population. Analysis of the adult AT population rate showed it to be significantly lower than the embryonic population. No clear differences in population doubling time can be inferred from this analysis.
Figure 6.
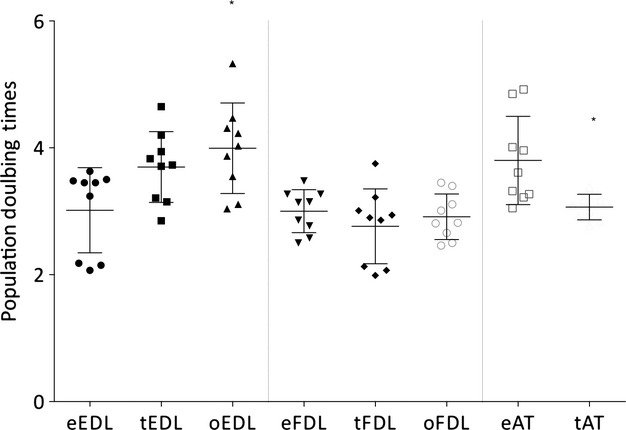
Comparative analysis of the growth rates of adult non-ossified and embryonic EDL, FDL and AT tenocytes (error bars of standard deviation included – n = 9 experiments). The growth rates of the EDL adult population show are not significantly different from that of the embryonic population. The oEDL growth rate is significantly higher. The growth rates of both adult FDL populations show no significant variation from the embryonic population. The adult AT population rate appears lower than that of the embryonic population.
An immunocytochemical profile characteristic of ossification
The cells from the non-ossified and ossified adult FDL were immunocytochemically analysed using a panel of tendon, cartilage and bone markers.
Both groups of cells were found to express tendon markers decorin and fibronectin in similar patterns to embryonic tenocytes (data not shown). The expression of pro collagen I and collagen III was seen at lower levels in both non-ossified (Fig. 7F,G) and ossified (Fig. 7K,L) adult FDL cells when compared with embryonic EDL tenocytes (Fig. 7A,B).
Figure 7.
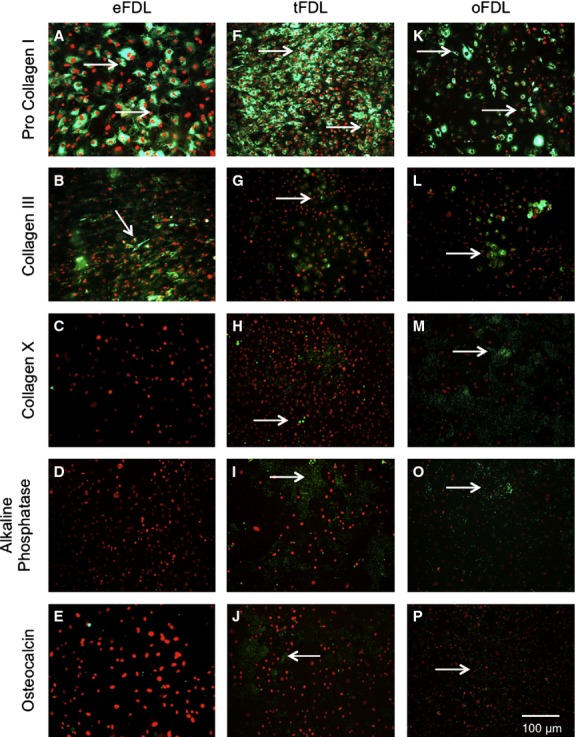
Immunological profile of cells from adult non-ossified FDL, adult ossified FDL and embryonic FDL. (A-E) Expression profile of embryonic tendon cells: a high expression of procollagen I (A) and collagen III (B), both part of the tendon extracellular matrix and no expression of collagen X (C), alkaline phosphatase (D) and osteocalcin (E), all markers for endochondral ossification and bone. (F-J) Expression profile of the non-ossified adult tendon cells: the expression of tendon markers is noticeably lowered (F,G), whereas bone and ossification markers have a slightly increased expression (H-J). (K-P) Expression profile of the ossified adult tendon cells: the expression of tendon markers is considerably lowered (K,L) and bone and ossification markers have a substantially strong expression (M-P).
Marker for cartilage collagen II was not expressed in either non-ossified adult or ossified adult cells (data not shown), whereas collagen X was expressed in both non-ossified (Fig. 7H) and ossified (Fig. 7M) adult FDL cells. The expression of bone markers alkaline phosphatase, msx2 and osteocalcin was positive in both non-ossified (Fig. 7I,J) and ossified adult FDL cells (Fig. 7O,P). Table 2 summarises the differences in immunocytochemical labelling between the cells from the embryonic and adult tendons.
