Abstract
Adult muscle size and fibre-type composition are heritable traits that vary substantially between individuals. We used inbred mouse strains in which soleus muscle mass varied by an order of magnitude to explore whether properties of muscle spindles can also be influenced by genetic factors. Skip-serial cross-sections of soleus muscles dissected from 15 male mice of BEH, BEL, C57BL/6J, DUH, LG/J and SM/J strains were analysed for number of muscle spindles and characteristics of intrafusal and extrafusal fibres following ATPase staining. The BEL and DUH strains determined the range of: soleus mean size, a 10-fold difference from 2.1 to 22.3 mg, respectively; the mean number of extrafusal fibres, a 2.5-fold difference from 497 to 1249; and mean fibre-cross-sectional area, three-fold difference, e.g. for type 1 fibres, from 678 to 1948 μm2. The range of mean proportion of type 1 fibres was determined by C57BL/6J (31%) and DUH (64%) strains. The mean number of spindles per muscle ranged between nine (LG/J) and 13 (BEL) (strain effect P < 0.02). Genetic correlations between spindle count and muscle weight or properties of extrafusal fibres were weak and not statistically significant. However, there was a strong correlation between the proportion of spindles with more than one bag2 fibre and the proportion of extrafusal fibres that were of type 1, and strain-dependent variation in the numbers of such spindles was statistically significant. The numbers of intrafusal fibres per spindle ranged from 2 to 8, with the most common complement of four found in 75.6% of spindles. There were no significant differences between the strains in the mean numbers of intrafusal fibres; however, the variance of the number was significantly less for the C57BL/6J strain than for any of the others. We conclude that abundance of muscle spindles and their intrafusal-fibre composition are substantially determined by genetic factors, which are different from those affecting muscle size and properties of the extrafusal fibres.
Keywords: genetics, intrafusal fibers, proprioceptors
Introduction
Mammalian muscle spindles, the length sensors of skeletal muscle essential for motor control, are remarkably stereotyped in their structure from muscle to muscle both within and between species. Each comprises an encapsulated bundle of highly specialised muscle fibres (intrafusal fibres) innervated by both sensory and motor nerve fibres, the capsule being an expansion of the perineurium of the supplying nerves (reviewed by Banks, 2005). In almost all spindles, three types of muscle fibre make up the intrafusal bundle: in their order of development they are bag2, bag1 and chain fibres, the most common complement being one bag2, one bag1 and two to several chain fibres, depending on species and muscle (Banks et al. 1977). Functionally, bag1 fibres enhance the dynamic response of the primary sensory ending when activated, whereas the static response of the ending is enhanced when bag2 and chain fibres are activated (Bessou & Pagés, 1975; Boyd, 1985). Differentiation of the intrafusal fibres during development seems not to be autonomous, but rather it depends on the sensory innervation. The two principal components of the spindle then become mutually interdependent for maintenance of the differentiated state, with intrafusal fibres as the source of neurotrophin-3 (NT-3) required by the sensory neurons for their continued viability (Copray & Brouwer, 1994; Ernfors et al. 1994). The number of spindles is set in the muscle primordium prior to birth, by the interaction between the sensory neurites and primary myotubes (Zelená, 1994). It is possible that there is an element of competition between sensory neurons and α-motoneurons to establish connexions with the primary myotubes.
The notion that the number of spindles is important and may vary from muscle to muscle in physiologically meaningful ways is as old as the recognition of the role of the spindle as a proprioceptor involved in motor control (Sherrington, 1894). Counts of spindles have usually been made by analysing serial or skip-serial sections of muscles from adults of smaller species such as the rat or guinea pig, or from neonates of larger species including the cat and human (see Banks, 2006 for a summary of the data from these species). When the numbers of spindles are considered in relation to the sizes of the adult muscles, the muscle weight or mass being the most widely used parameter, it is clear that there is a general trend for larger muscles to possess more spindles both between and within species. Until now, however, there has been no study of whether a similar relationship occurs in homonymous muscles of different strains of the same species, when those strains differ consistently in body and muscle size.
