Abstract
Particulate matter (PM) varies in chemical composition and mass concentration based on a number of factors including location, season, source and particle size. The aim of this study was to evaluate the in vitro and in vivo toxicity of coarse and fine PM simultaneously collected at three rural and two urban sites within the metropolitan New York City (NYC) region during two seasons, and to assess how particle size and elemental composition affect toxicity. Human pulmonary microvascular endothelial (HPMEC-ST1.6R) and bronchial epithelial (BEAS-2B) cell lines were exposed to PM (50 μg/mL) and analyzed for reactive oxygen species (ROS). Mice (FVB/N) were exposed by oropharyngeal aspiration to 50 μg PM, and lavage fluid was analyzed for total protein and PMN influx. The ROS response was greater in the HPMEC-ST1.6R cell line compared to BEAS-2B cells, but the responses were significantly correlated (p<0.01). The ROS response was affected by location, locale and the location:size interaction in both cell lines, and an additional association for size was observed from HPMEC-ST1.6R cells. Urban fine PM generated the highest ROS response. In the mouse model, inflammation was associated with particle size and by a season:size interaction, with coarse PM producing greater PMN inflammation. This study showed that the aerodynamic size, locale (i.e. urban versus rural), and site of PM samples affected the ROS response in pulmonary endothelial and epithelial cells and the inflammatory response in mice. Importantly, these responses were dependent upon the chemical composition of the PM samples.
Keywords: Aspiration, in vitro exposure, particulate matter, reactive oxygen species
Introduction
Ambient particulate matter (PM) of varying particle size ranges has been shown to originate from a number of anthropogenic and natural sources. Coarse thoracic PM (particles with aerodynamic diameters between 2.5 and 10 microns, PM10-2.5) tend to originate from resuspended dust, mechanical shearing and bioaerosols such as pollen and spores. Fine PM (aerodynamic diameters less than 2.5 microns, PM2.5) typically originate from fuel combustion, biomass burning and secondary particles resulting from atmospheric transformations of gaseous precursors, although there is some overlap between the above-mentioned sources and size fractions. Because the relative contributions of these sources vary with location, the chemical composition and concentration of PM is routinely observed to vary among sampling locations (Brunekreef & Forsberg, 2005; Sarnat et al., 2010; Shridhar et al., 2010).
Epidemiological studies have consistently found associations between ambient air PM concentrations and health effects. PM2.5 has been most closely associated with excess cardiovascular mortality (Dockery et al., 1993; Pope et al., 2002, 2004), lung cancer (Turner et al., 2011) and cardiac arrhythmias (Peters et al., 2000), and such effects vary in strength with sampling location, season and size fraction (Zanobetti & Schwartz, 2009). Coarse PM has been associated with respiratory disease (Host et al., 2008; Lin et al., 2005) and cardiopulmonary morbidity (Malig & Ostro, 2009), although some research studies have not been able to show any clear associations with health effects (Chen et al., 2011; Peng et al., 2008; Puett et al., 2009). Many of the contradictions from epidemiological work on coarse PM arise from findings that are confounded by the co-presence of fine PM, low exposure levels, and/or the lack of statistical power in single city or regional studies (Malig & Ostro, 2009; Perez et al., 2009).
Toxicological studies have confirmed the biological plausibility of the findings of epidemiology studies and demonstrated that PM-induced toxic effects are often dependent upon PM size and composition. In one study, size fractions of PM were collected at rural and urban locations in France. In vitro biomarkers of oxidative stress were affected in a composition- and size-dependent manner, with fine PM generating the greatest response (Stephanie et al., 2011). In a similar study conducted in the Netherlands, Steenhof et al. (2011) exposed a murine macrophage cell line to different size fractions of PM from eight different sampling sites and determined that the in vitro release of proinflammatory markers in response to the PM samples differed among locations and size fractions. Thus, the evidence to date suggests that toxicological responses differ between urban and rural PM samples as well as PM size fraction. These differences in toxicity might be due to different sources of PM within each size fraction. It must be noted, however, that the contribution of a PM pollution source is not exclusive for a given size range and, therefore, there can be an overlap between sources of fine and coarse PM.
