Abstract
Translationally controlled tumor protein (TCTP) is a housekeeping protein, highly conserved among various species. It plays a major role in cell differentiation, growth, proliferation, apoptosis and carcinogenesis. Studies reported so far on TCTP expression in different digestive organs have not led to any understanding of the role of TCTP in digestion, so we localized TCTP in organs of the mouse digestive system employing immunohistochemical techniques. Translationally controlled tumor protein was found expressed in all organs studied: tongue, salivary glands, esophagus, stomach, small and large intestines, liver and pancreas. The expression of TCTP was found to be predominant in epithelia and neurons of myenteric nerve ganglia; high in serous glands (parotid, submandibular, gastric, intestinal crypts, pancreatic acini) and in neurons of myenteric nerve ganglia, and moderate to low in epithelia. In epithelia, expression of TCTP varied depending on its type and location. In enteric neurons, TCTP was predominantly expressed in the processes. Translationally controlled tumor protein expression in the liver followed porto-central gradient with higher expression in pericentral hepatocytes. In the pancreas, TCTP was expressed in both acini and islet cells. Our finding of nearly universal localization and expression of TCTP in mouse digestive organs points to the hitherto unrecognized functional importance of TCTP in the digestive system and suggests the need for further studies of the possible role of TCTP in the proliferation, secretion, absorption and neural regulation of the digestive process and its importance in the physiology and pathology of digestive process.
Keywords: digestive system, enteric nervous system, immunohistochemistry, mice, translationally controlled tumor protein, Western blotting
Introduction
Translationally controlled tumor protein (TCTP, fortilin), a highly conserved protein, plays a major role in cell growth, cell cycle progression, resistance to stress, and apoptosis (Bommer, 2012), as well as in cancer, allergy and diabetes (Chan et al. 2012a). Previous studies demonstrated TCTP expression in lysates of digestive organs and cell lines both in normal and pathologic conditions. For example, TCTP mRNA was found expressed in normal human liver, small and large intestines, pancreas, and in colon carcinoma cells (Chung et al. 2000). High expression of TCTP mRNA was found in porcine liver and intestine (Yubero et al. 2009). In Northern blot experiments with mouse tissues, TCTP showed high expression in the liver (Fiucci et al. 2003). Expression of TCTP mRNA was significantly higher in cancer tissues than in the adjacent non-cancerous tissues of hepatocellular carcinoma patients. Increased expression of TCTP mRNA was also found for 12 h after partial hepatectomy in rats (Zhu et al. 2008). Overexpression of TCTP was found to contribute to mitotic defects in hepatocellular carcinoma cells (Chan et al. 2012b). In pancreatic beta-cells, TCTP was found to be regulated by glucose and by pro-apoptotic stimuli, besides protecting the cells against apoptosis (Diraison et al. 2011). These disparate findings implicated in the function, regeneration and as carcinogenesis of the digestive system, fail to provide an understanding of the role of TCTP in digestion, its cellular location or its role in disease. This study on the localization of TCTP in various organs and cells of the digestive system is a step towards providing this understanding.
Materials and methods
Animals and tissues
C57Bl/6 inbred male mice (8–9-week-old, n = 11) were purchased from Orient Bio, Korea, and maintained in a 12-h light/dark cycle and fed standard rodent chow and water ad libitum. Eleven animals were deeply anesthetized with ethyl ether and samples from tongues, salivary glands, esophaguses, stomachs, small intestines, large intestines, livers, gallbladders and pancreases were collected from five of them for immunohistochemical studies (IHC) and from six of them for Western blotting (WB), and processed. All animal procedures were performed according to the National Institutes of Health Publication No. 8523: Guide for the Care and Use of Laboratory Animals and were approved by Ewha Womans University's Institutional Animal Care and Use Committee (Approval ID: 2012-01-029). The tissue samples were rinsed with phosphate-buffered saline (PBS) and frozen in liquid nitrogen until use.
Cell culture
Human cervical cancer-derived HeLa cell and Jurkat cell lines were obtained from American Type Culture Collection (ATCC) and maintained in Dulbecco's minimal essential media (DMEM, Gibco), supplemented with 10% fetal bovine serum (FBS, Gibco), 100 units mL−1 penicillin, and 100 units mL−1 streptomycin. The cells were cultured at 37 °C in a humidified 5% CO2 atmosphere incubator. Prior to use, the HeLa cells and Jurkat were harvested with a scrapper, lysed and sonicated for 10 s on ice.
Preparation of human recombinant TCTP
The sequence coding human TCTP (hTCTP, residues 1–172) was amplified using the oligonucleotide primers for cloning into the bacterial expression vector, pET22b+ (Invitrogen). The forward primer was TGA GAT CCG GCT GCT AAC AAA GCC CGA-, and reverse primer ACA TTT TTC CAT CTC TAA ACC ATC CTT AAA GAA AAT CAT ATA TGG GGT-.
Escherichia coli BL21(DE3)pLysS cells were transformed with the pET22b+/TCTPs and the TCTPs overexpressed were purified using a cobalt-charged His-Bind column according to the manufacturer's protocol (ThermoScientific). The proteins were separated by fast protein liquid chromatography (FPLC) on a Mono Q HR 5/5 column (Amersham Pharmacia Biotech) using a NaCl gradient. Buffer A was: 20 mm Tris-HCl, 1 mm EDTA, 50 mm NaCl, pH 7.4, and Buffer B was: 20 mm Tris-HCl, 1 mm EDTA, 1 m NaCl, pH 7.4. A linear gradient from 0 to 15% Buffer B was run over five column volumes and this was followed by a linear gradient from 15 to 50% Buffer B over 25 column volumes to elute the TCTP. Each eluted fraction was desalted and transferred into PBS using PD-10 column (Amersham Pharmacia Biotech).
