Abstract
Background
Although photoplethysmography and cerebral state index (CSI) have been used as indices in monitoring vital signs perioperatively, there are only a few reports comparing the performance of photoplethysmography with CSI in monitoring anaesthesia depth. The aim of the present study was to clarify features of photoplethysmography in monitoring balanced general anesthesia compared with CSI.
Material/Methods
Forty-five patients undergoing elective operation under general anaesthesia were enrolled in this study. Anaesthesia was induced with target-controlled infusion propofol. The photoplethysmogram, CSI, Modified Observer’s Assessment of Alertness/Sedation Scale (MOAAS), and mean arterial pressure (MAP) were continuously monitored and recorded. Finger photoplethysmogram amplitude (PPGA) and pulse beat interval (PBI) were calculated off-line.
Results
For the period of time from pre-induction to pre-intubation, the coefficient of correlation between MOAAS and CSI was higher than those between MOAAS and PPGA, PBI, and MAP. CSI showed higher prediction probabilities (Pk) to differentiate the levels of MOAAS than did PPGA, PBI, and MAP. PPGA, PBI, and MAP values showed significant differences between before and after intubation, as well as pre- and post-incision (P<0.05), but no significant changes in cerebral state index (P>0.05).
Conclusions
The present study shows that photoplethysmography-derived parameters appear to be more suitable in monitoring the nociceptive component of balanced general anesthesia, while CSI performs well in detecting the sedation or hypnotic component of balanced general anesthesia.
Keywords: Photoplethysmogram Amplitude, Pulse Beat Interval, Cerebral State Index, Modified Observer’s Assessment of Alertness/Sedation Scale, Depth of Anaesthesia
Background
The moderate scheme of general anaesthesia consists of unconsciousness (hypnosis), analgesia, and immobilization [1,2]. During the peri-operative period, because nociception and hypnosis are partially inter-related, deep hypnosis reduces nociception and powerful nociception arouses awakening [3,4]. Clinically, the depth of anaesthesia has always been evaluated by observation of autonomic reactions, such as tachycardia, hypertension, sweating, lacrimation, and the presence of body movement. These indices are helpful measures, but they are not direct indicators of hypnotic and analgesia effects. There is no “gold standard” available for monitoring balanced anaesthesia [5]. Therefore, further direct and reliable methods for monitoring the depth of anaesthesia (both analgesia and hypnosis) are needed.
The photoplethysmogram (PPG) waveform is a non-invasive and readily available optical signal, which is able to measure pulsatile blood volume in the microvascular bed. PPG is inexpensive, noninvasive, and convenient, and has increasingly attracted attention from investigators from diverse domains of science [6]. The PPG waveform contains information related to the balance (or analgesia level) between nociception and anti-nociception undergoing general anaesthesia, which has been ascertained to result in physiological reactions of the autonomic nervous system (ANS) [7–9]. The cerebral state index (CSI), which is obtained by a cerebral state monitor (CSM™ Danmeter, Odense, Denmark), has been recommended as a measure for monitoring the hypnosis component of anaesthesia, but its application has not yet been fully evaluated [10–12]. Furthermore, there are no reports comparing the performance of PPG with CSI in monitoring anaesthetic depth under general anaesthesia.
The aim of the present explorative study was to detect the similarities and differences between finger PPG and CSM™ and to identify a reliable method for monitoring balanced anaesthesia.
Material and Methods
Patients
Sample size calculation was performed using G*Power software (version 3.1, Franz Faul, Universitat Kiel, Germany) [13]. We calculated that we would need to recruit at least 25 patients. The study was approved by the Ethics Committee of Nanjing Hospital, which is affiliated with Nanjing Medical University. With written informed consent from each patient, 45 patients (American Society of Anesthesiologists (ASA) physical status I or II) who were scheduled for elective thyroid or breast surgery were eligible for the research. The ages of these patients varied between 20 and 67 years old. The patient selection exclusion criteria included the following: a history of hypertension or diabetes or overweight; a history of hyperthyroidism or autonomic nerve dysfunction; chronic use of alcohol or psychoactive medication or substance abuse; abnormalities in cardiovascular, pulmonary, renal, hepatic, cerebral nerve system, or fingernail; and rescue medication administered for correcting low heart rate or blood pressure in the study.