Changes in the composition of PM due to temporal variations in source emissions, atmospheric chemistry, presence of allergens, temperature and meteorological conditions, may also contribute to fluctuations in health effects observed at different sites and/or during different seasons (Peng et al., 2005). As seen in work by Halatek et al. (2011), in which different size fractions from four European cities were analyzed during three different seasons, the seasonal effects showed significant differences for coarse and fine PM in terms of biological and cytological in vivo bioassay parameters such as MIP-2, TNF-α, total protein, neutrophils and macrophages.
The aim of this study was to evaluate the relative in vitro and in vivo toxicity of two different PM size fractions (coarse thoracic and fine PM) at both rural and urban sites in the New York City (NYC) metropolitan area during two seasons (summer and winter). In addition, PM greater than 10 microns (defined here as super-coarse PM), a poorly studied size fraction of ambient particles, was also evaluated for one rural and one urban sampling location. Furthermore, we sought to determine whether our in vitro findings were predictive of in vivo effects by evaluating urban and rural PM samples in a mouse model of lung inflammation and injury. Trace element and endotoxin concentrations of the PM samples were evaluated for correlations of toxicity-related endpoints with chemical composition. By restricting our sampling to 48 h at each location, we were able to achieve sufficient temporal discrimination for identifying sources and components of PM that contributed to the observed acute effects in the in vitro and in vivo bioassays. Although ultrafine PM (<0.1 μm) is an important sub-fraction of fine PM and has potentially unique toxicological properties, it was not evaluated separately in the current study.
Methods
PM collection
Sampling
Ambient coarse and fine PM samples were simultaneously collected every 48 h for one month in the NYC metropolitan area at two urban and three rural locations during the summer and winter. Three locations (Tuxedo, NY; Wallkill, NY; and Goshen, NY) were rural, and two locations (Bronx, NY and Manhattan, NY) were urban. The sampling location at Tuxedo is within 21 935 acres of a natural forest habitat in Orange County, NY. Sampling in Wallkill and Goshen were done at a small non-active farm and a rural residential area, respectively. All rural sampling was done at near-ground level. The urban samplers were located on the roof of a one-story building in Manhattan and at near-ground level in a residential area of the Bronx. Winter samples were collected in February/March 2009 and summer samples were collected in June/July 2009.
PM samples were simultaneously collected at the five locations with two-stage (PM10-2.5 and PM2.5) high volume impaction stages (ChemVol model 2400, BGI, Inc., Waltham, MA) at 900 L/min. The coarse PM fraction was collected on a polyurethane foam substrate (PUF; McMaster-Carr, Atlanta, GA) and the fine PM fraction was collected on 17 cm polypropylene substrate (G5300, Manadnock Non-Wovens, Mount Pocono, PA). Additionally, particles larger than 10 microns (super-coarse PM) were pre-collected on a PUF substrate on the uppermost stage of the cascade impactor at the Wallkill and Bronx sites during the summer. All PM collection substrates were pre-cleaned using alcohol and sterile water solutions and dried prior to sampling. Because the collected samples were to be used in in vitro and in vivo bioassays, the impactor stages were initially sterilized, and the substrates were handled under near-sterile conditions prior to and during operation in the field. All samples were stored at −20 ºC after collection. Prior to and following each collection, PUF substrates were weighed using standard operating procedures in an environmentally controlled room (20 ºC to 23 ºC and 38%–42% relative humidity) on a microbalance (XS105 DualRange, Mettler-Toledo, Columbus, OH) to determine the amount of collected PM for each size fraction.
Recovery of PM extracts
Coarse and super-coarse PUFs were transferred to 50 mL glass tubes and pre-wetted with 5 mL of 70% ethanol. Sterile Milli-Q water (25 mL for coarse PUF and 23 mL for super-coarse) was added to completely submerge the PUFs. 22 mL of 70% ethanol was used to pre-wet the fine substrate and 110 mL of Milli-Q was added to submerge it. Tubes were sonicated for 1 h, shaken and sonicated for another hour. Extracted PM was transferred to pre-weighed, sterile, polypropylene containers and archived in a −70 ºC freezer. Extracted samples were lyophilized, resuspended and aliquots were stored at −70 ºC.