SDS-PAGE and Western blotting
Prior to use for these procedures, the frozen tissues were ground in liquid nitrogen. The ground tissue, HeLa and Jurkat cells were lysed in a buffer containing 50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1 mm EDTA, 2 mm Na3VO4, 1 mm NaF, 0.25% deoxycholate, 1% Triton-X, and protease inhibitor cocktail (Roche, Mannheim, Germany), and sonicated for 10 s on ice. The lysates were centrifuged at 12 000 g for 20 min at 4 °C. Protein concentration in supernatants was determined using Bradford assay (BioRad, CA, USA), using bovine serum albumin (BSA) dilutions as standards. Optical densities of standards and samples were read by microplate reader at 595 nm. The samples were diluted with 4× SDS sample buffer containing 0.2 m Tris-HCl (pH 6.8), 40% sodium dodecyl sulfate (SDS), 40% glycerol, 0.2% bromophenol blue and 8% β-mercaptoethanol, and heated at 100 °C for 10 min. Human recombinant proteins were diluted with 4× SDS sample buffer and heated at 100 °C for 10 min. Ten micrograms of each sample were separated on 12% SDS-PAGE gel and transferred to nitrocellulose membrane (Whatman, Germany). The membrane was blocked with Tris-buffered saline containing 0.1% Tween-20 (TBST) and 3% BSA (USB, OH, USA) for 1 h, and incubated with polyclonal rabbit anti-TCTP antibody (Abcam Ab37506, dilution 1 : 4000) and with monoclonal rabbit anti-β-actin antibody (Sigma-Aldrich,dilution 1 : 1000) in TBST containing 3% BSA at 4 °C overnight. After washing in TBST, the membrane was incubated with secondary antibody for 1 h at room temperature, washed, and enhanced chemiluminescence was visualized using ECL prime Western blotting detection system (Amersham Bioscience, Germany). Protein expression levels were calculated using multi gauge 3.0 photo software (Fuji Film, Japan) and normalized to β-actin. Statistical analysis was done using one-way anova with Dunnett's Multiple Comparison test by graphpad prism 5 (Graphpad Software, CA, USA).
Immunohistochemistry
Samples for immunoperoxidase staining were taken following the perfusion of the animals with 10% formalin in PBS. Samples were then fixed by immersion in the same fixative for 24 h, dehydrated in ethanol and embedded in paraffin. Sections of 5–8 μm were cut on a rotary microtome and mounted on glass slides. After deparaffinization and hydration, the tissue sections were boiled for 15 min in sodium citrate buffer, pH 6.0, to retrieve the epitope. Endogenous peroxidase was blocked by incubation with 1% hydrogen peroxide for 15 min. Blocking of non-specific protein binding and immunostaining were performed with the ImmPress Reagent kit (Vector laboratories) according to the manufacturer's instructions. Rabbit polyclonal anti-TCTP antibody (Abcam, ab37506) was applied to the sections for 1 h at room temperature in 1 : 1000 dilution. Then, the sections were treated with HRP conjugate from the ImmPRESS kit for 30 min and stained with 0.025% DAB solution (Sigma-Aldrich). Cell nuclei were stained with Harris hematoxylin (Sigma-Aldrich) and sections were embedded with Permount (Fisher Scientific). Stained sections were analyzed under the microscope (Axio Scope, A1, Zeiss), and representative photographs were taken with a digital camera. TCTP expression (immunoreactivity) in cells was assessed as low, moderate or high based on staining intensity.
Mouse pancreas was used as a positive control for immunohistochemical staining because TCTP expression is high in this tissue. For the negative controls, sections were processed in the same way as described above, but TCTP antibody was replaced by an irrelevant rabbit polyclonal antibody at the same concentration. In addition, Western blotting with the same tissues was used as a control for primary antibody specificity.
Results
Western blot analysis of TCTP expression in various mouse digestive tissues
To measure total TCTP expression in each organ, we performed Western blotting using lysates of HeLa cells, Jurkat cells, mouse pancreatic tissue and human recombinant TCTP as positive controls. HeLa cell lysate was used as a reference. The Western blotting results presented in Fig. 1 show that TCTP is expressed in all organs examined. The highest TCTP expression (TCTP/β-actin) was in the pancreas (**P < 0.01) and the lowest in the esophagus and gallbladder.
Figure 1.
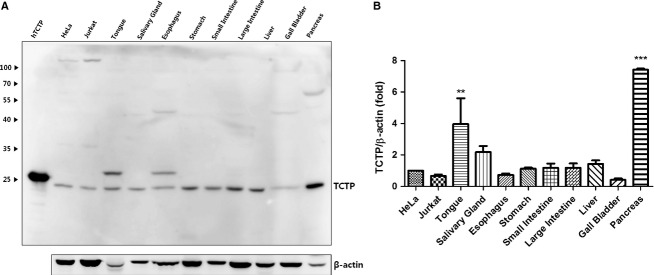
Western blotting analysis of translationally controlled tumor protein (TCTP) expression in the mouse digestive system. (A) TCTP expression in digestive organs and cell lysates (HeLa and Jurkat). Human recombinant TCTP (2 μg), HeLa and Jurkat cell lysates were used as positive controls. (B) Quantification by densitometry of Western blot images using HeLa as a control. Expression levels in organs are presented as multiples of HeLa expression level. Protein expression levels were calculated by multi gauge 3.0 photo software (Fuji Film, Japan) and normalized by β-actin. Statistical analysis was done using one-way anova with Dunnett's multiple comparison test by graphpad prism 5 (Graphpad Software, CA). **P < 0.01 and ***P < 0.001, comparing levels in organs with HeLa, n = 4.
Immunohistochemical localization of TCTP
Analysis of histological sections immunostained for anti-TCTP revealed varying levels of localization of TCTP, cytoplasmic in all cases. TCTP expression in all the organs examined was dependent on the cell type and location. Data on TCTP expression in different cells are summarized in the Table 1.
Table 1.