Table 2.
Summary of immunocytochemistry
| Antibody | Cell type |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| eEDL | tEDL | oEDL | eFDL | tFDL | oFDL | eAT | tAT | Bone | Cartilage | |
| Tenascin | ++ | ++ | + | ++ | ++ | + | ++ | + | ++ | ++ |
| Decorin | ++ | + | + | ++ | ++ | + | ++ | + | + | + |
| Procollagen I | ++ | + | + | ++ | + | + | ++ | + | + | ++ |
| Collagen III | ++ | + | + | ++ | + | + | ++ | + | − | + |
| Fibronectin | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | − |
| Collagen II | − | − | − | − | − | − | − | − | ++ | + |
| Collagen X | − | + | ++ | − | + | ++ | − | + | ++ | |
| Msx2 | − | + | ++ | − | + | ++ | − | + | ++ | − |
| Osteocalcin | − | + | ++ | − | + | ++ | − | + | ++ | ++ |
| Alkaline phosphatase | − | + | ++ | − | + | ++ | − | + | ++ | + |
e, embryonic; t, non-ossified adult tendon; o, ossified adult tendon.
A statistical analysis of the difference in immunocytochemical profiles revealed similar results: a decrease in Pro collagen I and collagen III from embryonic cells to adult ossified and non-ossified cells, and an increase in ossification markers collagen II, collagen X, msx2, osteocalcin and alkaline phosphatase (Fig. 8). It is to be noted that few of these changes were statistically significant following the quantification analysis of the immunocytochemistry.
Figure 8.
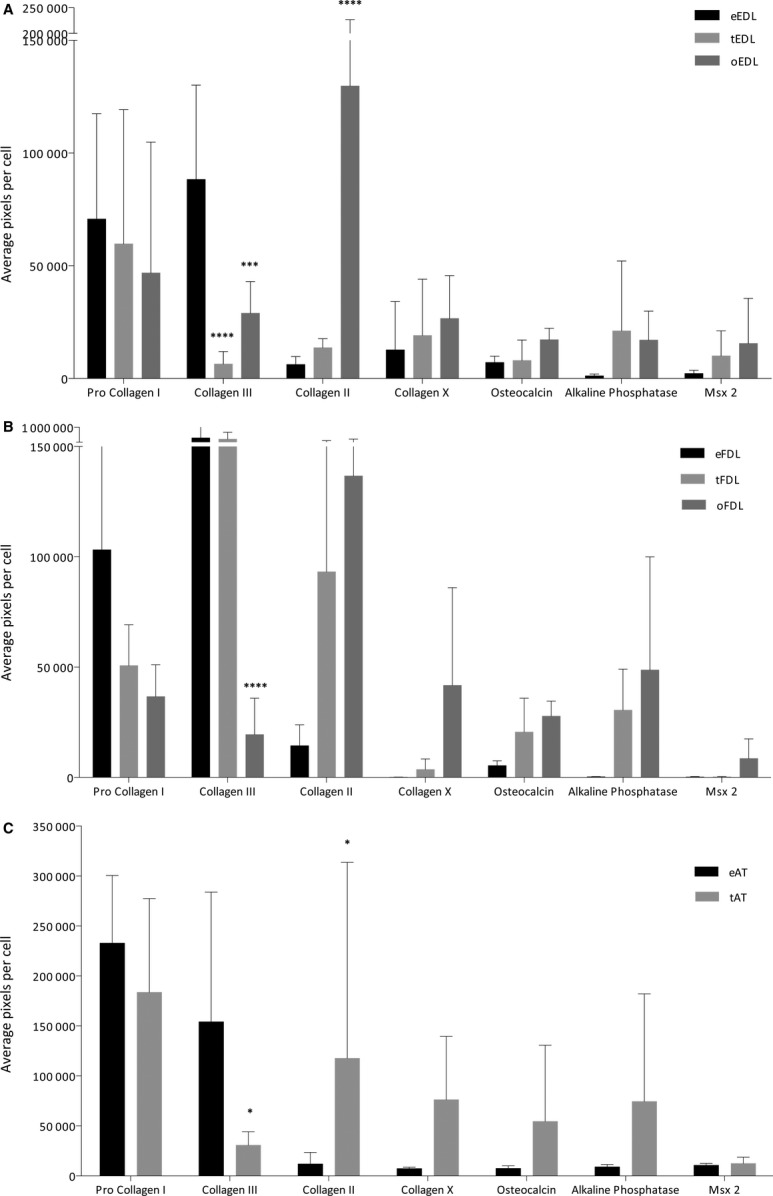
Immunocytochemistry quantification analysis (error bars of standard deviation included – n = 9). (A) Quantified immunocytochemical expression of cells from the adult non-ossified, adult ossified and embryonic EDL tendon. The expression of procollagen I and collagen III was lowered, whereas the expression of bone and cartilage markers collagen II, collagen X, osteocalcin, alkaline phosphatase and Msx2 was higher. (B) Quantified immunocytochemical expression of cells from the adult non-ossified, adult ossified and embryonic FDL tendon, with similar results to that for the EDL. (C) Quantified immunocytochemical expression of cells from the adult non-ossified and embryonic Achilles tendon, which also showed similar patterns of expression.