PM characterization
All filter extracts were microwave digested and analyzed by inductively coupled plasma mass-spectroscopy (ICP-MS) for trace element analysis. Thawed 1 mg/mL PM samples were sonicated in an ultrasonic water bath for 20 min. For each sample, 100 μL of PM and 200 μL of 16 M HNO3 (Optima-grade, Thermo Fisher Scientific, Waltham, MA) were added into pre-cleaned 6 mL (perfluoroalkoxy) PFA digestion vials. Two vials were added into large digestion vessels containing a Teflon spacer and 10 mL of Milli-Q water. The samples were digested with a 2-step microwave program using a MDS-200 Microwave Laboratory Digestion System (CEM Corp., Worcestershire, UK): (1) pressure =50 psi, ramp time =30 min, time at pressure =20 min; and (2) cool down with no power for 30 min. Following the digestion, 5 mL Milli-Q water was added to bring the final acid concentration to approximately 4%. The elemental composition was determined by magnetic sector ICP-MS at the Lamont-Doherty Earth Observatory of Columbia University. To 800 μL of each PM sample, 40 μL of 1 ppm indium (1% HNO3), 80 μL of 2% nitric acid and 680 μL of Milli-Q water were added. Standard curves were run both in selected samples (i.e. standard additions) and in clean 1% nitric acid by means of a mixed multi-element standard added in volumes of 0, 40 and 80 μL in place of an equivalent volume of 2% nitric acid. Samples were analyzed on an Axiom single-collector magnetic sector ICP-MS (VG Elemental, Winsford, UK). Indium-normalized sample data were quantified by comparison with standard curves. The elements measured were: Be, Mg, P, S, K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Sr, Ag, Cd, Sn, Sb, Cs, La, Pb and Tl. Procedural blanks and NIST standard reference 1648 samples were incorporated into every digestion run for quality assurance. Appropriate procedural blank subtractions were made as previously described (Kinney & Thurston, 1993).
Endotoxin concentrations were determined by a quantitative kinetic-chromogenic Limulus Amebocyte Lysate (LAL) assay (Lonza, Walkersville, MD). For the subset of samples used in the in vivo assay (n =60), 100 μL of 250 μg/mL PM extracts (in duplicate) and the endotoxin standards (Escherichia coli O55:B5) were added into a 96-well plate and incubated at 37 ºC for 10 min. After incubation, 100 μL of the LAL/Substrate reagent was added to each well. Absorption measurements were recorded at baseline and every 150 s at 405 nm on an automated plate reader (Spectra Max M2e, Molecular Devices Corp., Sunnyvale, CA). Reaction time was calculated as the time for the absorbance to increase 0.200 absorbance units above baseline value.
In vitro exposure
Cell culture
The human pulmonary microvascular endothelial cell line (HPMEC-ST1.6R) was kindly provided by Dr James Kirkpatrick and Dr Vera Krump-Konvalinkova at Johannes Gutenberg University (Mainz, Germany). Cells were maintained at 37 ºC in a humidified atmosphere of 5% CO2 and grown in Endothelial Growth Medium (EGM-2) containing 1% penicillin/streptomycin (Gibco, Grand Island, NY) and supplemented with an EGM-2 BulletKit and 5% fetal bovine serum (FBS) (Lonza).
The bronchial epithelial cell line (BEAS-2B) was obtained from the American Type Culture Collection (ATCC, Rockville, MD) and maintained in DMEM medium (Dulbecco’s Modified Eagle Medium; Gibco) with 10% FBS (Gemini Bio Products, Calasas, CA) and 1% penicillin/streptomycin (Gibco).
LDH assay
Cells were seeded in 96-well plates at a density of 8000 cells/well and allowed to adhere overnight. The following day, 100 μL fresh DMEM/F12 was added into each well. 25 μL of 250 μg/mL PM samples was thawed, sonicated for 30 min, added in triplicate to wells, and incubated for 24 h (final concentration of 50 μg/mL). LDH activity was determined using a colorimetric assay (Takara Bio Inc., Madison, WI) in which the LDH activity of each well was calculated as a percentage of total LDH activity (1% triton X-100-treated cells) and then corrected by subtracting baseline LDH in the supernatant of media-only treated cells.