Imunohistochemical localization of translationally controlled tumor protein (TCTP) in cells of mouse digestive organs
| Organ cells | TCTP expression level |
|---|---|
| Tongue cells | |
| Stratified epithelium (non-keratinized cells) | Moderate |
| Mucous acini of intramural salivary glands | Low to moderate |
| Serous acini of intramural salivary glands | Moderate to high |
| Parotid gland cells | |
| Serous acini | High |
| Ductal epithelium | Low |
| Submandibular gland cells | |
| Serous acini | High |
| Mucous acini | Low |
| Ductal epithelium | Low |
| Sublingual gland cells | |
| Mucous acini | Low to moderate |
| Ductal epithelium | Low |
| Esophagus cells | |
| Stratified epithelium (non-keratinized cells) | Moderate |
| Stomach cells | |
| Stratified epithelium (non-glandular portion of the stomach) | Moderate |
| Surface lining and neck cells of fundal glands | Low to moderate |
| Cells of bases of fundal glands | High |
| Surface lining and neck cells of pyloric glands | Moderate |
| Cells of bases of pyloric glands | Moderate |
| Neurons of myenteric nerve ganglia | High |
| Small intestine cells | |
| Epithelium of crypts | High |
| Absorptive epithelium of villi | Low to moderate |
| Goblet cells | Low to moderate |
| Neurons of myenteric nerve ganglia | High |
| Large intestine cells | |
| Epithelium of crypts | Moderate |
| Absorptive surface epithelium | Low to moderate |
| Goblet cells | Low to moderate |
| Neurons of myenteric nerve ganglia | High |
| Liver cells | |
| Hepatocytes (pericentral) | Moderate |
| Hepatocytes (periportal) | Low |
| Pancreas cells | |
| Epithelium of acini | High |
| Islet cells | Low to moderate |
Tongue
In the tongue, TCTP expression was found in the stratified epithelium (non-keratinized cells) and papillae, as well as in intramural salivary glands (Fig. 2). In the lingual epithelium, TCTP expression was higher in lower cell layers than in superficial layers (Fig. 2A,B). TCTP expression was also considerably higher in serous lingual glands than in mucous glands (Fig. 2C–F).
Figure 2.
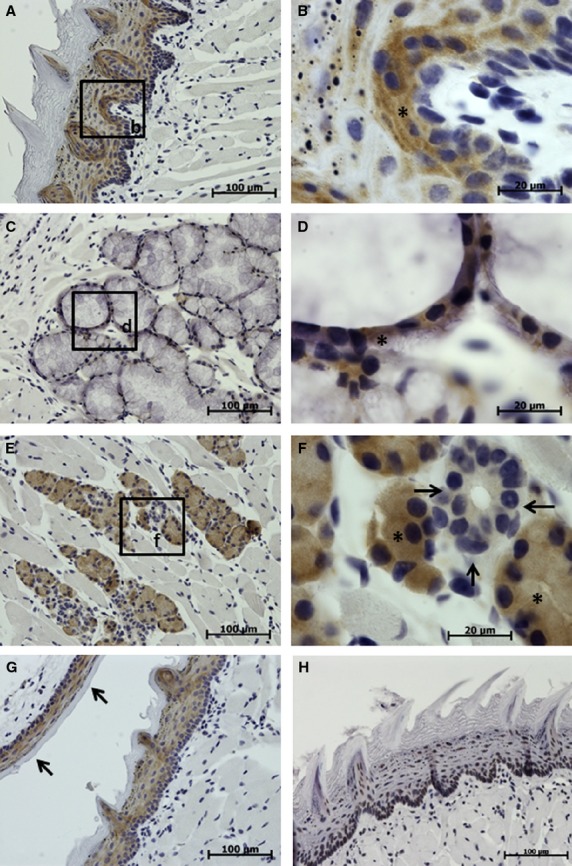
Immunohistochemical studies (IHC) localization of translationally controlled tumor protein (TCTP) in the tongue and soft palate. Translationally controlled tumor protein expression is shown in brown, the cell nuclei stained blue with hematoxylin. (A-G) Sections treated with TCTP antibody. (H) Control section treated with the irrelevant rabbit polyclonal antibody. (A,B) Tongue mucosa demonstrating more intense TCTP expression in the basal cell layers (asterisk). (C,D) Intramural lingual salivary gland (mucous), showing moderate TCTP expression in the epithelium of acinus (asterisk). (E,F) Intramural lingual salivary gland (serous), showing moderate to high TCTP expression in acini (asterisks) and no signal in the duct (arrows). (G) Tongue and soft palate mucosa (indicated with arrows) showing moderate intensity TCTP expression in non-keratinized cells of the epithelium.
Salivary glands
Translationally controlled tumor protein was found localized in all three big salivary glands – submandibular, sublingual and parotid. The highest expression of TCTP was found in the parotid gland and the lowest in the sublingual gland. In the parotid gland, epithelium of serous acini had a high level of TCTP expression, whereas ductal epithelium had a low expression (Fig. 3A,B). Submandibular gland containing both serous and mucous acini demonstrated a high expression in serous acini and a low expression in mucous acini (Fig. 3C,D). The sublingual gland showed moderate TCTP expression in mucous acini and low expression in ductal epithelium (Fig. 3E–G). In all glands, TCTP expression was much stronger in acini than in ductal epithelium and stronger in serous acini than in mucous ones.
Figure 3.
Immunohistochemical studies (IHC) localization of translationally controlled tumor protein (TCTP) in big salivary glands. TCTP is shown in brown, cell nuclei stained blue with hematoxylin. (A-G) Sections treated with TCTP antibody. (H) Control section of sublingual gland treated with irrelevant rabbit polyclonal antibody. (A,B) Parotid gland shows high TCTP expression in serous acini (arrow) and low expression in the duct (asterisk). (C,D) Submandibular gland shows high TCTP expression in serous acini (asterisk) and low expression in mucous acini (star). (E-H) Sublingual gland shows moderate TCTP expression in mucous acini (F) and low expression in the duct (G).
Esophagus
In the esophagus, moderate levels of TCTP were found in non-keratinized cells of stratified epithelium of mucosa (Fig. 4A,B). Basal layers of cells had a higher expression of TCTP than did superficial layers.
Figure 4.
