The purpose of this Commentary is to emphasize the powerful impact rigorous, interdisciplinary exercise medicine research can have on clinical practice and public health policy. Because exercise profoundly influences virtually all aspects of human biology, research on dose-response relationships, exercise-drug/device interactions, exercise genomics, personalized medicine, disease and population specificity, and behavioral medicine offers enormous potential for novel insights into health and disease. With this core concept in mind, here we: (i) briefly summarize the opposing powers of exercise and chronic inactivity; and (ii) discuss the decided advantages of innovative exercise research to advance translational science and ultimately global health.
Exercise vs. Inactivity: Powerful, Opposing Forces that Modulate Health Across the Lifespan
Over the last 100 years the science of exercise has grown from seminal discoveries demonstrating the effects of exercise intensity on vascular control, heat production, oxygen requirement, and lactic acid dynamics – which led to Nobel Prizes in Physiology or Medicine in 1920 (August Krogh, Denmark) and 1922 (A.V. Hill, Britain, and Otto Meyerhof, Germany) – to our modern-day understanding that one's cardiorespiratory fitness (CRF) (indexed by one's maximum rate of oxygen consumption, VO2max) is among the most powerful predictors of morbidity and mortality.1-3
Exercise training – regularly performed, prescriptive, dose titratable physical activity – is increasingly used as an adjuvant therapy across a wide range of human diseases consequent to now documented improvements in cardiac function, muscle oxidative capacity, metabolic health, glucose and lipid homeostasis, adiposity, inflammatory burden, muscle mass and strength, joint pain, mobility function, depression, anxiety, and cognition4, 5 (Figure 1). On the other hand, the evidence is now incontrovertible that poor fitness and low physical activity are major causes of chronic, non-communicable diseases (CNCDs) – e.g., heart disease, stroke, type 2 diabetes, chronic respiratory diseases, and some cancers – which account for approximately 60% of deaths worldwide.6-9 The economic burden attributed to CNCDs reaches hundreds of billions annually in lost productivity and healthcare expenditures; yet, ironically, the bulk of CNCD-related morbidity and mortality is preventable.6, 9
Figure 1.
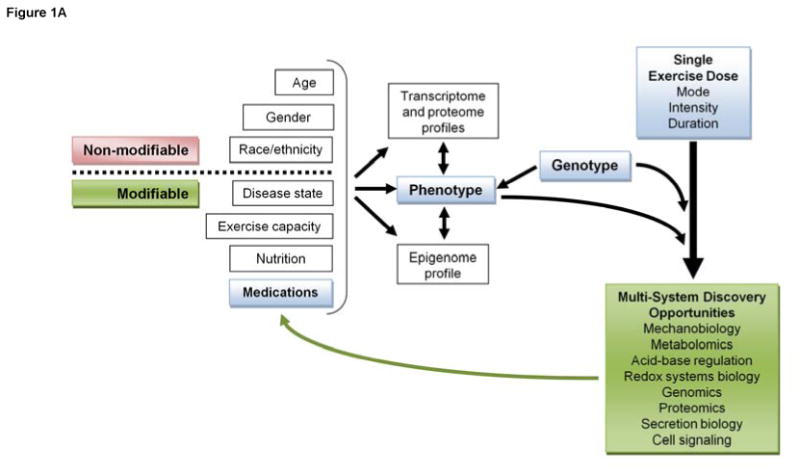
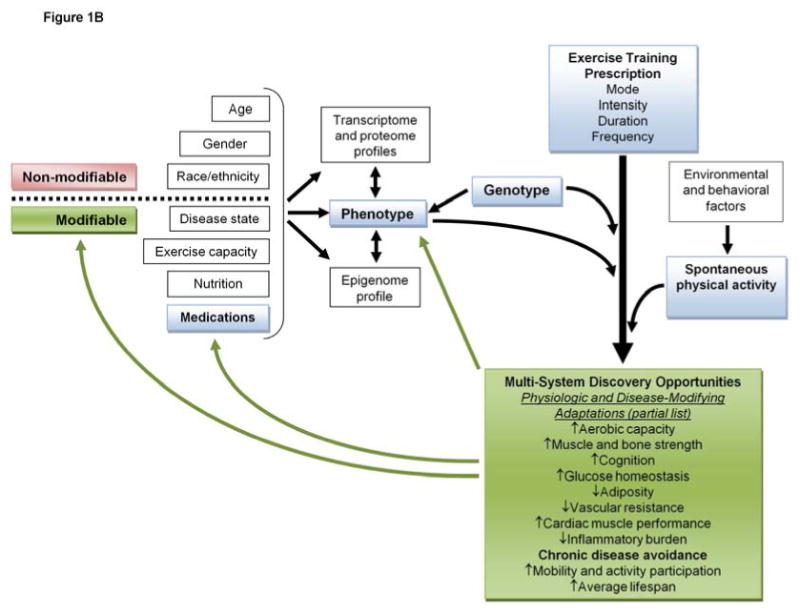
Discovery opportunities driven by (A) acute response exercise research, and (B) exercise training and physical activity research.