ROS assay
The oxidative potential of the collected PM samples was determined using a dichlorofluorescein diacetate (DCFH-DA) assay. Cells were seeded at 5000 cells/well in black 96-well plates and grown to confluence (approximately 2 days). 100 μL of 10 μM DCFH-DA (Invitrogen, Carlsbad, CA) in PBS was added to each well and incubated for 30 min at 37 ºC. The dye-loaded cells were washed two times with DMEM/F12 media and then 100 μL fresh DMEM/F12 media was added. 25 μL of sonicated 250 μg/mL PM samples was added to wells (in triplicate) for a final concentration of 50 μg/mL and fluorescence measurements were taken at 0 h and 3 h for the BEAS-2B cell line and 0 h and 5 h for the HPMEC-ST1.6R cell line using a HTS 7000 Microplate Reader (Perkin Elmer, Waltham, MA) at excitation and emission wavelengths of 485 and 535 nm, respectively. This dose was the highest non-toxic dose based upon the results of the LDH assay. ROS production was calculated as the increase in fluorescence intensity over time. All plate readings were normalized by subtracting the mean water vehicle blank from the change in fluorescence intensity on each plate. Positive controls (metal-rich fireworks PM) were included on each plate. To compare the toxicity of super-coarse PM to the other size fractions, additional plates of cells were treated with representative size samples of PM collected on the same day.
In vivo exposure
Animal care
Eight to 10 week old male and female FVB/N mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred at NYU. Mice were housed in polycarbonate cages with corn-cob bedding in temperature and humidity controlled rooms with a 12 h light/dark cycle. Animals were provided standard chow and water ad libitum. All animal procedures and handling were performed under the National Institutes of Health and Animal Welfare Act guidelines for the ethical treatment of animals and under a protocol approved by the NYU School of Medicine Institutional Animal Care and Use Committee.
A subset of extracted PM samples was selected for an in vivo bioassay of lung inflammation and injury in mice. These samples were the highest and lowest responding (in terms of ROS response) coarse PM samples for each season and location, as well as their corresponding fine PM samples collected on the same day (60 samples in total). Additional mice were treated with super-coarse PM samples taken at one rural (Wallkill, NY) and one urban (Bronx, NY) site and, for comparison, with the corresponding coarse and fine PM samples collected on the same day.
Oropharyngeal aspiration and bronchoalveolar lavage
An oropharyngeal aspiration technique (Rao et al., 2003) was used to disperse PM into the lungs of mice. Briefly, mice were anesthetized with isofluorane (Abbott Laboratories, King of Prussia, PA) and placed on a 45º angle slanted board. 50 μL of sterile water (negative control) or 50 μL of 1 mg/mL PM suspended in sterile water was placed at the base of the tongue just before a deep inhalation, for a total dose of 50 μg/mouse.
To investigate the role of PM-bound endotoxin in lung inflammation, we treated additional mice with E. coli 0127:B8 (Gibco) by oropharyngeal aspiration. Mice were treated with 0.1, 0.5, 1.0, 5.0 or 10.0 ng endotoxin/mouse and the lungs were examined 24 h later.
Twenty-four hours after aspiration, animals were sedated with an intraperitoneal (ip) injection of ketamine at a concentration of 0.1 mg/g of body weight and euthanized with a lethal dose of 0.26 mg/g of sodium pentobarbital (ip). The lungs were lavaged twice using PBS (phosphate buffered saline, Invitrogen) and cell counts, cell differentials and total protein (BCA Protein Assay Kit, Thermo Fisher Scientific) were measured in lavage fluid.
Statistical analysis
Prism 5.0 for Windows (GraphPad Software, San Diego, CA) was used for the analysis of data, which are reported as means ± standard error (SE), unless otherwise noted. Where appropriate, data were analyzed using unpaired t-tests. For in vitro experiments using more than two groups for analysis, one-way ANOVA, followed by Tukey’s Multiple Comparison Test, was used. For in vivo experiments using more than two groups for analysis, one-way ANOVA, followed by Dunnett’s Multiple Comparison Test, was used. Interaction terms were analyzed by three-dimensional non-additive fixed-effects ANOVA models using S-PLUS (MathSoft Inc., Cambridge, MA). Correlations were performed between trace element concentrations and in vitro and in vivo responses using Pearson’s Correlation Coefficient. The statistical significance was set for p ≤ 0.05.