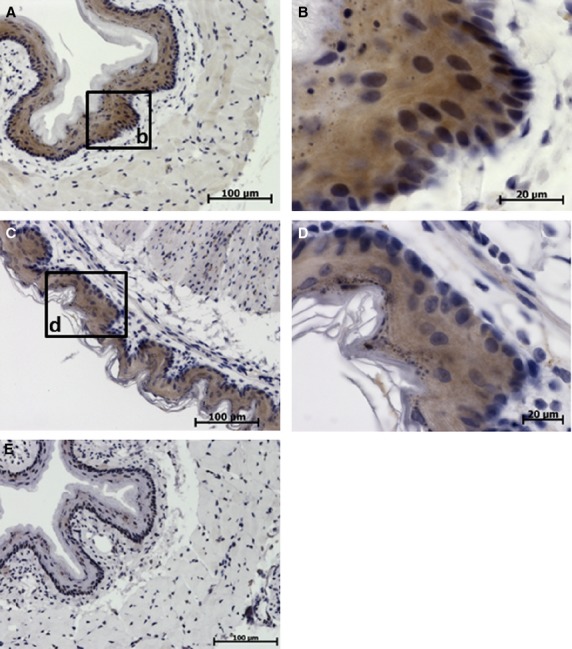
Immunohistochemical studies (IHC) localization of translationally controlled tumor protein (TCTP) in the esophagus and non-glandular portion of the stomach. Translationally controlled tumor protein expression is shown in brown, cell nuclei stained blue with hematoxylin. (A,B) Sections of esophagus. (C,D) Sections of non-glandular portion of the stomach. (E) Control section of esophagus treated with irrelevant rabbit antibody. (A) Low-power image of esophagus. (B) High-power image of esophageal mucosa, moderate to high TCTP expression in the stratified squamous epithelium. (C) Low-power image of non-glandular portion of the stomach. (D) High-power image of the non-glandular portion of the stomach, moderate TCTP expression in the epithelium.
Stomach
Translationally controlled tumor protein expression was found mainly in mucosa in both non-glandular and glandular portions of the stomach. In the non-glandular portion, TCTP was moderately expressed in non-keratinized cells of stratified epithelium (Fig. 4C,D). In gastric glands, TCTP expression was non-uniform and was considerably higher in the base of gland than in the neck, isthmus or surface lining epithelium (Fig. 5). This variance was greater in fundal glands (Fig. 5B,C) than in pyloric glands (Fig. 5E,F). A high level of TCTP expression was also found in cells of myenteric nerve ganglia (Fig. 5G).
Figure 5.
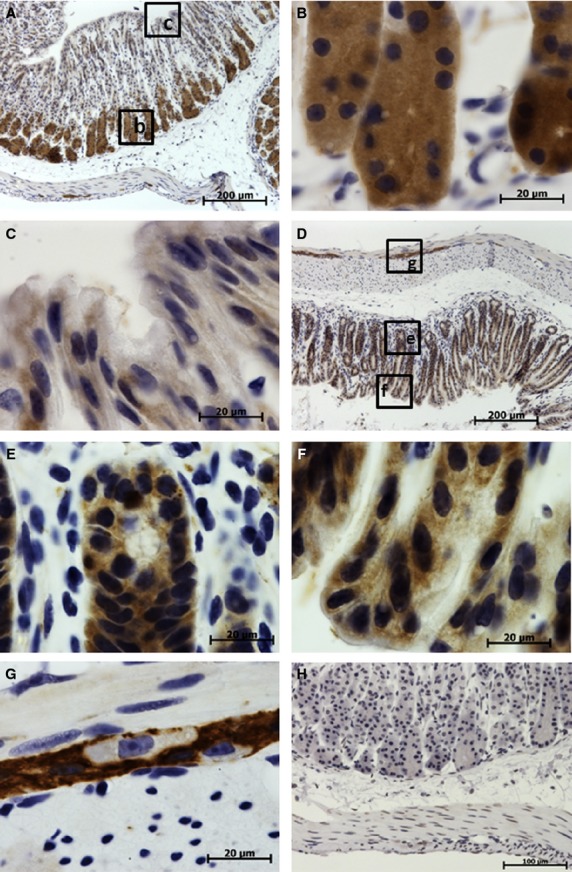
Immunohistochemical studies (IHC) localization of translationally controlled tumor protein (TCTP) in the glandular portion of the stomach. Translationally controlled tumor protein expression is shown in brown, cell nuclei stained blue with hematoxylin. (A-G) Sections treated with anti-TCTP antibody. (H) Control section treated with irrelevant rabbit polyclonal antibody. (A) Low-power image of stomach fundus demonstrating high TCTP expression in the base of gastric glands and low expression in the neck and surface lining cells. (B) High-power image of the base of fundal glands with high TCTP expression. (C) High-power image of necks of fundal glands with low TCTP expression. (D) Low-power image of pyloric portion of the stomach. (E) High-power image of the base of pyloric gland with moderate to high TCTP expression. (F) High-power image of necks of pyloric gland and gastric pits demonstrating moderate TCTP expression. (G) High-power image of the myenteric nerve ganglion in the stomach muscular layer with high TCTP expression, note the absence of TCTP in the perikaryon of neurons.
Small intestine
In the small intestine, a high expression of TCTP was found in the epithelia of crypts (Fig. 6B) and in myenteric nerve ganglia (Fig. 6D) within the muscle layer. TCTP expression in absorptive epithelium and goblet cells was low to moderate and decreased from the base of the villus toward the tip (Fig. 6C).
Figure 6.