The molecular profile of the adult cells confirms an endochondral ossification hypothesis
Corroborating with the cells' protein expression, an analysis of the gene expression of extracellular matrix proteins was performed (Fig. 9). The expression of tendon specific matrix proteins tenascin, decorin and fibronectin were not found to be significantly different in adult non-ossified and ossified tissue compared to the embryonic tissue in the EDL (Fig. 9A), FDL (Fig. 9B) or the Achilles tendon (Fig. 9C). In both the EDL and FDL cells, an increase in gene expression of collagen I was noted, although the variation between ossified, non-ossified and embryonic tendon was not found to be statistically significant. A statistically significant increase of collagen III expression was found in all three cell types between the embryonic and adult samples. A marked elevation of the expression of bone markers alkaline phosphatase and msx2 was found in the cells from ossifying tendons EDL and FDL, but not in the non-ossifying Achilles tendon.
Figure 9.
qRT-PCR mRNA expression analysis (error bars of standard deviation included – n = 9). (A) Gene expression of cells from the adult non-ossified, adult ossified and embryonic EDL tendon. (B) Gene expression of cells from the adult non-ossified, adult ossified and embryonic FDL tendon. (C) Shows the gene expression of cells from the adult non-ossified and embryonic Achilles tendon. The expression of tendon specific markers tenascin, decorin and fibronectin was similar in all samples from the EDL, FDL and Achilles tendons. Collagen I expression was found not to vary in a statistically significant manner as opposed to collagen III expression, which was significantly increased in the ossified and non-ossified samples from all three tendons. An increase in gene expression of bone markers alkaline phosphatase and Msx2 was found in the cells from the adult ossifying EDL and FDL tendons compared with the embryonic cells; however, a decrease of these same markers was found in the cells from the non-ossifying adult Achilles tendon.
Discussion
The present study investigated the cellular and extracellular basis of the ossified avian tendon. The morphological appearance of the tissue and cells including cell number and morphology and patterns of expression of extracellular matrix (ECM) proteins together lead to a theory of endochondral ossification for the ossified avian hindlimb tendons.
The ossified avian tendon tissue
Morphological analysis of the tissue demonstrated the presence of two distinct regions within the adult tendon: a non-ossified region and an ossified region. Both these regions displayed a loss in collagen crimp morphology in comparison with embryonic tendon, characteristic of ageing and injury in tendon (Lavagnino et al. 2012). The clear boundary between the ossified and non-ossified regions of the adult tendon, suggest tendon ossification could be patterned on the process of formation of the enthesis, as in cases of calcific tendinopathy (Benjamin et al. 2000). A development from the enthesis as a graded region of fibrocartilage could also be suggested (Roeder et al. 2012); however, the central localisation of the ossified regions suggests a different ossification mechanism. The presence of cartilage and fibrocartilage cells within the ossified region of the tendon confirms distinctive elements of an ossification process; however, it is unclear whether this is an endochondral or an intramembranous process.
When correlated to the total surface area of the tendons, cellularity data suggested that there are significantly more cells in total in the adult than the embryonic tendon. This would indicate that additional tendon cells are created following hatching of the chick but are reduced gradually with aging. Our histological analysis of the ossified region of the adult tendon confirmed typical patterns of ageing seen in other models (Lavagnino et al. 2012). A lack of crimp morphology was observed in all adult samples, as well as a reduced number of cells per mm2. It is unclear whether a reduction in the number of cells per mm2 in the adult tendon is enough to affect normal tendon mechanics and ultimately lead to a framework for tendon mineralisation.