Adult muscle size and fibre-type composition are highly variable between individuals, due at least in part to their plasticity. In humans, for example, factors such as the level and type of physical activity and age are important modifiers of the structural and functional properties of muscle. However, as heritability for muscle strength is estimated to be approximately 50% (Arden & Spector, 1997; Carmelli & Reed, 2000), genetic factors clearly play an important role in determining muscle mass, size and strength. Properties of muscle spindles are also subject to the influence of genetic factors. Gene knockout models have shown that disruption of Erbb2 (Andrechek et al. 2002) or the NT-3 coding gene, Ntf3, (Ernfors et al. 1994) in muscle led to the early degeneration or complete absence of spindles, with reduced numbers of spindles in heterozygotes. Conversely the disruption of the myostatin gene, a known suppressor of muscle growth (McPherron et al. 1997), increased both muscle size and spindle number (Elashry et al. 2011). Thus, genetic factors can substantially affect the number of spindles, not merely their presence or absence, and can do so sometimes in concert with extrafusal muscle properties.
The existence of many well-established inbred strains of mice, often with substantially different muscular phenotypes, including cross-sectional area, mass and fibre number, provides an opportunity to examine whether genetic variation influences the number of spindles or their complement of intrafusal fibres. In addition, this research model provides insight into the extent to which muscle size and properties of the spindles are driven by the same genetic factors. Here we describe the results of analysing the soleus muscle of six inbred mouse strains in terms of: the numbers of spindles and of the several types of intrafusal and extrafusal fibres; extrafusal fibre size; and muscle mass.
Methods
Soleus muscles dissected from 10- to-14-week-old mice were analysed. Tissue collection methods were in accordance with the Institution's guide for the care and use of laboratory animals. Males from six inbred strains, BEH (n = 2), BEL (n = 3), C57BL/6J (n = 3), DUH (n = 2), LG/J (n = 2) and SM/J (n = 3) were examined (see Results for definition of the strain abbreviations).
The muscles were frozen in isopentane cooled in liquid nitrogen. The entire length of the muscle was cryosectioned transversely at 10 μm thickness with a cryotome (Leica CM1850UV) at −20 °C. Due to its small size, the soleus in BEL strain was sectioned as a part of the triceps surae muscle group. Every fifth section was mounted on a glass slide and incubated for ATPase activity following acid preincubation at pH 4.47 (Brooke & Kaiser, 1970). The number, length and fibre-type composition of muscle spindles was then determined from the skip-serial sections using a Nikon Optiphot microscope. Micrographs to show details of intrafusal muscle fibres were made using a Hamamatsu C4742-95 digital camera attached to a Zeiss Axio Imager M1 microscope with ×63 PlanApochromat oil-immersion objective. The images were captured with Volocity 6.0 and exported as JPEG format.
Quantitative data are presented as mean ± SD unless stated otherwise. For statistical analysis, the data were compiled in prism5 software (v.5.04; GraphPad, San Diego, CA, USA) or in excel 2007. The agreement of the spindle count data with the normal distribution was examined using the Shapiro–Wilk test. The effect of strain was assessed using a one-way anova. A post-hoc Tukey test was then used to locate the difference. Pearson correlations were carried out to evaluate the association between variables. Genetic correlations were calculated between strain means of the variables (Blizard & Bailey, 1979). Chi-square tests were used to analyse evidence relating to strain-dependency of: (i) the intrafusal-fibre complements of spindles; and (ii) the incidence of spindles with more than one bag2 fibre.
Results
This panel of strains included a classical laboratory mouse strain, C57BL/6J, strains selected for divergent growth, Berlin high (BEH) and Berlin low (BEL) (Bunger et al. 2001a), a strain derived by long-term selection for large body weight, Dummerstorf (DUH) (Bunger et al. 2001b), and strains selected for high and low body weight, LG/J (Goodale, 1938) and SM/J (MacArthur, 1944), respectively. In addition, BEH strain is homozygous for the mutant myostatin gene, an allele known as compact (Varga et al. 2003). These strains were chosen to represent a broad range of variation in muscle mass, and in the number and histochemical properties of extrafusal muscle fibres present in soleus muscles of inbred mouse models (Table 1).
Table 1.