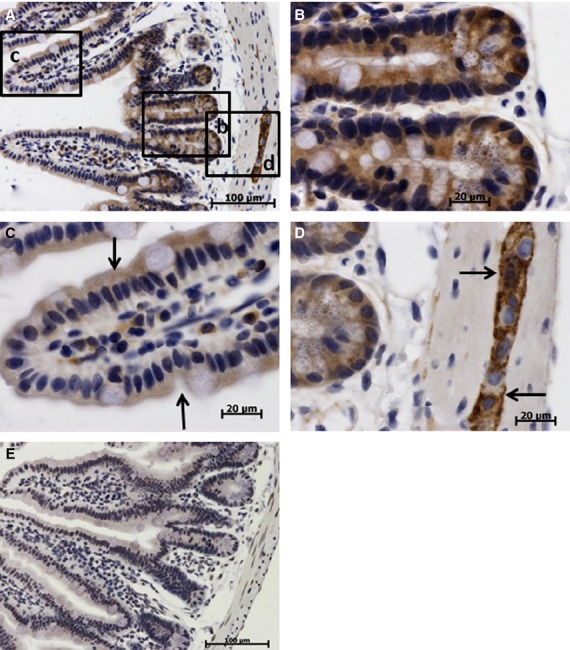
Immunohistochemical studies (IHC) localization of translationally controlled tumor protein (TCTP) in the small intestine (ileum). Translationally controlled tumor protein expression shown by brown staining, cell nuclei (blue) stained with hematoxylin. (A-D) Sections treated with anti-TCTP primary antibody. (E) Control section treated with irrelevant rabbit antibody. (A) Low-power image of intestinal villus and crypts. (B) High-power image of the ileum crypt, high TCTP expression in cryptal epithelium. (C) High-power image of the intestinal villus, absorptive epithelium (arrows) has moderate to low TCTP expression decreasing toward the tip of the villus. (D) High-power image of myenteric nerve ganglion (arrows); note high TCTP expression in the cytoplasm of neurons and the lack of the signal in perikaryon.
Large intestine
In the large intestine, TCTP expression was also cytoplasmic and was found in surface lining epithelium (Fig. 7B) and in epithelium of crypts (Fig. 6D). Neurons of myenteric nerve ganglia (Fig. 7C,E) had high levels of TCTP expression, like those of small intestine and stomach.
Figure 7.
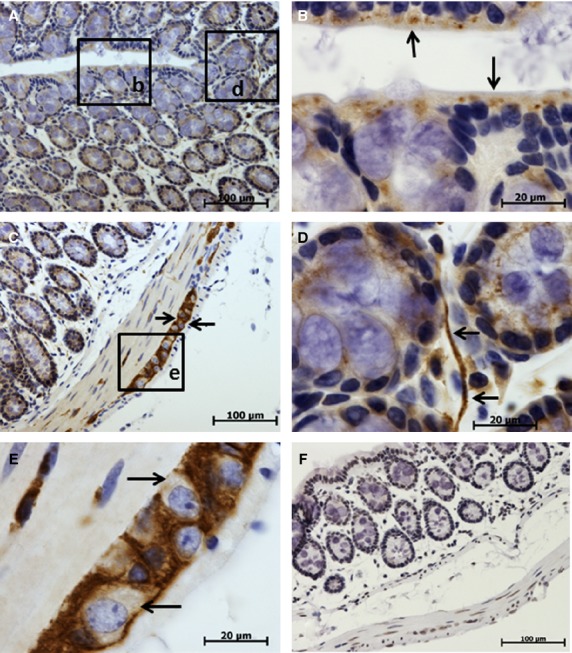
Immunohistochemical studies (IHC) localization of translationally controlled tumor protein (TCTP) in the large intestine (colon). TCTP expression shown by brown staining, cell nuclei (blue) stained with hematoxylin. (A–E) Sections treated with anti-TCTP primary antibody. (F) Control section treated with irrelevant rabbit antibody. (E) High-power image of myenteric nerve ganglion with intense TCTP expression (note the absence of TCTP expression in the perikaryon of neurons marked by arrows). (A) Low-power photo of colon mucosa. (B) High-power image of the colon epithelium and part of the crypt, moderate TCTP expression in the lining epithelium indicated with arrows. (C) Low-power image of colon wall, arrows point to the myenteric nerve ganglion with intense TCTP expression. (D) High-power image of the colon crypt, TCTP expression in epithelial cells of crypts and in nerve fiber (arrows).
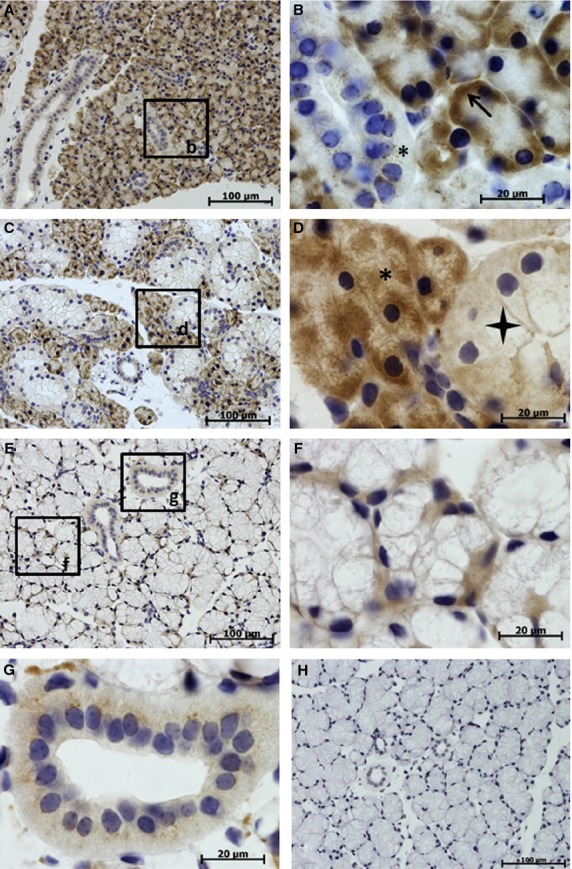
Liver
In the liver, moderate TCTP expression was found in hepatocytes with the expression level depending on the location of the hepatocytes. Expression was higher in pericentral (zones 2 and 3 of hepatic acinus) hepatocytes (Fig. 8A,B) than in periportal (zone 1) hepatocytes (Fig. 8A,C). TCTP immunoreactivity was not detected in bile ducts.
Figure 8.