Results
PM mass and trace element characterization
Among all five locations, the PM mass concentrations were highest at the two urban locations (Bronx and Manhattan) compared to the three rural locations (Goshen, Tuxedo, Wallkill) for the three size fractions (Table 1). For the coarse PM size fraction, there appeared to be little difference in concentration between the summer and winter seasons, whereas winter mass concentrations were greater than summer for fine PM at all five locations. Across all locations and both seasons, fine PM had an equal or higher mass concentration than coarse PM. The super-coarse mass concentration was lower in Wallkill when compared to the corresponding Wallkill coarse and fine PM, whereas the super-coarse PM mass concentration was comparable to the corresponding coarse PM in the Bronx. The Bronx super-coarse PM mass concentration was 2.5 times higher than that for the Wallkill super-coarse PM fraction.
Table 1.
Mass concentrations for super-coarse, coarse and fine particulate matter from five distinct locations.
| Super-coarse | Coarse | Fine | |
|---|---|---|---|
| Wallkill (rural) | |||
| Summer | 2.9 ± 0.5 | 5.2 ± 1.0 | 5.2 ± 1.7 |
| Winter | ND | 4.7 ± 2.4 | 8.9 ± 5.1 |
| Goshen (rural) | |||
| Summer | ND | 5.8 ± 1.3 | 6.6 ± 2.5 |
| Winter | ND | 6.5 ± 2.7 | 10.3 ± 5.4 |
| Tuxedo (rural) | |||
| Summer | ND | 5.0 ± 1.5 | 5.8 ± 2.5 |
| Winter | ND | 4.4 ± 2.4 | 7.8 ± 5.1 |
| Bronx (urban) | |||
| Summer | 7.2 ± 2.5 | 7.3 ± 2.3 | 9.2 ± 3.9 |
| Winter | ND | 8.9 ± 4.0 | 14.0 ± 7.1 |
| Manhattan (urban) | |||
| Summer | ND | 8.0 ± 2.4 | 9.5 ± 3.5 |
| Winter | ND | 11.9 ± 5.1 | 14.0 ± 5.5 |
Average mass concentration (μg/m3) ± standard deviation for PM stratified by location, size fraction, locale and season. ND =not determined.
The trace metal concentrations were assessed at the five locations, and compared by size fraction, season, location and locale. Compared to fine PM, elevated concentrations of Mg and Mn were observed for all coarse PM winter samples. The urban locales had higher Co, Ni and Zn in their winter coarse PM fractions, while the summer coarse PM fractions for urban locales demonstrated elevated Mg and Cu. Sb was present in higher concentrations at both urban locations, regardless of season or size fraction, whereas Ti was elevated in only the coarse urban PM samples. Cd, S and Se were observed to have elevated concentrations in all fine PM samples, and Fe and Sn were highest in all coarse PM samples, regardless of season. Correlations between trace metal concentrations and in vitro and in vivo responses, as well as discussions on suspected sources, are presented in subsequent sections.
In vitro toxicity – ROS response
ROS responses are reported for the two cell lines, BEAS-2B epithelial cells and HPMEC-ST1.6R endothelial cells, at equal mass concentration for all of the collected PM samples. The LDH assay was completed first to assess cell cytotoxicity, and no samples exhibited a significant decrease in cell viability at a concentration of 50 μg/mL (data not shown), which was the concentration used throughout the remainder of the experiment.
The ROS response was examined in the two cell types to determine if the PM-stimulated oxidative stress response was significantly related to sampling location, season, particle size range and/or locale (rural versus urban), as well as interactions between the factors. The ROS response in the HPMEC-ST1.6R cell line was much greater than in the BEAS-2B cell line, although the responses between the two cell types were significantly correlated (Pearson’s correlation coefficient, R =0.22, p =0.0007). In both the BEAS-2B and HPMEC-ST1.6R cells, the ROS response was significantly affected by location, locale (i.e. urban versus rural), and the interaction of location and particle size range (Table 2). In the HPMEC-ST1.6R cells, an additional association was observed for particle size range (Table 2).