Taking Advantage of Exercise Research to Advance Translational Science
While there is general consensus that exercise is integral to the prevention and treatment of chronic disease (i.e. “exercise is medicine”10), the field is ideally positioned for innovative research that would bolster current efforts to transform the translational research enterprise11 by addressing key knowledge gaps.
Discovering new molecules and pathways
Exercise activates a complex array of coordinated cellular and molecular processes involving a wide array of signaling networks and transcriptional regulators that differentially affect virtually every human tissue and organ system12. As such, even a single exercise bout can be used experimentally as a quantifiable, reproducible perturbation to cellular homeostasis (Figure 1A) that may well reveal novel mechanisms regulating biological adaptations to long-term exercise training (Figure 1B) that prevent or manage disease. Taking advantage of exercise potency for such discovery purposes is exemplified by the seminal 2012 discovery that exercise-induced autophagy plays a key role in glucose homeostasis and is largely controlled by B-cell lymphoma 2 (BCL2)13. Moreover, researchers in the exercise field have been largely responsible for the recent discoveries that skeletal muscle is an endocrine organ, that secretion of growth factors and novel myokines is sensitive to the level of contractile activity, and that exercise-induced myokine secretion may play important disease-fighting roles14-16. This area is ripe for future research expected to contribute enormously to the translation of science into real benefit for humans.
Exercise-drug/device interactions
It is important to consider levels of physical activity and carefully evaluate disease-specific exercise interventions prior to medication usage, and certainly as an integral component in the development and evaluation of any drug or device therapy. There is a solid rationale for such a strategy, particularly considering that a lifestyle intervention (i.e. moderate intensity physical activity combined with a reduced calorie diet) out-performed metformin in a large population of obese individuals at risk for type 2 diabetes, and the benefits of physical activity + diet (vs. metformin) were actually underestimated due to intent-to-treat analysis17. The United States Food and Drug Administration (FDA) seems to concur with this approach. In its draft guidance document released in 2007 for industry developing weight loss drugs, the FDA recommended that “the use of weight-management products should be contemplated only after a sufficient trial of lifestyle modification (diet and exercise) has failed.”18 Furthermore, the FDA recommended that Phase 3 trials for weight loss products be conducted with a lifestyle modification as the “placebo” condition.18 In our view this approach should prevail throughout all phases of drug/device development including the pre-clinical studies in laboratory animals. The sedentary, overfed state of typical laboratory mice and rats leads to obesity, metabolic syndrome, and early mortality compared to counterparts in the wild, raising the question of whether this “couch potato” state of caged laboratory animals is an appropriate control condition since its profound effects may skew and even mislead drug efficacy results.19 On the other hand, for some applications one could argue that this is a realistic pre-clinical model based on the current prevalence of obesity and sedentary behavior among U.S. adults and children.
Exercise can profoundly influence drug pharmacokinetics20; thus there is a need for adjunct exercise + drug testing to determine if exercise training enhances or interferes with drug outcomes, or vice versa. For example, in HIV+ patients with insulin resistance and central adiposity, exercise training enhanced the insulin sensitizing effects of pioglitazone.21 On the other hand, 40 mg/d simvastatin completely abrogated beneficial endurance exercise training adaptations including enhanced CRF and skeletal muscle mitochondrial function.22 Exercise performance can also serve as a valuable biomarker of drug/device efficacy. For example, among heart failure patients, the acute heart rate response to a single bout of moderate exercise is a valuable, independent predictor of failure to respond appropriately to cardiac resynchronization therapy.23 The popular understanding that exercise improves health is reflected (albeit, often with damaging hyperbole) in media attention given to exercise discoveries that attract some to suggest that single-target drugs can mimic the complex, multi-system effects of actual exercise training (Figure 1). Clearly there is no single mediator capable of recapitulating all of the physiological adaptations to exercise and assertions that promote “exercise pills”24 are misleading. On the other hand, exercise research can very effectively inform and perhaps expedite the search for medicines that enhance the benefits of physical activity in patients with physical (e.g., mobility impairment25, 26) or physiological (e.g. abnormal pressor responses27, 28) constraints that otherwise limit exercise tolerance and thus prescription options.