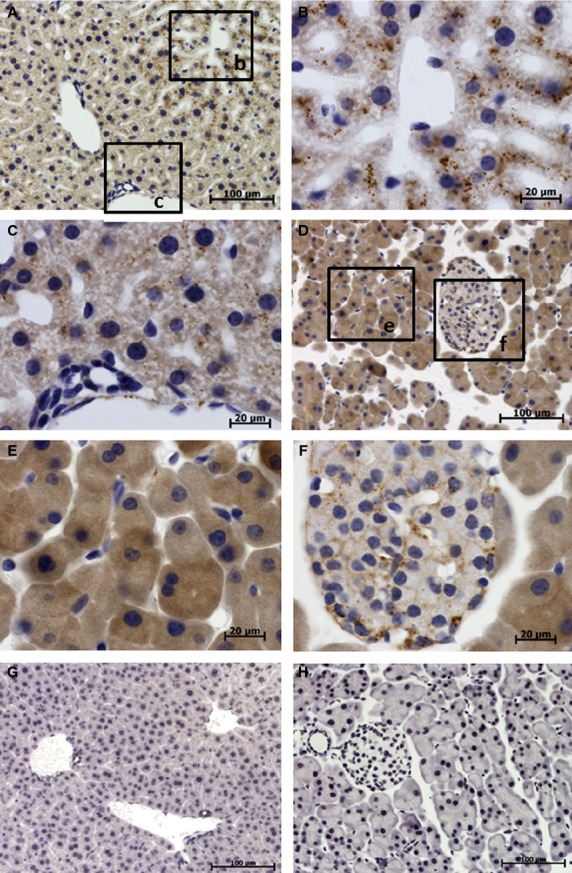
Immunolocalization of translationally controlled tumor protein (TCTP) in liver and pancreas. Translationally controlled tumor protein expression shown by brown staining, cell nuclei (blue) stained with hematoxylin. (A–C,G) Liver sections. (D-F,H) Pancreas sections. (A) Low-power image of liver section. (B) High-power image of pericentral hepatocytes demonstrating moderate TCTP expression in the cytoplasm of hepatocytes shown as brown dots. (C) High-power image of periportal hepatocytes shows low TCTP expression compared with that in pericentral hepatocytes. (D) Low-power image of pancreas section. (E) High-power image of pancreas section demonstrating intense diffuse TCTP expression in acinar cells. (F) High-power image of pancreatic islet demonstrating low to moderate TCTP expression in islet cells. (G) Control liver section treated with irrelevant antibody. (H) Control pancreas section treated with irrelevant antibody.
Pancreas
In the pancreas, TCTP was found expressed in both exocrine and endocrine portions. Exocrine acini showed strong diffuse localization of TCTP (Fig. 8D,E), whereas islet cells exhibited low to moderate levels of TCTP localization (Fig. 8D,F). TCTP was not detected in pancreatic ducts or blood vessels.
Discussion
This study, the first systematic attempt to localize TCTP in organs and cells of mouse digestive system, clearly shows that the degree of TCTP expression depends on the location and function of the cell or organ in question.
Previous studies conducted in various species using different methods, also reported varying levels of TCTP mRNA or protein expressions in different digestive organs. High levels of TCTP or its mRNA were found in the pancreas, small intestine and liver, and low levels in stomach and colon (Li et al. 2001). Employing Western blotting, Amson et al. (2012) found TCTP expression in the liver and intestines of mice. We found significant expression of TCTP both in the small and large intestines, pancreas, liver and stomach of mice (Fig. 1). Our study appears to be the first to look for or find TCTP expression in tongue, salivary glands and esophagus of mice. The high TCTP we found in mouse tongue may be a consequence of its high expression in the mucosa and in intramural salivary glands revealed by IHC photography (Fig. 2).
Our study of the alimentary canal wall revealed the predominant expression of TCTP in mucosa, and a lower expression in submucosa, tunica muscularis and serosa. TCTP expression in stratified squamous epithelia of the tongue, esophagus and non-glandular portion of the stomach was higher than that in surface lining cells of the glandular portion of stomach and absorptive epithelium of intestine categorized as columnar epithelium. Moreover, TCTP levels were higher in basal than surface layers of cells of stratified epithelia. We observed a similar pattern of TCTP expression in the transitional epithelium of ureter and urinary bladder of mice (Sheverdin et al. 2012). Higher TCTP expression in the basal cell layer than in intermediate layer was also reported in human corneal epithelium (Batisti et al. 2012). These authors also observed that in herpetic keratitis, TCTP expression increased in all layers of corneal epithelium. Wu et al. (2012) also found TCTP expression in stratified epithelium and in normal skin as well as in squamous cell skin carcinoma (Wu et al. 2012). Taken together, these results support our conclusion that higher TCTP expression in basal layers of stratified epithelium is a general phenomenon which may be related to the higher proliferative activity of these cells. Taken in conjunction with the report of Sanchez et al. (1997) that TCTP is highly expressed in actively dividing cells, our findings suggest that TCTP may play a role in physiological as well as reparative regeneration of epithelial cells.
TCTP levels in columnar epithelia also depend on their location, and thus on functions of columnar epithelial cells. In the stomach, fundic glands had higher levels of TCTP than did pyloric glands, and small intestinal crypts had higher levels than large intestinal crypts. Translationally controlled tumor protein expressions were different in different glands, as well as in cells located in different parts of the same gland. Epithelial cells in the base of the gastric gland and intestinal crypt had higher TCTP levels than did surface lining cells both in stomach and intestines. This suggests that TCTP expression in columnar epithelium is related to secretion, with actively secreting cells having higher TCTP levels. Lower levels of TCTP in absorptive cells may indicate less involvement of TCTP in the absorption process.
We found moderate to high TCTP levels in all digestive glands examined, intramural as well as extramural. The highest expression was observed in serous glands such as parotid gland, pancreas, gastric glands and intestinal crypts, which primarily secrete proteins. Mucous glands, which primarily secrete glycoproteins, had a much lower expression. Submandibular gland, which has both serous and mucous acini, contained higher levels of TCTP. These findings seem to suggest some relationship or correlation between TCTP expression in the cell and the chemical nature of its secretions.