Properties of soleus muscle in the panel of examined strains
| Weight, mg | Fibre count | CSA 1, μm2 | CSA 2A, μm2 | % type 1 | |
|---|---|---|---|---|---|
| BEH*** | 16.6 ± 2.2 | 1063 ± 54 | 1673 ± 158 | 1932 ± 139 | 35 ± 6 |
| BEL*** | 2.1 ± 0.8 | 497 ± 122 | 678 ± 115 | 812 ± 180 | 56 ± 8 |
| C57BL/6J** | 9.8 ± 1.3 | 949 ± 61 | 1423 ± 155 | 1393 ± 160 | 31 ± 2 |
| DUH*** | 22.3 ± 3.7 | 1249 ± 231 | 1948 ± 323 | 2671 ± 427 | 64 ± 11 |
| LG/J* | 10.1 ± 0.9 | 586 ± 62 | 1405 ± 299 | 1423 ± 262 | 45 ± 3 |
| SM/J* | 4.8 ± 0.5 | 562 ± 102 | 875 ± 190 | 957 ± 195 | 37 ± 4 |
Whole muscle properties
Recently reported histological and morphological properties of the soleus muscle from the strains of the selected panel (Lionikas et al. 2010; Carroll et al. 2011; Kilikevicius et al. 2012) showed a great diversity (Table 1). Soleus mass varied over a 10-fold range, from 2.1 ± 0.8 mg (BEL) to 22.3 ± 3.7 mg (DUH), whereas variability in the number of extrafusal fibres was much smaller (2.5× range), from 497 ± 122 to 1249 ± 231 per muscle, with the same strains defining the range. In general, the discrepancy between mass and fibre number was offset by a three-fold difference in fibre cross-sectional area between the two strains, e.g. for type 1 fibres, from 678 ± 115 to 1948 ± 323 μm2. Some of the properties were very closely correlated (Table 2); the range of cross-sectional areas of type 1 and 2A fibres followed a very similar direct relationship both to muscle mass (P < 0.001) and also to each other (P < 0.01). The range was again defined by the same strains, cross-sectional areas being smallest in BEL and largest in DUH. Thus, muscle mass, fibre number and cross-sectional areas covered a considerable range and the genetic correlations, i.e. correlations between the strain means of these indices, were positive and statistically significant (Table 2, P < 0.05 for each possible combination). In contrast, although the proportion of type 1 (slow-twitch, postural) muscle fibres varied two-fold, from 31 ± 2% (C57BL/6J) to 64 ± 11% (DUH), it did not correlate with muscle mass (P = 0.615).
Table 2.
Genetic correlations of muscle spindles with other indices of soleus muscle. Correlation coefficient, r, and associated P value (below)
| Fibre number | CSA1 | CSA2A | % type 1 | Spindles | |
|---|---|---|---|---|---|
| WEIGHT | 0.923 | 0.970 | 0.993 | 0.262 | −0.198 |
| 0.009 | 0.001 | 0.00008 | 0.615 | 0.706 | |
| Fibre number | 0.900 | 0.915 | 0.120 | 0.038 | |
| 0.015 | 0.011 | 0.821 | 0.944 | ||
| CSA1 | 0.948 | 0.112 | −0.313 | ||
| 0.004 | 0.832 | 0.546 | |||
| CSA2A | 0.367 | −0.143 | |||
| 0.474 | 0.788 | ||||
| % type 1 | 0.344 | ||||
| 0.504 | |||||
CSA1, cross-sectional area of type 1 fibres; CSA2A, cross-sectional area of type 2A fibres; % type 1, percentage of type 1 fibres.
Statistically significant correlations are in bold.
Spindle count
The number of muscle spindles in individual solei ranged from 8 to 14. The within-strain variability was small (the largest difference between the most and least spindle-rich muscles of the same strain was two spindles), conferring adequate statistical power for testing strain effect with available samples sizes. Because the data distribution did not deviate from normality (Shapiro–Wilk test, P = 0.075), a one-way anova was applied to assess whether the variation in the number of spindles was affected by between-strain factors. This analysis revealed that the number of muscle spindles did indeed vary significantly (P < 0.02) among strains. Interestingly, soleus of the smallest strain (BEL strain) consistently contained more spindles, 13 ± 1, than either SM/J strain, 10 ± 1 or LG/J, 9 ± 1 (both P < 0.05; Fig. 1), which were 2.3-and 4.8-fold greater in mass, respectively.
Figure 1.