Different forms of mineralisation, ossification and calcification were observed in the adult tendons. As collagen I provides an accurate template for the binding of minerals (Landis & Silver, 2009), it is possible the age-related structural changes provide the correct scaffold for mineralisation and the deposit of calcium in the tendon tissue. The absence of calcium deposits or mineralisation in the Achilles tendon suggests that the cartilage and fibrocartilage cells present are not solely responsible for the mineralisation of the other tendons. However, they could be a stepping stone to bone-forming cells inducing ossification.
Whereas calcification is the deposit of material on tissue, ossification involves bone formation from within the tissue. The presence of cartilage cells within the ossified tissue could be a marker for endochondral ossification, as opposed to a suggested intramembranous ossification process, as intramembranous ossification does not require a cartilage template, with mesenchymal cells differentiating into bone-forming osteoblasts (Karsenty & Wagner, 2002).
The foundation of the tendon's ability to stretch and withstand strong forces is dependent on the ECM, which forms the main body of the tendon. Our analysis of ECM proteins expressed in the ossified avian tendon revealed large changes in the normal profile. Although key tendon proteins were continuously expressed throughout the adult tendon, a significant decrease in expression of the collagen I was found compared with the embryonic tendon. This decrease in the tendon material could explain the lack of crimp morphology found in the tissue as well as the stiffness in the ossified regions. The lowered expression of collagen I and increased expression of collagen III in the adult tendon could lead to a less organised and weaker tendon, previously described in other models accompanied by chondrocyte markers (Sharma & Maffulli, 2005; Maffulli et al. 2006; de Mos et al. 2007; Lui et al. 2010). It would appear this depletion of collagen occurs as a result of the ossification process, with more bone-like material replacing the tendon's inherent collagens. Increased collagen II and collagen X in addition to a decrease in collagen I has been found around calcific deposits in calcific tendinopathy (Lui et al. 2009b).
The cells behind ossified avian tendon
To better elucidate the basis of the ossification process in the adult tendon, cells from ossified and non-ossified regions of the adult tendon were harvested and analysed. Cells displaying osteogenic, chondrogenic and tenogenic morphology were found in all samples. The finding that there were no significant differences in the growth rates between adult groups or between adult and embryonic tenocytes suggests the mineralisation process does not affect cell growth. Analysis of the cells from the adult tendon revealed similar expression profiles to that of the tissue: a decrease in production of collagen I, an increase in production of collagen III, and an increase in expression of bone-specific markers all correlate with a model of endochondral ossification. The same analysis at the molecular level revealed the protein expression visibly correlated to the gene production in the cells.
The variety of cells found in the samples as well as the ECM expression profile indicates that a cellular process such as the differentiation of tenocytes is likely to be responsible for the ossification progression. Tendon derived stem-like cells (TDSCs) have been isolated from tendon in a variety of species (Rui et al. 2010a) and appear capable of differentiating into chondrocytes and osteoblasts (Salingcarnboriboon et al. 2003). These cells demonstrate stem cell-like characteristics and can induce ectopic bone formation in response to bone morphogenetic protein-2, 4 and 7 (BMP-2, BMP-4 AND BMP-7; Hashimoto et al. 2007; Lui et al. 2009a; Rui et al. 2010b, 2011b,2011c; Zhang & Wang, 2011). It is therefore possible tendon ossification could be caused by the erroneous differentiation of these cells (Rui et al. 2011a). This has been suggested in other disorders of ectopic calcification including vascular (Speer et al. 2009), skin (Kim et al. 2008), lung (Chan et al. 2002) and skeletal disorders (Lounev et al. 2009).
Various theories have been proposed as to the driving force behind pathological and non-pathological ossification. Although ossification has been observed in some tendons, particularly those with wrap-around regions, the presence of mineralisation in the tensional regions of tendons refutes a model of homeostatic adaptation as an interpretation of the mineralisation of the tendons of the chicken hindlimb (Benjamin et al. 2008). The biomechanics of the tendon must have a strong influence on its development and function. As the structure–function relationship of tendons varies greatly depending on their anatomical location and biomechanical purpose, different tendons should also be considered separately in the study of their adaptation and cellular behaviour (Screen et al. 2013). It is important to understand why this process is natural in the chicken hindlimb as well as those of other birds like turkey (Spiesz et al. 2012) so that we might better understand the role this mineralisation plays in pathology. Recent evidence reveals ossified tendons in dinosaurs (Klein et al. 2012). As chickens are close to dinosaurs on the evolutionary tree, could this provide some evolutionary advantage?