Monitoring
The patients were given no premedication. After the patients arrived at the operating room, transfusion of Ringer’s lactated solution was started. The sensor of a reusable pulse oximeter was placed on the index finger of the non-dominant hand for tracing photoplethysmogram, which were recorded automatically by using a serial cable and S/5 Collect™ software (GE Healthcare) running on a portable computer. The CSI monitor composite electrodes were attached according to manufacturer’s instruction to the midline of the patient’s forehead, laterally on the forehead, and on the mastoid process behind the ear. After a basic control for electrode impedance, the monitor computed the index from the primary EEG signals, which offers numerical indices from 0 to 100. The finger photoplethysmogram and cerebral state index were continuously monitored and recorded every 2–4 s. The Modified Observer’s Assessment of Alertness/Sedation Scale was recorded every 30 s to examine the sedation level in the study period. Finger photoplethysmogram amplitude (PPGA) and pulse beat interval (PBI) were calculated off-line. Under general anaesthesia, routine monitoring contains heart rate, MAP, electrocardiography (ECG), saturation of pulse oximetry, and concentration of inspiratory oxygen.
Anaesthesia
Anaesthesia was induced with a target-controlled infusion (TCI) of propofol. The initial concentration of target effect-site was set at 0.5mg/L, regulated in steps of 0.5 mg/L, and adjusted every 3 min until 3 min after the patient lost consciousness and did not respond to pain stimulation (MOAAS 0). Propofol was administered by Diprifusor software (version 2; AstraZeneca), which consists of the pharmacokinetic model depicted by Marsh et al. [14]. After anesthesia induction began, manually operated normo-ventilation was started. Endotracheal intubation was placed after patients were administered fentanyl 4 μ/kg and cisatracurium 0.2 mg/kg i.v. and maintained serum concentration of remifentanil at 4 ng/ml to keep MOAAS at 0. The recording was accomplished 3 min after the skin was incised, and subsequent anaesthesia was adjusted according to clinical need. The operation room temperature was maintained at 23–25°C.
Data collection and statistical analysis
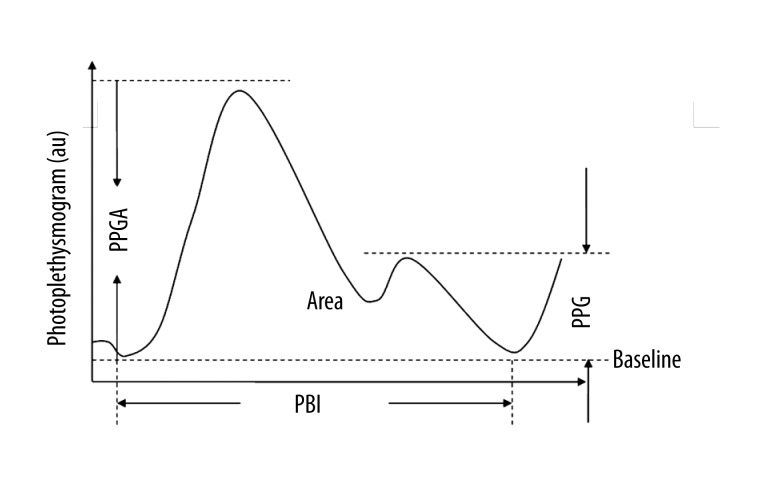
The baseline of all observation indices were recorded during the last 1 min before propofol transfusion was started. Sedation level was estimated every 30 s according to the Modified Observer’s Assessment of Alertness/Sedation (MOAAS) rating scale until loss of response to stimulation in the study [15]. The photoplethysmogram data stored in the portable computer previously was used to compute PPGA and PBI off-line. The baseline of photoplethysmogram amplitude (PPGA(0)) was recorded as 1 arbitrary unit (au), and the PPGA of other points was recorded as the ratio of PPGA(n)/PPGA(0), and the PBI was extracted from photoplethysmogram waveform. Figure 1 clarifies the parameters from a standard photoplethysmogram wave that responds to nociception: photoplethysmogram amplitude (PPGA), notch amplitude (PPG n), pulse beat interval (PBI), and area under the curve (AUC) [16,17]. CSI and MAP were consecutively monitored and recorded. Because the consecutive measurements within 1 subject are correlated, a single value was calculated for each patient as the mean value during the specified period. The statistical comparisons were performed using these single values from different subjects.