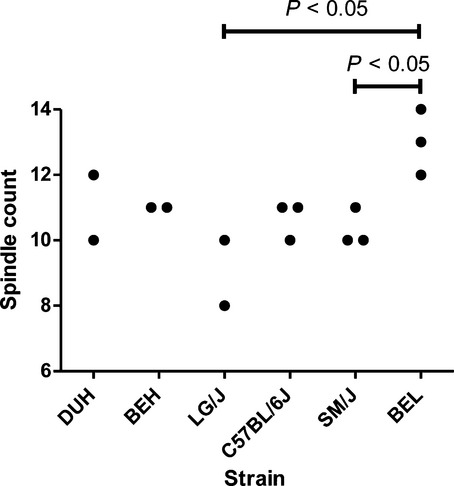
Abundance of spindles in individual soleus muscles from males of six inbred mouse strains.
We then examined the genetic correlation of the number of muscle spindles with other variables characterizing the soleus muscles of the different strains. The r values ranged between −0.31 and 0.33, but none of them approached statistical significance (Table 2; P value range 0.504–0.944).
Intrafusal muscle fibres
The number of intrafusal muscle fibres in each spindle (regarding each spindle as a single encapsulated structure) ranged from 2 to 8, with four the most common complement (124/164 overall, 75.6%). The proportion of spindles with four intrafusal fibres ranged from 68% in SM/J to 87.5% in C57BL/6J, the next most common complements being 3 (9.8% overall) and 5 (6.7% overall) (Fig. 2). All other complements are too rare for any strain-dependent differences that might be present to reach statistical significance with our sample (e.g. the contingency table, Supporting Information Table S1). The mean number of intrafusal fibres per spindle was 4.1 overall, ranging from 3.9 (C57BL/6J and SM/J) to 4.5 (LG/J). One-way anova failed to demonstrate any difference between the strains in this respect. However, C57BL/6J stands out from the rest as being much the least variable; with a coefficient of variation of 5.5% it was significantly different even from the next least variable, SM/J (coefficient of variation 16.0%) by the F test (F = 0.357, P < 0.01 for 31, 30 df), despite the two strains having essentially identical means.
Figure 2.
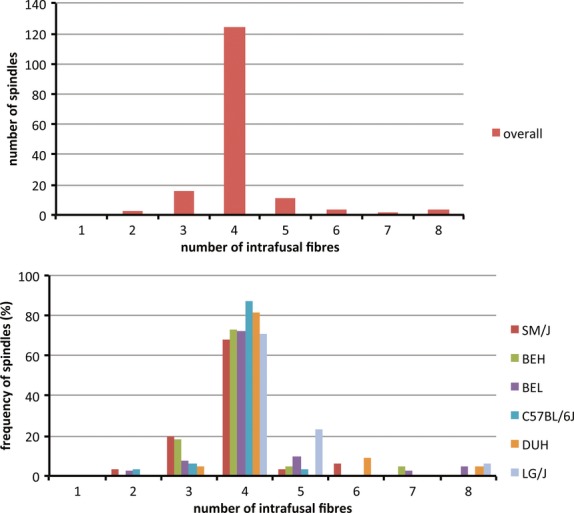
(Top) Bar chart showing the distribution of spindles with different numbers of intrafusal fibres for the complete sample. (Bottom) Histogram comparing the frequency distributions by strain for spindles with different numbers of intrafusal fibres.
It was usually possible to identify all three types of intrafusal fibre by their particular staining intensities, which are similar to those of other mammals (see Banks and Barker, #joa12076-bib-04002004): bag2 fibres exhibit an acid-stable ATPase over almost their entire length, apart from the equatorial (sensory) region; bag1 fibres only show a moderately intense acid-stable ATPase activity close to their extreme ends; chain fibres are almost always shorter than either type of bag fibre and show no acid-stable ATPase at all (Fig. 3). If we regard the complement of one bag2, one bag1, and one or more chain fibres as standard, then all spindles of three intrafusal fibres were of standard composition, as were most (99/124, 79.8%) spindles of four fibres. However, only 4/11 (36.4%) spindles with five fibres were standard, and none of those with more than five fibres was standard. For spindles with four or more intrafusal fibres, all but one of which contained at least one chain fibre, deviations from the standard composition were due to additional bag fibres. This occurred in 41 spindles, of which 35 had two or more bag2 fibres and 15 had two or more bag1 fibres. There was a very strong correlation (r = 0.88) in the proportions of spindles with non-standard complements of bag2 and bag1 fibres among the different strains, as there was between the proportions of type 1 extrafusal fibres and either bag2 (r = 0.89) or bag1 (r = 0.78) (Fig. 4). The strain dependency of the proportion of spindles containing additional bag2 fibres was significant P < 0.05; Table 3), with C57BL/6J having the lowest proportion with more than one bag2 fibre (6.3%) and LG/J the highest proportion (35.3%); but there were insufficient degrees of freedom to test the data for bag1 after grouping to provide sufficiently large expectations.