Table 2.
p Values for the effect of location, season size and locale.
| HPMEC-ST1.6R Endothelial cells | BEAS-2B Epithelial cells | % PMNs | Total protein | |
|---|---|---|---|---|
| Location | <0.01 | <0.01 | 0.20 | 0.01 |
| Season | 0.11 | 0.64 | 0.97 | 0.18 |
| Size | <0.01 | 0.86 | <0.01 | 0.68 |
| Locale | <0.01 | <0.01 | 0.45 | 0.55 |
| Location: Season | 0.27 | 0.96 | 0.48 | 0.15 |
| Location: Size | <0.01 | <0.01 | 0.67 | 0.60 |
| Season: Size | 0.66 | 0.24 | 0.03 | 0.43 |
p Values associated with the effect of location, season, size and locale (and interactions) on the in vitro ROS response in both HPMEC-ST1.6R and BEAS-2B cell types as well as % PMNs and total protein on the in vivo lung inflammation and injury response, using two-way ANOVA for location, season, size and locale and three-way ANOVA for interactions. Values in bold designate a statistically significant effect.
Due to the significant correlation between cell types, only ROS values for the HPMEC-ST1.6R vascular endothelial cells are reported. As shown in Figure 1(A), significant differences in ROS production were observed among all sites and the Manhattan site, as well as among all location sites and the Bronx site; no significant differences in ROS were observed amongst any of the rural locations. Season had no significant effect on ROS production (Table 2 and Figure 1B), whereas size (fine PM>coarse PM; Figure 1C) and locale (urban PM>rural PM; Figure 1D) had significant effects.
Figure 1.
ROS activity in HPMEC-ST1.6R endothelial cells treated (in triplicate) with 50 μg/mL PM for 5 h. For each box plot, the box boundaries represent the 25–75 percentiles, the line represents the median, the whiskers represent the 5–95 percentiles, and the closed circles represent outliers. The figure is separated by (A) Location, (B) Season, (C) Size fraction and (D) Locale. *p Value ≤0.05, ***p value ≤0.001 (A: One-way ANOVA, Tukey’s Multiple Comparison Test; B, C, D: unpaired t test).
To examine temporal changes, PM samples were collected every 48 h (three days over weekends) at each location during each season. For the Bronx winter samples, the fine PM fraction elicited a greater ROS response than the coarse PM fraction, but both had significant sample-to-sample variability, which was temporally similar between the particle size fractions (Figure 2). Similar associations were observed for the other locations (data not shown).
Figure 2.
ROS activity in HPMEC-ST1.6R endothelial cells treated with 50 μg/mL of coarse (A) or fine (B) PM collected over 48 h (weekdays) or 72 h (weekend) at the Bronx sampling site during the Winter. Data represent the mean (±SE) relative fluorescence.
As stated above, neither significant seasonal effect was observed nor were there significant interactions between season and particle size or location (Table 2). However, when separated by location and particle size range, the Manhattan and Tuxedo coarse PM as well as Wallkill fine PM showed significant seasonal differences (Figure 3). Additionally, although there were significant location-dependent differences for the coarse PM samples, there was less variation across all locations for the coarse PM samples compared to the fine PM samples, in which the urban locations appeared to dominate the ROS responses.
Figure 3.

The effect of season on ROS production by coarse (A) and fine (B) PM samples. Vascular endothelial cells were exposed to 50 μg/mL PM (in triplicate) for 5 h. Data represent the mean relative fluorescence unit ±SE. *p Value ≤0.05, ***p value ≤0.001 (unpaired t test, Bonferroni corrected).
Numerous trace elements were positively associated with the ROS response in the vascular endothelial cells (Table 3). Elements originating in the burning of residual oil (i.e. S, V and Ni), as well as elements originating in brake and tire wear (i.e. Cu, Zn and Sb), showed significant positive associations with ROS. By contrast, there were negative correlations for markers of resuspended road dust/soil (i.e. Mg and Ca).
Table 3.