Figure 1.
Demonstration of a typical photoplethysmogram (PPG) waveform and its parameters. PPGA (peak amplitude) is defined as the distance from baseline to peak, PPGn (notch amplitude) is defined as the distance from baseline to notch, PBI (pulse beat interval) is defined as the time interval of the adjacent beat-to-beat waveform of PPG, and Area (area under the curve) is above the baseline. The amplitude is measured in arbitrary units (au).
Values are reported as mean ± standard deviation unless otherwise indicated. All data were tested for normal distribution using the Kolmogorov-Smirnov test. We employed the paired-samples T Test to compare these single mean values before and after tracheal intubation, pre-incision, and post-incision. Correlations were calculated between MOAAS and PPGA, PBI, MAP, and CSI, and Spearman correlation coefficients were computed. We calculated the probability that the PPGA, PBI, MAP, and CSI could predict MOAAS using the jack-knife method [18]. A prediction probability (Pk) value of 1 means perfect prediction, whereas a (Pk) value of 0.5 would mean the indicator is useless for predicting the depth of anaesthesia. All tests were 2-tailed, and the threshold of statistical significance was P<0.05. Statistical analyses were all performed using SPSS (version 16.0; SPSS Inc., Chicago, IL, USA).
Results
Four cases were excluded due to technical failure, and another 2 cases were excluded due to rescue medication administration for correcting low heart rate or blood pressure. No patient reported recall of intraoperative awareness when interviewed just prior to discharge. Data from 39 subjects (7 men and 32 women) out of the original 45 were analyzed. The mean (SD) value of their age, weight, and height were 48.4 (11.0) years, 61.7 (6.5) kg, and 164 (5.8) cm, respectively.
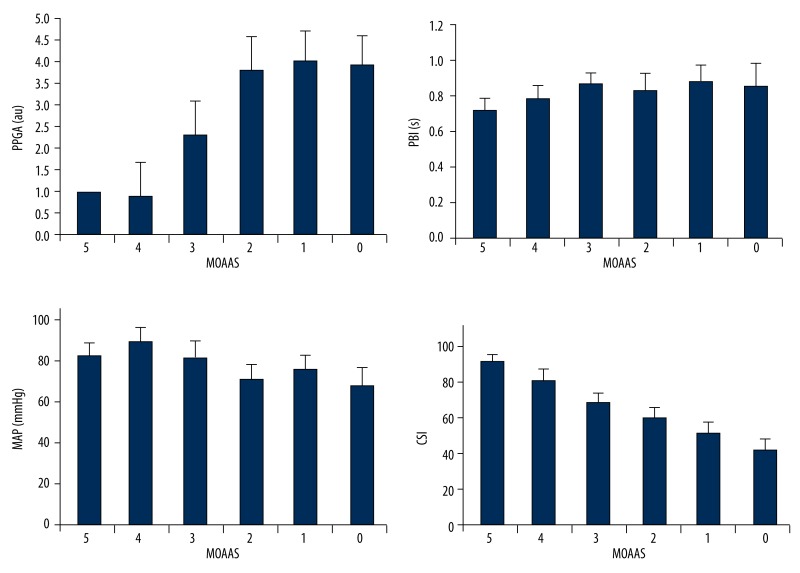
For the period of time from pre-induction to pre-intubation, CSI decreased after induction of anaesthesia. The increase in sedation (MOAAS decreasing from 5 to 0) correlated best with decreases in the values of CSI (from 99 to 41), but was not associated with other indices (Figure 2). The coefficients of correlation among MOAAS and CSI were higher than those between MOAAS and PPGA, PBI, and MAP. CSI showed higher prediction probabilities (Pk) to differentiate the levels of MOAAS than PPGA, PBI, and MAP. The results of Spearman rank correlation among PPGA, PBI, MAP, CSI and the level of MOAAS, and the Pk values of MOAAS for PPGA, PBI, MAP, and CSI are shown in Table 1.