Figure 3.
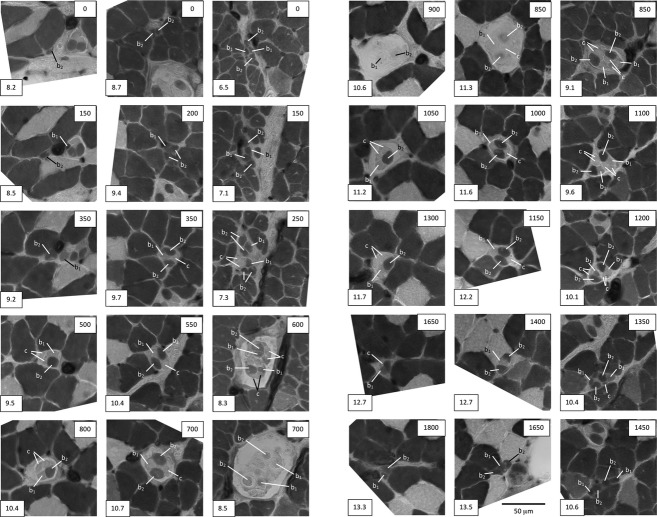
Representative cross-sections taken at intervals throughout three sample spindles from a BEL soleus muscle to show the identification of intrafusal-fibre types, and to illustrate: (left column) the most common complement of one bag2, one bag1 and two chain fibres; (middle column) a spindle with two bag2, one bag1 and one chain fibre; and (right column) one of the largest spindles found in any strain with a complement of two bag2, two bag1 and four chain fibres. The box at the lower left of each micrograph gives the reference for the section in the format: slide number.section number. The box at the upper right gives the nominal distance from the first section in μm. In the spindle shown in the right column, the two bag2 fibres are thought to have branched somewhere between sections 7.1 and 7.3, resulting in the appearance of four profiles in 7.3. Acid-stable ATPase following pre-incubation at pH 4.47; differential staining properties of the three types of intrafusal fibre are described in the text.
Figure 4.
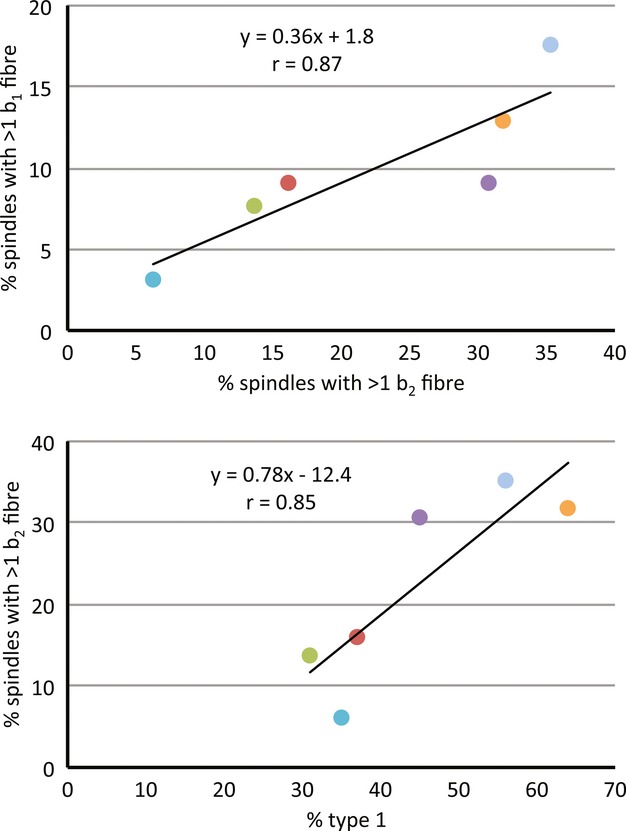
Scatter plots of strain means with least-squares fitted regression lines to show by strain the relationships between: (top) the proportions of spindles with > one bag1 fibre and those with > one bag2 fibre; (bottom) the proportions of spindles with > one bag2 fibre and extrafusal fibres of type 1. A similar relationship was observed between the proportions of spindles with > one bag1 fibre and extrafusal fibres of type 1 (not shown). Symbols are colour-coded to indicate strain as in Fig. 2.