Concluding remarks
This study characterised ossified avian tendon tissue found in the chicken species and suggests that a process akin to endochondral ossification is responsible for the non-pathological mineralisation of the tendon. This process is most likely instigated by the cells present in the tendon tissue; however, the nature of the cells responsible and the mechanism by which tenocytes are replaced by cells of an osteogenic and chondrogenic lineage remain unclear. Further investigation into the plasticity of these cells should lead to a better understanding of this process and, as such, tendon mineralisation in injury, ageing and disease.
Acknowledgments
The authors would like to thank The Anatomical Society for funding the studentship under which this study was completed. They would also like to thank Miss Verity Monk for her preparation of materials for the cellularity analysis. The (hybridoma or monoclonal antibody) developed by [Investigator(s)] was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Conflict of interest
The authors have no conflict of interest to declare.
Authors' contribution
N.A.A. contributed to the design of the study, acquisition of data, data analysis and interpretation and writing of the manuscript. D.J.R.E. and R.L.S. contributed to the concept and design of the study, critical revision of the manuscript and supervised the project.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Antibodies used in the present study.
Table S2. Primers designed and used in the present study (Sigma-Aldrich).
Table S3. Taqman primer probes used in the present study (Life Technologies).
References
- Abdalla O. Ossification and mineralization in the tendons of the chicken (Gallus domesticus. J Anat. 1979;129:351–359. [PMC free article] [PubMed] [Google Scholar]
- Adams JS, Organ CL. Histologic determination of ontogenetic patterns and processes in Hadrosaurian ossified tendons. J Vertebr Paleontol. 2005;25:614–622. [Google Scholar]
- Aksoy MC, Surat A. Fracture of the ossified Achilles tendon. Acta Orthop Belg. 1998;64:418–421. [PubMed] [Google Scholar]
- Archer RS, Bayley JRL, Archer CW. Cell and matrix changes associated with pathological calcification of the human rotator cuff tendons. J Anat. 1993;182:1–11. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. Tendons in health and disease. Man Ther. 1996;1:186–191. doi: 10.1054/math.1996.0267. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. The cell and developmental biology of tendons and ligaments. Int Rev Cytol. 2000;196:85–130. doi: 10.1016/s0074-7696(00)96003-0. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Rufai A, Ralphs JR. The mechanism of formation of bony spurs (enthesophytes) in the achilles tendon. Arthritis Rheum. 2000;43:576–583. doi: 10.1002/1529-0131(200003)43:3<576::AID-ANR14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Kaiser E, Milz S. Structure-function relationships in tendons: a review. J Anat. 2008;212:211–228. doi: 10.1111/j.1469-7580.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Stuelten CH, Kilts T. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- Brown H, Ehrlich HP, Newbern PM. Para osteo arthropathy – ectopic ossification of healing tendon about the rodent ankle joint: histologic and type V collagen changes. Proc Soc Exp Biol Med. 1986;183:214–220. doi: 10.3181/00379727-183-42407. [DOI] [PubMed] [Google Scholar]
- Chan ED, Morales DV, Welsh CH. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med. 2002;165:1654–1669. doi: 10.1164/rccm.2108054. [DOI] [PubMed] [Google Scholar]
- Clegg PD, Strassburg S, Smith RK. Cell phenotypic variation in normal and damaged tendons. Int J Exp Pathol. 2007;88:227–235. doi: 10.1111/j.1365-2613.2007.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel MB, Zerlotti E. Changes in cells, matrix and water of calcifying turkey leg tendons. Am J Anat. 1967;120:489–525. doi: 10.1002/aja.1001200306. [DOI] [PubMed] [Google Scholar]
- Erdogan F, Aydingoz O, Kesmezacar H. Calcification of the patellar tendon after ACL reconstruction. A case report with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2004;12:277–279. doi: 10.1007/s00167-003-0424-1. [DOI] [PubMed] [Google Scholar]