Figure 2.
(A) PPGA, (B) PBI, (C) MAP, and (D) CSI, during different sedation levels (MOAAS 5 to 0).
Table 1.
Spearman rank correlations for four indices vs. the level of MOAAS, and prediction probability (Pk) for four indices to predict MOAAS levels.
| Index | r | Pk |
|---|---|---|
| PPGA | −0.57 | 0.68±0.12 |
| PBI | −0.28 | 0.53±0.14 |
| CSI | 0.92 | 0.94±0.05 |
| MAP | 0.42 | 0.67±0.14 |
Results are shown as mean or mean ±SD. R – Spearman Rank Correlation; Pk – PK for OAA/S Levels; PPGA –photoplethysmogram amplitude; PBI – pulse beat interval; CSI – cerebral state index; MAP – mean arterial pressure.
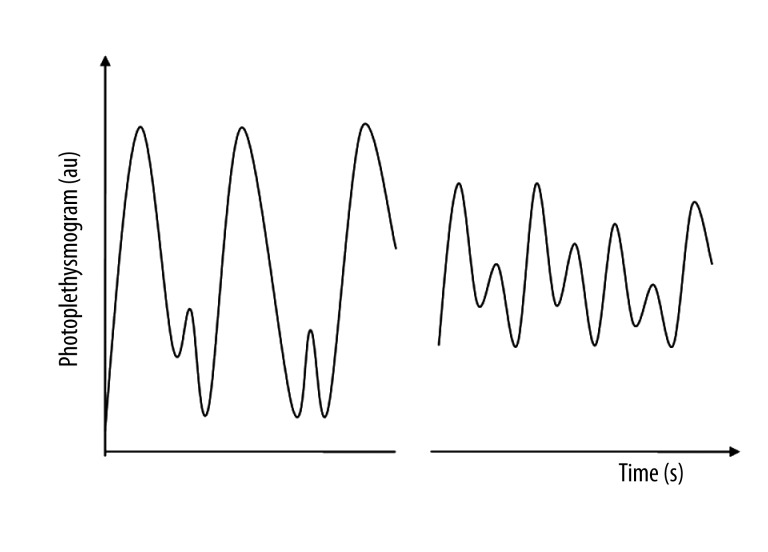
PPGA and PBI decreased and MAP increased during tracheal intubation and skin incision, and the shape flattens and narrows. Figure 3 displays a simulated representative photoplethysmogram waveform. PPGA, PBI, and MAP values showed significant differences between pre- and post-tracheal intubation, as well as pre- and post-incision (P<0.05), but no significant changes in CSI (P>0.05) (Table 2).
Figure 3.
Demonstration of the photoplethysmographic waveform at pre- and post-intubation. The peak amplitude was decreased in the post-intubation, and the shape of the waveform was flattened and narrowed. This was also the case at pre- and post- skin-incision.
Table 2.
Values before and after intubation, before and after skin incision, n=39.
| Endotracheal intubation | Skin incision | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| PPGA | 4.14±1.06 | 2.19±0.6* | 3.8±1.1 | 1.9±0.7* |
| PBI(s) | 0.91±0.11 | 0.82±0.06* | 0.89±0.02 | 0.80±0.08* |
| CSI | 44.3±5.5 | 45.9±5.8 | 44.9±6.3 | 46.6±5.7 |
| MAP (mmHg) | 63.5±8.3 | 72.4±9.1* | 68.7±9.2 | 74.6±8.9* |
Data are presented as mean ±SD. Comparisons by Paired-Samples T Test and * P<0.05 was considered statistically significant. PPGA – photoplethysmogram amplitude; PBI – pulse beat interval; CSI – cerebral state index; MAP – mean arterial pressure.