Table 3.
Chi-square goodness-of-fit test showing the strain dependency of the proportion of spindles with > one bag2 fibre. Strains have been grouped to ensure that the expected number of spindles was at least five, the minimum required by the criteria for this test. Expected numbers equal the total number of spindles for the strain (or grouped strains) multiplied by the overall number observed with > one b2 fibres and divided by the overall total number of spindles
| Strain | Total no.of spindles | No. of spindles with > one b2 fibres |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Observed | Proportion | Expected | (o − e)2/e | ||||||
| C57BL/6J and BEH | 54 | 5 | 0.09 | 11.6 | 3.75 | ||||
| SM/J | 31 | 5 | 0.16 | 6.7 | 0.41 | ||||
| BEL | 39 | 12 | 0.31 | 8.4 | 1.57 | ||||
| DUH and LG/J | 39 | 13 | 0.33 | 8.4 | 2.55 | ||||
| Overall | 163 | 35 | 0.21 | χ2 | 8.29 | P < 0.05 for 2 df | |||
Discussion
Effects of genetic variants
Comparison of phenotypes between inbred strains facilitates assessment of the effects of genetic and environmental factors on the trait of interest. Because mice from the same strain are highly isogenic, the within-strain variability indicates the outcome of environmental or random influences. In contrast, the phenotypic differences between strains are largely attributable to genetic differences, which range from hundreds of thousands of single nucleotide polymorphisms (SNP; Frazer et al. 2007) to larger structural variations of the genomes of different strains (Yalcin et al. 2012). Previous studies using the gene knockout approach demonstrated that abundance of muscle spindles can be affected by a variety of factors, particularly growth and trophic factor signalling, including ErbB2, NT-3 and myostatin (Ernfors et al. 1994; Andrechek et al. 2002; Elashry et al. 2011). The finding in the present study, that BEL strain has ≈40% more spindles than the LG/J strain, provides evidence that naturally occurring genetic variability also influences the abundance of these proprioceptors. Whether this involves variability in growth/trophic factor levels is a matter for further study. However, it is interesting that the greater spindle abundance in BEL is occurring independently of either size (smallest cross-sectional area among all the strains) or number (comparable with LG/J and SM/J strains) of the extrafusal fibres.
Muscle size and its underlying indices (fibre number and size) substantially differ among the six studied strains (Table 1), implying a significant contribution of genetic variability to this difference. However, this notion combined with the lack of appreciable genetic correlation (Table 2) between muscle size and spindle number indicates that, whereas genetic factors are important determinants of both these parameters, different sets of genes must be responsible for each of them.
Muscle size and spindle count
Although it has long been appreciated that, in general, larger muscles contain more spindles than smaller ones, the consequences of the non-linearity in this general relationship have only recently been clarified (Banks, 2006). The precise relationship depends on whether comparisons are being made between samples of homonymous muscles from species differing one from another in body mass by an order of magnitude or more, or between the individual muscles comprising almost the whole complement of just one species. In either case, particular muscles may have greater or lesser abundance of spindles than the regression value for a hypothetical muscle of the same mass, when spindle counts are plotted against muscle mass as their logarithmic transforms. In the across-species comparison of homonymous muscles, the overall fractional power function relating spindle number to muscle mass has an exponent very close to 1/3, indicating that the fundamental connexion during evolutionary changes in body (and muscle) size is isometric with respect to linear dimensions (Banks, 2006). The reports of an approximately two-fold increase in muscle mass (McPherron et al. 1997) and 34% increase in spindle counts (mean of 26 and 41%; Elashry et al. 2011) in the myostatin null mouse are consistent with this relationship (20.33 = 1.257). It is interesting, therefore, that in the within-species analysis of the six strains of mice the same relationship is not found between soleus muscles differing in mass by up to an order of magnitude. Indeed, the greatest number of spindles is found in the strain with the smallest muscles, indicating that there is no fundamental causative relationship between the two indices.