- Fenwick S, Harrall R, Hackne R. Endochondral ossification in Achilles and patella tendinopathy. Rheumatology (Oxford) 2002;41:474–476. doi: 10.1093/rheumatology/41.4.474. [DOI] [PubMed] [Google Scholar]
- Fink RJ, Corn RC. Fracture of an ossified Achilles tendon. Clin Orthop Relat Res. 1982;169:148–150. [PubMed] [Google Scholar]
- Fisher TR, Woods CG. Partial rupture of the tendo calcaneus with heterotopic ossification. Report of a case. J Bone Joint Surg Br. 1970;52:334–336. [PubMed] [Google Scholar]
- Goyal S, Vadhva M. Fracture of ossified Achilles tendon. Arch Orthop Trauma Surg. 1997;116:312–314. doi: 10.1007/BF00390061. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Developmental Dynamics. 1951;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Yoshida G, Toyoda H. Generation of tendon-to-bone interface ‘enthesis’ with use of recombinant BMP-2 in a rabbit model. J Orthop Res. 2007;25:1415–1424. doi: 10.1002/jor.20447. [DOI] [PubMed] [Google Scholar]
- Hatori M, Kita A, Hashimoto Y. Ossification of the Achilles tendon: a case report. Foot Ankle Int. 1994;15:44–47. doi: 10.1177/107110079401500109. [DOI] [PubMed] [Google Scholar]
- Hatori M, Matsuda M, Kokubun S. Ossification of Achilles tendon – report of three cases. Arch Orthop Trauma Surg. 2002;122:414–417. doi: 10.1007/s00402-002-0412-9. [DOI] [PubMed] [Google Scholar]
- Houshian S, Tscherning T, Riegels-Nielsen P. The epidemiology of Achilles tendon rupture in a Danish county. Injury. 1998;29:651–654. doi: 10.1016/s0020-1383(98)00147-8. [DOI] [PubMed] [Google Scholar]
- Hutchinson JR. The evolution of hindlimb tendons and muscles on the line to crown-group birds. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1051–1086. doi: 10.1016/s1095-6433(02)00158-7. [DOI] [PubMed] [Google Scholar]
- Järvinen M, Jozsa L, Kannus P. Histopathological findings in chronic tendon disorders. Scand J Med Sci Sports. 1997;7:86–95. doi: 10.1111/j.1600-0838.1997.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Joshi N, Diaz E, Massons J. Achilles tendon ossification. Acta Orthop Belg. 1994;60:432–433. [PubMed] [Google Scholar]
- Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Kim SY, Choi HY, Myung KB. The expression of molecular mediators in the idiopathic cutaneous calcification and ossification. J Cutan Pathol. 2008;35:826–831. doi: 10.1111/j.1600-0560.2007.00904.x. [DOI] [PubMed] [Google Scholar]
- Klein N, Christian A, Sander PM. Histology shows that elongated neck ribs in sauropod dinosaurs are ossified tendons. Biol Lett. 2012;8:1032–1035. doi: 10.1098/rsbl.2012.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus R, Stahl JP, Meyer C. Frequency and effects of intratendinous and peritendinous calcifications after open Achilles tendon repair. Foot Ankle Int. 2004;25:827–832. doi: 10.1177/107110070402501113. [DOI] [PubMed] [Google Scholar]
- Landis WJ, Silver FH. The structure and function of normally mineralizing avian tendons. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1135–1157. doi: 10.1016/s1095-6433(02)00248-9. [DOI] [PubMed] [Google Scholar]
- Landis WJ, Silver FH. Mineral deposition in the extracellular matrices of vertebrate tissues: identification of possible apatite nucleation sites on type I collagen. Cells Tissues Organs. 2009;189:20–24. doi: 10.1159/000151454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavagnino M, Gardner K, Arnoczky SP. Age-related changes in the cellular, mechanical, and contractile properties of rat tail tendons. Connect Tissue Res. 2012;54:70–75. doi: 10.3109/03008207.2012.744973. [DOI] [PubMed] [Google Scholar]
- Leppilahti J, Puranen J, Orava S. Incidence of Achilles tendon rupture. Acta Orthop Scand. 1996;67:277–279. doi: 10.3109/17453679608994688. [DOI] [PubMed] [Google Scholar]
- Levi N. The incidence of Achilles tendon rupture in Copenhagen. Injury. 1997;28:311–313. doi: 10.1016/s0020-1383(96)00200-8. [DOI] [PubMed] [Google Scholar]