Drug repurposing
Exercise fits into the overall public health interests of lower cost, readily available therapies, and should be considered within the context of primary prevention, cure acceleration, and drug development. In the U.S., for example, it is an average of 14 years from target discovery to FDA approval of a new drug, failure rate exceeds 95 percent, and the cost per successful drug is more than $1 billion, after adjusting for all of the failures.11 Inclusion of exercise as a comparator or adjunct therapy in pre-clinical and clinical efficacy studies would likely streamline the process and ensure that the outcomes are translatable.
A major research priority in translational science is to expedite the time to effective cures or disease management by finding new, alternative uses of currently approved medications. Exercise can and should be used as a powerful tool in this process, as exemplified by the elegant, pre-clinical work of Srinivasan et al.29 In their search for effective ways to prevent osteoporosis, these investigators found that supplementing exercise-mediated mechanical loading with low-dose cyclosporin A completely rescued loading-induced bone formation in the aged skeleton. One can envision capitalizing on any number of putative as well as novel physiological adaptations to specific modes of exercise (Figure 1) to advance drug repurposing.
Dose-response relationships and exercise genomics
Like any other treatment the desired outcomes of exercise training are highly dependent on the type and dose of the prescription and genetic variation, with substantial gaps in knowledge remaining.4 An individual's “trainability” – ability to adapt optimally and thus reap the full benefits of any specific exercise training prescription – is likely determined by genetic, environmental, and phenotypic factors. Molecular signatures predictive of trainability are emerging,30 opening a new frontier of “exercise genomics”31 research that is positioned to advance personalized medicine in both prevention and therapeutics. Additionally, well-controlled exercise trials comparing doses (e.g.,32) in a disease-specific manner are sorely needed and should take full advantage of advances in genomics, epigenetics, proteomics, metabolomics, stem cell biology, and imaging technologies to shed light on the complexity of exercise adaptation. These tools present rich opportunities for innovative and translational research.
Exercise adherence and behavioral medicine
A major challenge in the field that affects clinical application of exercise medicine among healthcare providers is a perception among many that, despite the proven benefits, adherence to unsupervised exercise prescription is poor. In addition to patient outcomes, exercise adherence rates profoundly influence hypothesis testing in the research setting.33 The 16-center Look AHEAD trial included a number of behavioral strategies to promote adherence to an at-home lifestyle intervention (diet + moderate physical activity) in obese and overweight type 2 diabetics, and several encouraging secondary outcomes were noted in the short term.34 On the other hand, the discouraging incidence (not different from education control) of cardiovascular events (primary outcome) in the long term led to early closure of Look AHEAD following a futility analysis.35 While adherence to the clinic visit schedule during the first year (1-3 group meetings and 1 individual session per month) was ∼80%,34 it is certainly possible that variable adherence to the daily/weekly at-home diet and exercise recommendations over the long term played a significant role in the futile result. There is a remarkable need, and therefore opportunity, for interdisciplinary research in behavioral medicine to identify key factors influencing exercise adherence, as well as intervention strategies that foster lasting behavioral change. In fact, understanding inter-individual variability in exercise adherence is an emerging science, and a few genetic and non-genetic predictors of adherence have been described recently.33
Exercise is powerful medicine
Apart from the attractive opportunities to exploit the exercise stimulus for discovery and in drug/device development, it must be emphasized that exercise itself is a proven and readily available therapy that has demonstrated a high therapeutic index in a number of disease states with little to no side effects.4 While the issue of adherence cannot be overlooked, the power of exercise in CNCD treatment and protection from CNCD-related morbidity and mortality is profound.4 In fact, in their recent synthesis of current data on the protective effects of exercise training against cardiovascular disease, Joyner and Green36 demonstrated that exercise-mediated protection far exceeds the degree of protection predicted based on traditional risk factors. For example, exercise-mediated reductions in low-density lipoprotein (LDL) cholesterol or increases in high-density lipoprotein (HDL) are only modest;37, 38 yet risk of cardiovascular disease is reduced upwards of 50%, which rivals or exceeds the cardioprotection associated with statins39 without the adverse effects. This poorly understood “risk factor gap” shows the potency of exercise, and points to the significant opportunities for potentially pivotal exercise-related discoveries.