Muscle size is a composite variable depending on the properties of extrafusal fibres constituting the muscle, i.e. their number, diameter and length. Each of these properties is affected by distinct mechanisms. For instance, the number of extrafusal fibres in mouse is set during embryogenesis and remains stable through most of its lifespan (Wirtz et al. 1983; Ontell et al. 1988), whereas the diameter of the fibres changes dramatically during postnatal growth (Wirtz et al. 1983) and can be influenced by a wide variety of systemic stimuli. The length of the fibres, on the other hand, is largely determined by mechanisms related to elongation of the bone, which might not be directly related to growth of muscle tissue per se. Therefore, associations of muscle size with other traits should be interpreted with the above mentioned complexity in mind.
Intrafusal muscle fibres
The slight differences in mean number of intrafusal muscle fibres per spindle between the different strains were not statistically significant with our sample sizes. However, the number of intrafusal fibres in each spindle was significantly less variable in C57BL/6J than in all the other strains. Moreover, the only clear relationship between any intrafusal and extrafusal phenotypic character among the different strains was the highly correlated variation in proportions of type 1 extrafusal fibres and of spindles with more than one bag2 fibre. This association could be due to the fact that both type 1 extrafusal and bag2 intrafusal fibres are derived from the same population of primary myotubes during development (see Banks, 2005, for a brief review of spindle development). There is no obvious reason to suppose that the occurrence of additional bag2 fibres in this strain-dependent manner is in any way functionally adaptive; however, it might enhance the static (or tonic) component of the primary endings' responses to stretch in those spindles possessing the additional fibres, given the effects of bag2 activation by static γ stimulation (Banks, 1991).
Conclusions
Our study has revealed that genetic variation plays an important role in determining number of spindles in muscle tissue as well as aspects of their intrafusal-fibre complements. We have also shown that variables such as size of the muscle, number and size of the extrafusal fibres, and proportion of different fibre types, are not associated with the abundance of muscle spindles. The close correlation between the proportions of type 1 extrafusal fibres and of spindles with more than one bag2 fibre may reflect a common developmental mechanism. We conclude that abundance of muscle spindles is determined by genetic factors which are significantly different from those affecting muscle size and properties of the extrafusal fibres.
Acknowledgments
This work was supported by Marie Curie International Reintegration Grant 249156 (A.L.) and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR056280 (A.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors are thankful to Mr. Gordon Stables for the formatting of the figures.
Author contributions
A.L., L.B., R.W.B. and G.S.B. – designed the study, carried out data analyses and wrote the manuscript; C.J.S. and T.L.S. – processed the samples, imaged and analysed image, contributed to writing.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Contingency-table analysis of the numbers of spindles with either the most frequent complement of intrafusal muscle fibres (4) or any other complement (<4 or >4) in different strains of mouse.
References
- Andrechek ER, Hardy WR, Girgis-Gabardo AA. ErbB2 is required for muscle spindle and myoblast cell survival. Mol Cell Biol. 2002;22:4714–4722. doi: 10.1128/MCB.22.13.4714-4722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden NK, Spector TD. Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study. J Bone Miner Res. 1997;12:2076–2081. doi: 10.1359/jbmr.1997.12.12.2076. [DOI] [PubMed] [Google Scholar]
- Banks RW. The distribution of static γ-axons in the tenuissimus muscle of the cat. J Physiol. 1991;442:489–512. doi: 10.1113/jphysiol.1991.sp018805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks RW. The muscle spindle. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. 4th edn. Philadelphia: W. B. Saunders; 2005. pp. 131–150. [Google Scholar]
- Banks RW. An allometric analysis of the number of muscle spindles in mammalian skeletal muscles. J Anat. 2006;208:753–768. doi: 10.1111/j.1469-7580.2006.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks RW, Barker D. The muscle spindle. In: Engel AG, Franzini-Armstrong C, editors. Myology. 3rd edn. New York: McGraw-Hill; 2004. pp. 489–509. [Google Scholar]