Discussion
Depth of anaesthesia is a complicated concept, which contains 3 components: hypnosis, analgesia, and relaxation [19]. It is difficult to measure because there is still no “gold standard” for monitoring and defining balanced anaesthesia. PPG technology and EEG-derived variables are rapidly being developed. PPG and CSI are non-invasive, inexpensive, and convenient, which is advantageous in facilitating long-term continuous monitoring. However, there are no reports regarding the similarities and differences between finger PPG and CSM™ in monitoring balanced anaesthesia in Chinese adults.
Photoplethysmography is a non-invasive optical technology that is able to measure blood volume and flow in the peripheral vascular bed. The principle of photoplethysmography is that a light from a light-emitting diode be made to irradiate the skin, then is absorbed, scattered, and reflected by the blood and other tissues. The intensity of the reflected or transmitted light that arrives at the detector is surveyed, and the variations in the photo-probe current are supposed to be sensitive to blood volume and flow change at the measurement site [7,20]. The algorithm for computing CSI adopts 4 sub-parameters of the electroencephalogram: the beta ratio, alpha ratio, beta ratio-alpha ratio, and burst suppression ratio. The virtue of CSI is that a fuzzy inference system was used to formulate the index. CSI is a dimensionless unit scale from 0 to 100, where 0 means a flat electroencephalographic signal and 100 suggests the waking state [21,22].
A standard unit for measuring photoplethysmogram waveform amplitude does not exist, thus the index of the PPGA can only be recorded according to the tendency of context [17]. The baseline of photoplethysmogram waveform amplitude (PPGA(0)) was recorded as 1 arbitrary unit, and PPGA of other points recorded as the ratio of PPGA(n)/PPGA(0), and the PBI was extracted from the photoplethysmogram waveform. Although the MOAAS score has its limitations, it is still used clinically to evaluate a patients’ state of awaking or calm because it shows a good correlation with the hypnotic component of anaesthesia [23]. The Pk is widely used in studying the overall relative performance of the various electroencephalogram-derived indexes to monitor the soporific constituent of anesthesia [24]. Therefore, Pk was employed in the data analysis in the present study.
In our study, the declining level of MOAAS, attributed to the induction with propofol alone, did not correlate with the magnitude change in PPGA, PBI, and MAP, but correlated well with the magnitude of change in CSI. Accordingly, CSI, but not PPGA, PBI, or MAP, was able to accurately differentiate the difference between every 2 adjacent levels of MOAAS. CSI showed a higher Spearman rank correlation coefficient for the level of MOAAS than did PPGA, PBI, and MAP. In addition, the CSI exhibited a significantly higher prediction probability (Pk>0.9) for differentiating different levels of MOAAS than the other 3 indices (P<0.05). Additionally, in the present study, PPGA and PBI decreased and MAP increased during tracheal intubation and skin incision, but there was no significant change in CSI. This is probably because harmful stress response under general anaesthesia is primarily related to the spinal cord, but CSI has a close correlation with hypnosis, which occurs in the cerebral cortex [25]. Noxious stimuli, such as intubation or skin incision, activate the sympathetic nervous system, which in turn increases heart rate and causes peripheral vasoconstriction [26], and then results in changing in PPGA, PBI, and MAP. The findings of present study suggest that photoplethysmography-derived parameters appear to be more suitable for monitoring the nociceptive component of balanced general anesthesia, while the CSI performs well in monitoring the sedation or hypnotic component of balanced general anesthesia. These results are consistent with those of other studies [27,28].
Our study is one of the first clinical investigations to clarify features of photoplethysmography in monitoring balanced general anesthesia, compared with CSI, in Chinese adults. Our study has several limitations. Firstly, we only studied 39 patients; to determine how useful the parameters in monitoring balanced general anesthesia might be, more patients should be studied. Secondly, elderly or overweight patients, and patients with a known history of uncontrolled hypertension, diabetes, a history of hyperthyroidism or dysfunction of autonomic nerve system, a history of neurological or connective tissue diseases, or a history of alcohol or substance abuse were not included in the study. Thirdly, we only used tracheal intubation and skin incision as stimuli, and the intensity of stimulation probably varied between participants. The differences between the responses of parameters to other stimuli should be studied.