We found that the distribution of TCTP in the liver was irregular, and depended on the position of hepatocytes within the hepatic lobule (acinus). TCTP expression was considerably higher in pericentral (zones 2 and 3 of hepatic acinus) than in periportal (zone 1) hepatocytes. Functional heterogeneity of hepatocytes, as well as heterogeneity of proteins they express, is well known (Katz, 1992). However, heterogeneity in TCTP expression has not been described hitherto and further studies are needed to understand this phenomenon. TCTP expression in the pancreas is high, and is probably linked to the high protein secretion in this organ, as discussed earlier. Our finding of TCTP both in pancreatic acini and in the endocrine portion of the gland suggests TCTP involvement in endocrine secretion. However, TCTP expression in islet cells was considerably lower than in acini.
A relationship between TCTP and cytoskeleton components has also been demonstrated. TCTP was shown to be a tubulin binding protein, which is temporarily associated with microtubules and the spindle apparatus, and its overexpression leads to microtubule stabilization and to distortions in the cell division processes (Gachet et al. 1999). Actin is colocalized with TCTP in ovarian cell lines, in the granules in the vicinity of the nucleus, and in focal adhesions. TCTP was also localized in the vicinity of cytokeratin filaments (Kloc et al. 2012). TCTP also interacts with F-actin, which is indirectly related to microtubules and regulates cell shape in a cytoskeleton-dependent manner (Bazile et al. 2009). It is colocalized with tubulin during mitosis in prostate epithelial cells (Arcuri et al. 2004). In this study, we observed that TCTP appears in different patterns within the cytoplasm of different cells, being diffuse in the cytoplasm in most of epithelial cells and granular in duct epithelium of salivary glands, in hepatocytes and in pancreatic islet cells. In the case of myenteric neurons, we observed little or no TCTP in perikaryon, suggesting that it is localized predominantly in neuronal processes (Figs 5G, 6D and 7E). We observed high TCTP expression in both myenteric ganglia and nerve fibers penetrating mucosa (Fig. 7D,E). In this regard it should be noted that in Aplysia, the composition and biochemical properties of the cytoskeletal proteins from neuron cell bodies were different from those of axons, and this led to the suggestion that at least two distinct cytoskeletal networks exist, one specific for the cell body, and the other specific for the axon (Drake & Lasek, 1984). Our findings, together with data from the literature, allow us to speculate that variations in TCTP distribution patterns in cells are related to differences in cytoskeleton organization and its functions. Further studies are needed to confirm and explain these relationships.
Our observation of high TCTP expression in myenteric nerve ganglia of stomach and intestine is in agreement with previous reports on TCTP expression in nerve tissue. TCTP expression was found in the human cerebellum, cerebral cortex, thalamus and caudate nucleus, and temporal cortex (Kim et al. 2001). TCTP mRNA was detected in normal human brain (Li et al. 2001). Also, TCTP was found expressed during neural differentiation of mouse embryonic stem cells (Wang & Gao, 2005) and in rat hippocampal tissue obtained by surgical microdissection (Corti et al. 2008). High TCTP expression in myenteric neurons suggests that TCTP may play an important role in neural regulation of gastrointestinal tract. Further studies focused on TCTP in the enteric nervous system should help elucidate its functions in neurons and regulation of digestion.
In this study we found that cellular localization of TCTP was confined to the cytoplasm, confirming a previous report that cytoplasmic localization is a characteristic of TCTP (Arcuri et al. 2004). But clearly there are many studies that demonstrated nuclear localization of the protein. TCTP was detected in both the cytoplasm, and the nuclei of ovarian epithelial cells and ovarian cancer cells (Kloc et al. 2012). In normal and tumor cell lines its translocation varied between normal and tumor cell lines at different time points (Ma & Zhu, 2012). TCTP was detected not only in cytoplasm but also in the nuclei of ovarian epithelial cells and ovarian cancer cells (Kloc et al. 2012). It was expressed with highest intensity in the spermatogonia nuclei in the postnatal testes (Guillaume et al. 2001). It was shown that sumoylation of TCTP is a mechanism for nuclear transport of TCTP, where it may have a role in protecting the DNA and the cell from oxidative stress (Munirathinam & Ramaswamy, 2012). Nuclear localization of TCTP as well as its translocation were observed in cultured cells, tumor cells, cells undergoing stress stimuli and proliferating cells of gonads. Cytoplasmic localization was demonstrated in mature somatic cells of normal tissues residing in their natural environment. However, the mechanisms underlying translocation of TCTP from cytoplasm to nuclei are not yet clear.
Conclusions
In summary, this study shows that: In the digestive system, TCTP is predominantly expressed in epithelia and enteric nervous system, suggesting its involvement in secretion, absorption and neural regulation of digestion. TCTP expression levels directly correlate with proliferative and secretory activities of epithelial cells; the levels are higher in actively proliferating cells and in cells secreting proteinaceous product.TCTP is expressed both in exocrine and endocrine portions of the pancreas.There is a porto-central gradient of TCTP expression in the liver, with higher expression in pericentral hepatocytes.
Acknowledgments
This study was supported by a grant from the Korea Health Technology R&D Project, Ministry of Health & Welfare (A111417) and the National Research Foundation grant funded by the Ministry of Science, ICT & Future Planning (2012R1A1A2042142) (2012M3A9A8053272).
Author contributions
V.S. carried out the immunohistochemical analysis and contributed to the writing of the manuscript. J.J. performed the Western blot analysis as well as data analysis. K.L. participated in the conception and design of the study and the writing of the manuscript. All authors read and approved the final manuscript.