- Liden M, Ejerhed L, Sernert N. The course of the patellar tendon after reharvesting its central third for ACL revision surgery: a long-term clinical and radiographic study. Knee Surg Sports Traumatol Arthrosc. 2006;14:1130–1138. doi: 10.1007/s00167-006-0167-x. [DOI] [PubMed] [Google Scholar]
- Lotke PA. Ossification of the Achilles tendon. Report of seven cases. J Bone Joint Surg Am. 1970;52:157–160. [PubMed] [Google Scholar]
- Lounev VY, Ramachandran R, Wosczyna MN. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui PPY, Chan KM. Tendon-derived stem cells (TDSCs): from basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Rev. 2011;7:883–897. doi: 10.1007/s12015-011-9276-0. [DOI] [PubMed] [Google Scholar]
- Lui PPY, Chan LS, Cheuk YC. Expression of bone morphogenetic protein-2 in the chondrogenic and ossifying sites of calcific tendinopathy and traumatic tendon injury rat models. J Orthop Surg Res. 2009a;4:27. doi: 10.1186/1749-799X-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui PPY, Fu SC, Chan LS. Chondrocyte phenotype and ectopic ossification in collagenase-induced tendon degeneration. J Histochem Cytochem. 2009b;57:91–100. doi: 10.1369/jhc.2008.952143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui PPY, Chan LS, Lee YW. Sustained expression of proteoglycans and collagen type III/type I ratio in a calcified tendinopathy model. Rheumatology (Oxford) 2010;49:231–239. doi: 10.1093/rheumatology/kep384. [DOI] [PubMed] [Google Scholar]
- Lui PPY, Cheuk YC, Le YW. Ectopic chondro-ossification and erroneous extracellular matrix deposition in a tendon window injury model. J Orthop Res. 2011;30:37–46. doi: 10.1002/jor.21495. [DOI] [PubMed] [Google Scholar]
- Mady F, Vajda A. Bilateral ossification in the Achilles tendon: a case report. Foot Ankle Int. 2000;21:1015–1018. doi: 10.1177/107110070002101206. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Waterston SW, Squair J. Changing incidence of Achilles tendon rupture in Scotland: a 15-year study. Clin J Sport Med. 1999;9:157–160. doi: 10.1097/00042752-199907000-00007. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Reaper J, Ewen SW. Chondral metaplasia in calcific insertional tendinopathy of the Achilles tendon. Clin J Sport Med. 2006;16:329–334. doi: 10.1097/00042752-200607000-00008. [DOI] [PubMed] [Google Scholar]
- McNeilly CM, Banes AJ, Benjamin M. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J Anat. 1996;189:593–600. [PMC free article] [PubMed] [Google Scholar]
- Moller A, Astron M, Westlin N. Increasing incidence of Achilles tendon rupture. Acta Orthop Scand. 1996;67:479–481. doi: 10.3109/17453679608996672. [DOI] [PubMed] [Google Scholar]
- de Mos M, van El B, DeGroot J. Achilles tendinosis: changes in biochemical composition and collagen turnover rate. Am J Sports Med. 2007;35:1549–1556. doi: 10.1177/0363546507301885. [DOI] [PubMed] [Google Scholar]
- Nakase T, Takeuchi E, Sugamoto K. Involvement of multinucleated giant cells synthesizing cathepsin K in calcified tendinitis of the rotator cuff tendons. Rheumatology (Oxford) 2000;39:1074–1077. doi: 10.1093/rheumatology/39.10.1074. [DOI] [PubMed] [Google Scholar]
- O'Brien EJ, Frank CB, Shrive NG. Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int J Exp Pathol. 2012;93:319–331. doi: 10.1111/j.1365-2613.2012.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva F, Barisani D, Grasso A. Gene expression analysis in calcific tendinopathy of the rotator cuff. Eur Cell Mater. 2011a;21:548–557. doi: 10.22203/ecm.v021a41. [DOI] [PubMed] [Google Scholar]
- Oliva F, Via AG, Maffulli N. Calcific tendinopathy of the rotator cuff tendons. Sports Med Arthrosc. 2011b;19:237–243. doi: 10.1097/JSA.0b013e318225bc5f. [DOI] [PubMed] [Google Scholar]
- Organ CL, Adams J. The histology of ossified tendon in dinosaurs. J Vertebr Paleontol. 2005;25:602–613. [Google Scholar]
- Parton MJ, Walter DF, Ritchie DA. Case report: fracture of an ossified Achilles tendon – MR appearances. Clin Radiol. 1998;53:538–540. doi: 10.1016/s0009-9260(98)80179-7. [DOI] [PubMed] [Google Scholar]
- Ranvier L. 1875. pp. 349–366. Tendons et expansions tendineuses. Traite technique d'histology.