- Banks RW, Harker DW, Stacey MJ. A study of mammalian intrafusal muscle fibres using a combined histochemical and ultrastructural technique. J Anat. 1977;123(Pt 3):783–796. [PMC free article] [PubMed] [Google Scholar]
- Bessou P, Pagés B. Cinematographic analysis of contractile events produced in intrafusal muscle fibres by stimulation of static and dynamic fusimotor axons. J Physiol. 1975;252:397–427. doi: 10.1113/jphysiol.1975.sp011150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA, Bailey DW. Genetic correlation between open-field activity and defecation: analysis with the CXB recombinant-inbred strains. Behav Genet. 1979;9:349–357. doi: 10.1007/BF01066973. [DOI] [PubMed] [Google Scholar]
- Boyd IA. Intrafusal muscle fibres in the cat and their motor control. In: Barnes WJP, Gladden MH, editors. Feedback and Motor Control in Invertebrates and Vertebrates. London: Croom Helm; 1985. pp. 123–144. [Google Scholar]
- Brooke MH, Kaiser KK. Three ‘myosin adenosine triphosphatase’ systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Bunger L, Laidlaw A, Bulfield G. Inbred lines of mice derived from long-term growth selected lines: unique resources for mapping growth genes. Mamm Genome. 2001a;12:678–686. doi: 10.1007/s00335001-3018-6. [DOI] [PubMed] [Google Scholar]
- Bunger L, Renne U, Buis RC. Body weight limits in mice – long-term selection and single gene. In: Reeve ECR, editor. Encyclopedia of Genetics. London: Fitzroy Dearbom Publishers; 2001b. pp. 337–360. [Google Scholar]
- Carmelli D, Reed T. Stability and change in genetic and environmental influences on hand-grip strength in older male twins. J Appl Physiol. 2000;89:1879–1883. doi: 10.1152/jappl.2000.89.5.1879. [DOI] [PubMed] [Google Scholar]
- Carroll AM, Palmer AA, Lionikas A. QTL analysis of type I and type IIA fibers in soleus muscle in a cross between LG/J and SM/J mouse strains. Front Genet. 2011;2:99. doi: 10.3389/fgene.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copray JC, Brouwer N. Selective expression of neurotrophin-3 messenger RNA in muscle spindles of the rat. Neuroscience. 1994;63:1125–1135. doi: 10.1016/0306-4522(94)90578-9. [DOI] [PubMed] [Google Scholar]
- Elashry MI, Otto A, Matsakas A. Axon and muscle spindle hyperplasia in the myostatin null mouse. J Anat. 2011;218:173–184. doi: 10.1111/j.1469-7580.2010.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Kucera J. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Frazer KA, Eskin E, Kang HM. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature. 2007;448:1050–1053. doi: 10.1038/nature06067. [DOI] [PubMed] [Google Scholar]
- Goodale HD. A study of the inheritance of body weight in the albino mouse by selection. J Hered. 1938;29:101–112. [Google Scholar]
- Kilikevicius A, Venckunas T, Zelniene R. Divergent physiological characteristics and responses to endurance training among inbred mouse strains. Scand J Med Sci Sports. 2012 doi: 10.1111/j.1600-0838.2012.01451.x. Doi: 10.1111/j.1600-0838.2012.01451.x. [DOI] [PubMed] [Google Scholar]
- Lionikas A, Cheng R, Lim JE. Fine-mapping of muscle weight QTL in LG/J and SM/J intercrosses. Physiol Genomics. 2010;42A:33–38. doi: 10.1152/physiolgenomics.00100.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur JW. Genetics of body size and related characters. I. Selection of small and large races of the laboratory mouse. Am Nat. 1944;78:142–157. [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Ontell M, Hughes D, Bourke D. Morphometric analysis of the developing mouse soleus muscle. Am J Anat. 1988;181:279–288. doi: 10.1002/aja.1001810306. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. On the anatomical constitution of nerves of skeletal muscles; with remarks on recurrent fibres in the ventral spinal nerve-root. J Physiol. 1894;17:211–258. doi: 10.1113/jphysiol.1894.sp000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga L, Muller G, Szabo G. Mapping modifiers affecting muscularity of the myostatin mutant (Mstn(Cmpt-dl1Abc)) compact mouse. Genetics. 2003;165:257–267. doi: 10.1093/genetics/165.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz P, Loermans HM, Peer PG. Postnatal growth and differentiation of muscle fibres in the mouse. I. A histochemical and morphometrical investigation of normal muscle. J Anat. 1983;137:109–126. [PMC free article] [PubMed] [Google Scholar]
- Yalcin B, Wong K, Bhomra A. The fine-scale architecture of structural variants in 17 mouse genomes. Genome Biol. 2012;13:R18. doi: 10.1186/gb-2012-13-3-r18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelená J. Nerves and Mechanoreceptors. London: Chapman & Hall; 1994. Development; pp. 38–55. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contingency-table analysis of the numbers of spindles with either the most frequent complement of intrafusal muscle fibres (4) or any other complement (<4 or >4) in different strains of mouse.