Conclusions
In conclusion, the present study shows that photoplethysmography-derived parameters appear to be more suitable in monitoring the nociceptive component of balanced general anesthesia, while CSI performs well in detecting the sedation or hypnotic component of balanced general anesthesia. This suggests that synthesized information from various origins may be necessary for monitoring balanced general anaesthesia. However, further investigations of different or larger groups of patients or all kinds of anaesthesia schemes are needed to clarify features of different monitors and explore the optimal combination of indices in monitoring balanced general anaesthesia.
Footnotes
Source of support: Departmental sources
Conflict of interest disclosures
No sources of funding were used in the preparation of this manuscript. The authors have indicated that they have no conflicts of interests that are relevant to the content of this manuscript.
References
- 1.Wennervirta J, Hynynen M, Koivusalo AM, et al. Surgical stress index as a measure of nociception/antinociception balance during general anesthesia. Acta Anaesthesiol Scand. 2008;52(8):1038–45. doi: 10.1111/j.1399-6576.2008.01687.x. [DOI] [PubMed] [Google Scholar]
- 2.Lamas A, López-Herce J, Sancho L, et al. Responsiveness to stimuli of bispectral index, middle latency auditory evoked potentials and clinical scales in critically ill children. Anaesthesia. 2008;63(12):1296–301. doi: 10.1111/j.1365-2044.2008.05654.x. [DOI] [PubMed] [Google Scholar]
- 3.Antognini JF, Carstens E, Sudo M, Sudo S. Isoflurane depresses electroencephalographic and medial thalamic responses to noxious stimulation via an indirect spinal action. Anesth Analg. 2000;91(5):1282–88. [PubMed] [Google Scholar]
- 4.Röpcke H, Rehberg B, Koenen-Bergmann M, et al. Surgical stimulation shifts EEG concentration-response relationship of desflurane. Anesthesiology. 2001;94(3):390–99. doi: 10.1097/00000542-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Bowdle TA. Depth of anesthesia monitoring. Anesthesiol Clin. 2006;24(4):793–822. doi: 10.1016/j.atc.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Elgendi M. On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rev. 2012;8(1):14–25. doi: 10.2174/157340312801215782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahni R. Noninvasive monitoring by photoplethysmography. Clin Perinatol. 2012;39(3):573–83. doi: 10.1016/j.clp.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Hamunen K, Kontinen V, Hakala E, et al. Effect of pain on autonomic nervous system indices derived from photoplethysmography in healthy volunteers. Br J Anaesth. 2012;108(5):838–44. doi: 10.1093/bja/aes001. [DOI] [PubMed] [Google Scholar]
- 9.Høiseth LØ, Hoff IE, Skare O, Kirkebøen KA, et al. Photoplethysmographic and pulse pressure variations during abdominal surgery. Acta Anaesthesiol Scand. 2011;55(10):1221–30. doi: 10.1111/j.1399-6576.2011.02527.x. [DOI] [PubMed] [Google Scholar]
- 10.Han DW, Linares-Perdomo OJ, Lee JS, et al. Comparison between cerebral state index and bispectral index as measures of electroencephalographic effects of sevoflurane using combined sigmoidal E(max) model. Acta Pharmacol Sin. 2011;32(10):1208–14. doi: 10.1038/aps.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuentes R, Cortínez LI, Struys MM, et al. The dynamic relationship between end-tidal sevoflurane concentrations, bispectral index, and cerebral state index in children. Anesth Analg. 2008;107(5):1573–78. doi: 10.1213/ane.0b013e318181ef88. [DOI] [PubMed] [Google Scholar]
- 12.Hoymork SC, Hval K, Jensen EW, Raeder J. Can the cerebral state monitor replace the bispectral index in monitoring hypnotic effect during propofol/remifentanil anaesthesia? Acta Anaesthesiol Scand. 2007;51(2):210–16. doi: 10.1111/j.1399-6576.2006.01213.x. [DOI] [PubMed] [Google Scholar]