Today, rehabilitative approaches after surgery of various types focus heavily on early mobility and prescriptive exercise (e.g., coronary bypass40), as opposed to prescribed long-term disuse or bed rest just a few decades ago. In fact, the success of exercise training in cardiac rehabilitation has led to the current push for insurance-sponsored prevention programs that would extend to a number of cardiometabolic disease states.40 Beyond treatment of CNCDs, exercise prescription has shown great promise in rare and orphan diseases, which presents an enormous opportunity for future research. Pharmacological therapies clearly are a necessity for some diseases and health crises. However, for many conditions, exercise can serve as a positive behavioral and physiological modifier of the disease process. For example, myopathies such as limb-girdle muscular dystrophy,41 as well as neurodegenerative diseases (e.g., Parkinson's disease42), benefit from exercise. Unfortunately data are lacking to guide exercise prescriptions for many diseases; thus large-scale (Phase 3 equivalent) research focused on dose-response efficacy would have a powerful impact.
Summary and Recommendations for Future Research
While a host of benefits are undeniable, to fully capitalize on exercise pluripotency, key knowledge gaps demand robust research in (at least) five priority areas: 1) optimizing exercise dosing in a disease or population-specific manner to streamline clinical care; 2) a better understanding of exercise-drug/device interaction, synergism, or antagonism; 3) genetic and non-genetic determinants of both exercise responsiveness and adherence, enabling personalized treatments and advances in behavioral medicine; 4) mechanisms by which physical inactivity across the lifespan fosters development and progression of CNCDs; and 5) taking advantage of the potent exercise stimulus to discover new molecular targets and potential re-purposing uses for currently available medications. In summary, we strongly recommend that exercise medicine research be embraced as a grand opportunity to advance knowledge that will help abate the global health crises driven largely by common CNCDs, and to find effective therapies for rare and neglected diseases.
Acknowledgments
The ideas and opinions expressed here are solely those of the authors and have not been endorsed by any government or private entity. The authors declare no competing interests. Manuscript preparation was supported in part by the UAB Center for Exercise Medicine (MMB) and R01DK074825 (PDN). Editorial review of the manuscript prior to submission was supported by the UC Irvine CTSA Grant UL1TR000153 and UAB CTSA Grant UL1TR00165.
List of Abbreviations
- VO2max
maximum rate of oxygen consumption
- CRF
cardiorespiratory fitness
- CNCDs
chronic, non-communicable diseases
- FDA
United States Food and Drug Administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999 Oct 27;282(16):1547–1553. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 2.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009 May 20;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 3.Kaminsky LA, Arena R, Beckie TM, et al. The importance of cardiorespiratory fitness in the United States: the need for a national registry: a policy statement from the American Heart Association. Circulation. 2013 Feb 5;127(5):652–662. doi: 10.1161/CIR.0b013e31827ee100. [DOI] [PubMed] [Google Scholar]
- 4.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011 Jul;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 5.Bateman LA, Slentz CA, Willis LH, et al. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise - STRRIDE-AT/RT) Am J Cardiol. 2011 Sep 15;108(6):838–844. doi: 10.1016/j.amjcard.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daar AS, Singer PA, Persad DL, et al. Grand challenges in chronic non-communicable diseases. Nature. 2007 Nov 22;450(7169):494–496. doi: 10.1038/450494a. [DOI] [PubMed] [Google Scholar]
- 7.Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 8.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004 Mar 10;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 9.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ACSM. Exercise is Medicine: American College of Sports Medicine and American Medical Association. 2011 http://www.exerciseismedicine.org/index2.htm [2007; http://www.exerciseismedicine.org/index2.htm.
- 11.NIH. Restructuring the National Institutes of Health to advance translational science 2011. [Accessed 30 November 2011];2011 http://www.nih.gov/about/director/ncats/NCATSbudget.pdf.