References
- Amson R, Pece S, Lespagnol A. Reciprocal repression between P53 and TCTP. Nat Med. 2012;18:91–99. doi: 10.1038/nm.2546. [DOI] [PubMed] [Google Scholar]
- Arcuri F, Papa S, Carducci A. Translationally controlled tumor protein (TCTP) in the human prostate and prostate cancer cells: expression, distribution, and calcium binding activity. Prostate. 2004;60:130–140. doi: 10.1002/pros.20054. [DOI] [PubMed] [Google Scholar]
- Batisti C, Ambrosio MR, Rocca BJ. Translationally controlled tumour protein (TCTP) is present in human cornea and increases in herpetic keratitis. Diagn Pathol. 2012;7:90. doi: 10.1186/1746-1596-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazile F, Pascal A, Arnal I. Complex relationship between TCTP, microtubules and actin microfilaments regulates cell shape in normal and cancer cells. Carcinogenesis. 2009;30:555–565. doi: 10.1093/carcin/bgp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommer UA. Cellular function and regulation of the translationally controlled tumour protein TCTP. Open Allergy J. 2012;5:19–32. [Google Scholar]
- Chan TH, Chen L, Guan XY. Role of translationally controlled tumor protein in cancer progression. Biochem Res Int. 2012a doi: 10.1155/2012/369384. doi: 10.1155/2012/369384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TH, Chen L, Liu M. Translationally controlled tumor protein induces mitotic defects and chromosome missegregation in hepatocellular carcinoma development. Hepatology. 2012b;55:491–505. doi: 10.1002/hep.24709. [DOI] [PubMed] [Google Scholar]
- Chung S, Kim M, Choi W. Expression of translationally controlled tumor protein mRNA in human colon cancer. Cancer Lett. 2000;156:185–190. doi: 10.1016/s0304-3835(00)00460-2. [DOI] [PubMed] [Google Scholar]
- Corti V, Sanchez-Ruiz Y, Piccoli G. Protein fingerprints of cultured CA3-CA1 hippocampal neurons: comparative analysis of the distribution of synaptosomal and cytosolic proteins. BMC Neurosci. 2008;9:36. doi: 10.1186/1471-2202-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diraison F, Hayward K, Sanders KL. Translationally controlled tumour protein (TCTP) is a novel glucose-regulated protein that is important for survival of pancreatic beta cells. Diabetologia. 2011;54:368–379. doi: 10.1007/s00125-010-1958-7. [DOI] [PubMed] [Google Scholar]
- Drake PF, Lasek RJ. Regional differences in the neuronal cytoskeleton. J Neurosci. 1984;4:1173–1186. doi: 10.1523/JNEUROSCI.04-05-01173.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiucci G, Lespagnol A, Stumptner-Cuvelette P. Genomic organization and expression of mouse Tpt1 gene. Genomics. 2003;81:570–578. doi: 10.1016/s0888-7543(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Gachet Y, Tournier S, Lee M. The growth-related, translationally controlled protein P23 has properties of a tubulin binding protein and associates transiently with microtubules during the cell cycle. J Cell Sci. 1999;112:1257–1271. doi: 10.1242/jcs.112.8.1257. [DOI] [PubMed] [Google Scholar]
- Guillaume E, Pineau C, Evrard B. Cellular distribution of translationally controlled tumor protein in rat and human testes. Proteomics. 2001;1:880–889. doi: 10.1002/1615-9861(200107)1:7<880::AID-PROT880>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Katz NR. Metabolic heterogeneity of hepatocytes across the liver acinus. J Nutr. 1992;122:843–849. doi: 10.1093/jn/122.suppl_3.843. [DOI] [PubMed] [Google Scholar]
- Kim SH, Cairns N, Fountoulakisc M. Decreased brain histamine-releasing factor protein in patients with Down syndrome and Alzheimer's disease. Neurosci Lett. 2001;300:41–44. doi: 10.1016/s0304-3940(01)01545-2. [DOI] [PubMed] [Google Scholar]
- Kloc M, Tejpal N, Sidhu J. Inverse relationship between TCTP/RhoA and p53/cyclin A/actin expression in ovarian cancer cells. Folia Histochem Cytobiol. 2012;50:358–367. doi: 10.5603/19745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Zhang D, Fujise K. Characterization of fortilin, a novel antiapoptotic protein. J Biol Chem. 2001;276:47542–47549. doi: 10.1074/jbc.M108954200. [DOI] [PubMed] [Google Scholar]
- Ma YP, Zhu WL. Cytoplasmic and nuclear localization of TCTP in normal and cancer cells. Biochem Res Int. 2012;2012:871728. doi: 10.1155/2012/871728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munirathinam G, Ramaswamy K. Sumoylation of human translationally controlled tumor protein is important for its nuclear transport. Biochem Res Int. 2012;2012:831940. doi: 10.1155/2012/831940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JC, Schaller D, Ravier F. Translationally controlled tumor protein: a protein identified in several nontumoral cells including erythrocytes. Electrophoresis. 1997;18:150–155. doi: 10.1002/elps.1150180127. [DOI] [PubMed] [Google Scholar]
- Sheverdin V, Bae SY, Shin DH. Expression and localization of translationally controlled tumor protein in rat urinary organs. Microsc Res Tech. 2012;75:1576–1581. doi: 10.1002/jemt.22103. [DOI] [PubMed] [Google Scholar]
- Wang D, Gao L. Proteomic analysis of neural differentiation of mouse embryonic stem cells. Proteomics. 2005;5:4414–4426. doi: 10.1002/pmic.200401304. [DOI] [PubMed] [Google Scholar]
- Wu D, Guo Z, Min W. Upregulation of TCTP expression in human skin squamous cell carcinoma increases tumor cell viability through anti-apoptotic action of the protein. Exp Ther Med. 2012;3:437–442. doi: 10.3892/etm.2011.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yubero N, Esteso G, Cardona H. Molecular cloning, expression analysis and chromosome localization of the Tpt1 gene coding for the pig translationally controlled tumor protein (TCTP) Mol Biol Rep. 2009;36:1957–1965. doi: 10.1007/s11033-008-9405-2. [DOI] [PubMed] [Google Scholar]
- Zhu WL, Cheng HX, Han N. Messenger RNA expression of translationally controlled tumor protein (TCTP) in liver regeneration and cancer. Anticancer Res. 2008;28:1575–1580. [PubMed] [Google Scholar]