- 13.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–60. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 14.Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth. 1991;67(1):41–48. doi: 10.1093/bja/67.1.41. [DOI] [PubMed] [Google Scholar]
- 15.Jensen EW, Litvan H, Revuelta M, et al. Cerebral state index during propofol anesthesia: a comparison with the bispectral index and the A-line ARX index. Anesthesiology. 2006;105(1):28–36. doi: 10.1097/00000542-200607000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Korhonen I, Yli-Hankala A. Photoplethysmography and nociception. Acta Anaesthesiol Scand. 2009;53(8):975–85. doi: 10.1111/j.1399-6576.2009.02026.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Chen X, Ye S, et al. Changes in the photoplethysmogram with tracheal intubation and remifentanil concentration. Anaesthesia. 2012;67(12):1332–36. doi: 10.1111/anae.12001. [DOI] [PubMed] [Google Scholar]
- 18.Smith WD, Dutton RC, Smith NT. Measuring the performance of anesthetic depth indicators. Anesthesiology. 1996;84(1):38–51. doi: 10.1097/00000542-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Guignard B. Monitoring analgesia. Best Pract Res Clin Anaesthesiol. 2006;20(1):161–80. doi: 10.1016/j.bpa.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson L, Goscinski T, Kalman S, et al. Combined photoplethysmographic monitoring of respiration rate and pulse: a comparison between different measurement sites in spontaneously breathing subjects. Acta Anaesthesiol Scand. 2007;51(9):1250–57. doi: 10.1111/j.1399-6576.2007.01375.x. [DOI] [PubMed] [Google Scholar]
- 21.Anderson RE, Barr G, Jakobsson JG. Cerebral state index during anaesthetic induction: a comparative study with propofol or nitrous oxide. Acta Anaesthesiol Scand. 2005;49(6):750–53. doi: 10.1111/j.1399-6576.2005.00737.x. [DOI] [PubMed] [Google Scholar]
- 22.Brás S, Gouveia S, Ribeiro L, et al. Fuzzy logic model to describe anesthetic effect and muscular influence on EEG Cerebral State Index. Res Vet Sci. 2013;94(3):735–42. doi: 10.1016/j.rvsc.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Jensen EW, Litvan H, Struys M, et al. Pitfalls and challenges when assessing the depth of hypnosis during general anaesthesia by clinical signs and electronic indices. Acta Anaesthesiol Scand. 2004;48(10):1260–67. doi: 10.1111/j.1399-6576.2004.00521.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith WD, Dutton RC, Smith NT. Measuring the performance of anesthetic depth indicators. Anesthesiology. 1996;84(1):38–51. doi: 10.1097/00000542-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Eger EI, II, Koblin DD, Harris RA, et al. Hypothesis: inhaled anesthetics produce immobility and amnesia by different mechanisms at different sites. Anesth Analg. 1997;84(4):915–18. doi: 10.1097/00000539-199704000-00039. [DOI] [PubMed] [Google Scholar]
- 26.Paloheimo MPJ, Sahanne S, Uutela KH. Autonomic nervous system state: the effect of general anaesthesia and bilateral tonsillectomy after unilateral infiltration of lidocaine. Br J Anaesth. 2010;104:587–95. doi: 10.1093/bja/aeq065. [DOI] [PubMed] [Google Scholar]
- 27.Pilge S, Kreuzer M, Kochs EF, et al. Monitors of the hypnotic component of anesthesia-correlation between bispectral index and cerebral state index. Minerva Anestesiol. 2012;78(6):636–45. [PubMed] [Google Scholar]
- 28.Pirneskoski J, Harjola VP, Jeskanen P, et al. Critically ill patients in emergency department may be characterized by low amplitude and high variability of amplitude of pulse photoplethysmography. Scand J Trauma Resusc Emerg Med. 2013;24:21–28. doi: 10.1186/1757-7241-21-48. [DOI] [PMC free article] [PubMed] [Google Scholar]