- 12.Booth FW, Shanely RA. The biochemical basis of the health effects of exercise: an integrative view. Proc Nutr Soc. 2004 May;63(2):199–203. doi: 10.1079/pns2004337. [DOI] [PubMed] [Google Scholar]
- 13.He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012 Jan 11; doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem. 2012 Apr 6;287(15):11968–11980. doi: 10.1074/jbc.M111.336834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012 Aug;8(8):457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 17.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002 Feb 7;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.FDA. Draft guidance for industry developing products for weight management. HHS. 2007 [Google Scholar]
- 19.Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci U S A. 2010 Apr 6;107(14):6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenz TL. The effects of high physical activity on pharmacokinetic drug interactions. Expert Opin Drug Metab Toxicol. 2011 Mar;7(3):257–266. doi: 10.1517/17425255.2011.553190. [DOI] [PubMed] [Google Scholar]
- 21.Yarasheski KE, Cade WT, Overton ET, et al. Exercise training augments the peripheral insulin-sensitizing effects of pioglitazone in HIV-infected adults with insulin resistance and central adiposity. Am J Physiol Endocrinol Metab. 2011 Jan;300(1):E243–251. doi: 10.1152/ajpendo.00468.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikus CR, Boyle LJ, Borengasser SJ, et al. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol. 2013 Apr 10; doi: 10.1016/j.jacc.2013.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maass AH, Buck S, Nieuwland W, Brugemann J, van Veldhuisen DJ, Van Gelder IC. Importance of heart rate during exercise for response to cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2009 Jul;20(7):773–780. doi: 10.1111/j.1540-8167.2008.01422.x. [DOI] [PubMed] [Google Scholar]
- 24.Narkar VA, Downes M, Yu RT, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008 Aug 8;134(3):405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamb SE, Guralnik JM, Buchner DM, et al. Factors that modify the association between knee pain and mobility limitation in older women: the Women's Health and Aging Study. Ann Rheum Dis. 2000 May;59(5):331–337. doi: 10.1136/ard.59.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh JA, Lewallen DG. Predictors of activity limitation and dependence on walking aids after primary total hip arthroplasty. J Am Geriatr Soc. 2010 Dec;58(12):2387–2393. doi: 10.1111/j.1532-5415.2010.03182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyai N, Arita M, Miyashita K, Morioka I, Shiraishi T, Nishio I. Blood pressure response to heart rate during exercise test and risk of future hypertension. Hypertension. 2002 Mar 1;39(3):761–766. doi: 10.1161/hy0302.105777. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler MG, Ruiz-Ramon P, Shapiro MH. Abnormal stress responses in patients with diseases affecting the sympathetic nervous system. Psychosom Med. 1993 Jul-Aug;55(4):339–346. doi: 10.1097/00006842-199307000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasan S, Ausk BJ, Prasad J, et al. Rescuing loading induced bone formation at senescence. PLoS Comput Biol. 2010;6(9) doi: 10.1371/journal.pcbi.1000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timmons JA, Knudsen S, Rankinen T, et al. Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. J Appl Physiol. 2010 Jun;108(6):1487–1496. doi: 10.1152/japplphysiol.01295.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouchard C. Overcoming barriers to progress in exercise genomics. Exerc Sport Sci Rev. 2011 Oct;39(4):212–217. doi: 10.1097/JES.0b013e31822643f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slentz CA, Bateman LA, Willis LH, et al. Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab. 2011 Nov;301(5):E1033–1039. doi: 10.1152/ajpendo.00291.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herring MP, Sailors MH, Bray MS. Genetic factors in exercise adoption, adherence and obesity. Obes Rev. 2013 Sep 15; doi: 10.1111/obr.12089. [DOI] [PubMed] [Google Scholar]
- 34.Unick JL, Beavers D, Jakicic JM, et al. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the Look AHEAD trial. Diabetes Care. 2011 Oct;34(10):2152–2157. doi: 10.2337/dc11-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013 Jul 11;369(2):145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol. 2009 Dec 1;587(Pt 23):5551–5558. doi: 10.1113/jphysiol.2009.179432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halverstadt A, Phares DA, Wilund KR, Goldberg AP, Hagberg JM. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metabolism. 2007 Apr;56(4):444–450. doi: 10.1016/j.metabol.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Green DJ, O'Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol. 2008 Aug;105(2):766–768. doi: 10.1152/japplphysiol.01028.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008 Nov 20;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 40.Kwan G, Balady GJ. Cardiac rehabilitation 2012: advancing the field through emerging science. Circulation. 2012 Feb 21;125(7):e369–373. doi: 10.1161/CIRCULATIONAHA.112.093310. [DOI] [PubMed] [Google Scholar]
- 41.Sveen ML, Jeppesen TD, Hauerslev S, Krag TO, Vissing J. Endurance training: an effective and safe treatment for patients with LGMD2I. Neurology. 2007 Jan 2;68(1):59–61. doi: 10.1212/01.wnl.0000250358.32199.24. [DOI] [PubMed] [Google Scholar]
- 42.Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2008 Apr 15;23(5):631–640. doi: 10.1002/mds.21922. [DOI] [PubMed] [Google Scholar]