- Richards PJ, Braid JC, Carmont MR. Achilles tendon ossification: pathology, imaging and aetiology. Disabil Rehabil. 2008;30:1651–1665. doi: 10.1080/09638280701785866. [DOI] [PubMed] [Google Scholar]
- Roeder RK, Schwartz AG, Pasteris JD. Mineral distributions at the developing tendon enthesis. PLoS ONE. 2012;7:e48630. doi: 10.1371/journal.pone.0048630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney P, Walker D, Grant ME. Cartilage and bone formation in repairing Achilles tendons within diffusion chambers: evidence for tendon-cartilage and cartilage-bone conversion in vivo. J Pathol. 1993;169:375–381. doi: 10.1002/path.1711690315. [DOI] [PubMed] [Google Scholar]
- Rui YF, Lui PPY, Li G. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A. 2010a;16:1549–1558. doi: 10.1089/ten.TEA.2009.0529. [DOI] [PubMed] [Google Scholar]
- Rui YF, Lui PPY, Ni M. Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. J Orthop Res. 2010b;29:390–396. doi: 10.1002/jor.21218. [DOI] [PubMed] [Google Scholar]
- Rui YF, Lui PPY, Chan LS. Does erroneous differentiation of tendon-derived stem cells contribute to the pathogenesis of calcifying tendinopathy? Chin Med J (Engl) 2011a;124:606–610. [PubMed] [Google Scholar]
- Rui YF, Lui PPY, Lee YW. Higher BMP receptor expression and BMP-2-induced osteogenic differentiation in tendon-derived stem cells compared with bone-marrow-derived mesenchymal stem cells. Int Orthop. 2011b;36:1099–1107. doi: 10.1007/s00264-011-1417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui YF, Lui PPY, Rolf CG. Expression of chondro-osteogenic BMPs in clinical samples of patellar tendinopathy. Knee Surg Sports Traumatol Arthrosc. 2011c;20:1409–1417. doi: 10.1007/s00167-011-1685-8. [DOI] [PubMed] [Google Scholar]
- Ruzzini L, Abbruzzese F, Rainer A. Characterization of age-related changes of tendon stem cells from adult human tendons. Knee Surg Sports Traumatol Arthrosc. 2013 doi: 10.1007/s00167-013-2457-4. doi: 10.1007/s00167-013-2457-4. [DOI] [PubMed] [Google Scholar]
- Salingcarnboriboon R, Yoshitake H, Tsuji K. Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp Cell Res. 2003;287:289–300. doi: 10.1016/s0014-4827(03)00107-1. [DOI] [PubMed] [Google Scholar]
- Sandelin J, Santavirta S, Lattila R. Sports injuries in a large urban population: occurrence and epidemiological aspects. Int J Sports Med. 1988;9:61–66. doi: 10.1055/s-2007-1024980. [DOI] [PubMed] [Google Scholar]
- Screen HRC. Investigating load relaxation mechanics in tendon. J Mech Behav Biomed Mater. 2008;1:51–58. doi: 10.1016/j.jmbbm.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Screen HRC, Toorani S, Shelton JC. Microstructural stress relaxation mechanics in functionally different tendons. Med Eng Phys. 2013;35:96–102. doi: 10.1016/j.medengphy.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- Speer MY, Yang HY, Brabb T. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiesz EM, Roschger P, Zysset PK. Influence of mineralization and microporosity on tissue elasticity: experimental and numerical investigation on mineralized turkey leg tendons. Calcif Tissue Int. 2012;90:319–329. doi: 10.1007/s00223-012-9578-5. [DOI] [PubMed] [Google Scholar]
- Stanley RL, Fleck RA, Becker DL. Gap junction protein expression and cellularity: comparison of immature and adult equine digital tendons. J Anat. 2007;211:325–334. doi: 10.1111/j.1469-7580.2007.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers AP, Koob TJ. The evolution of tendon – morphology and material properties. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1159–1170. doi: 10.1016/s1095-6433(02)00241-6. [DOI] [PubMed] [Google Scholar]
- Uhthoff HK, Loehr JW. Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis, and management. J Am Acad Orthop Surg. 1997;5:183–191. doi: 10.5435/00124635-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Young MF, Bi Y, Ameye L. Biglycan knockout mice: new models for musculoskeletal diseases. Glycoconj J. 2002;19:257–262. doi: 10.1023/A:1025336114352. [DOI] [PubMed] [Google Scholar]
- Yu JS, Witte D, Resnick D. Ossification of the Achilles tendon: imaging abnormalities in 12 patients. Skeletal Radiol. 1994;23:127–131. doi: 10.1007/BF00563207. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang JHC. BMP-2 mediates PGE2-induced reduction of proliferation and osteogenic differentiation of human tendon stem cells. J Orthop Res. 2011;30:47–52. doi: 10.1002/jor.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Antibodies used in the present study.
Table S2. Primers designed and used in the present study (Sigma-Aldrich).
Table S3. Taqman primer probes used in the present study (Life Technologies).