Correlation coefficients between metal concentrations and responses.
|
In vitro response
|
In vivo response
|
|||
|---|---|---|---|---|
| HPMEC | BEAS-2B | % PMNs | Protein | |
| Be | 0.48 | 0.00 | −0.13 | 0.23 |
| Mg | −0.17 | −0.10 | 0.37 | 0.12 |
| P | 0.16 | 0.00 | 0.61 | 0.07 |
| S | 0.35 | 0.05 | −0.72 | −0.09 |
| K | 0.02 | 0.08 | 0.31 | −0.17 |
| Ca | −0.14 | −0.12 | 0.21 | 0.20 |
| Sc | −0.01 | −0.03 | 0.25 | 0.16 |
| Ti | −0.08 | −0.01 | 0.30 | 0.15 |
| V | 0.52 | 0.00 | −0.36 | 0.15 |
| Cr | 0.01 | 0.02 | 0.02 | −0.01 |
| Mn | −0.03 | −0.06 | 0.25 | 0.13 |
| Fe | −0.08 | 0.02 | −0.05 | −0.02 |
| Co | 0.51 | 0.02 | −0.15 | 0.18 |
| Ni | 0.53 | 0.03 | −0.21 | 0.16 |
| Cu | 0.25 | 0.12 | 0.06 | 0.07 |
| Zn | 0.50 | 0.04 | −0.30 | 0.17 |
| As | 0.16 | −0.02 | −0.62 | −0.18 |
| Se | 0.14 | 0.05 | −0.63 | 0.09 |
| Sr | 0.12 | 0.08 | 0.14 | −0.05 |
| Ag | −0.05 | 0.02 | −0.05 | 0.16 |
| Cd | −0.07 | −0.07 | −0.50 | −0.05 |
| Sn | −0.07 | 0.01 | 0.62 | 0.01 |
| Sb | 0.58 | 0.07 | −0.21 | 0.10 |
| Cs | −0.27 | −0.11 | 0.55 | −0.03 |
| La | 0.06 | −0.01 | 0.15 | 0.17 |
| Pb | 0.06 | 0.10 | −0.68 | −0.16 |
| Tl | −0.09 | 0.06 | 0.05 | 0.22 |
| Endotoxin | 0.05 | −0.13 | 0.56 | 0.18 |
Pearson correlation coefficients between metal concentrations and both in vitro and in vivo responses. Values in bold designate a statistically significant effect.
In vivo toxicity – % PMNs, total protein
The in vivo toxicity data were generated in a mouse model using a subset of PM samples of coarse and fine PM (n =60). As noted above, the greatest in vitro ROS responses were observed in cells treated with the urban fine PM fraction (Figure 4A and B). In comparison, however, a greater degree of inflammation occurred in the mouse lungs, represented by % PMNs in lavage fluid, treated with the coarse PM fraction. In fact, coarse PM samples produced significantly greater inflammation than the corresponding fine PM samples for all locations (Figure 4C). Additionally, % PMNs in mice treated with the coarse PM were significantly greater than that measured in control animals treated with sterile water. Only for the Wallkill (rural) and Bronx (urban) sampling sites did the fine PM produce a significant increase in % PMNs compared to controls. In terms of locale, coarse and fine PM from both urban and rural locales produced significant increases in % PMNs compared to controls, although coarse PM responses were also significantly greater than the corresponding fine PM responses (Figure 4D). In terms of lung injury, the coarse PM from each location except Tuxedo produced significantly elevated total protein in lavage fluid compared to control. For the fine PM fraction, total protein was significantly elevated only at the Wallkill and Manhattan locations (Figure 4E). No significant differences between coarse and fine PM at the urban and rural locales were observed for total protein count (Figure 4F). Overall in the mice, particle size significantly affected % PMNs and location affected total protein, with a significant interaction observed only between season and size for % PMNs (Table 2).
Figure 4.
Comparison of the effect of PM treatment on ROS activity in vascular endothelial cells and lavage fluid PMNs and total protein in mice. Location (A, C; sampling site) and locale (B, D; urban or rural) had significant effects on both in vitro and in vivo endpoints. Data represent the mean relative fluorescence unit ±SE (A, B; in triplicate), % PMNs (C, D; n =6 per group) and total protein (E, F; n =6 per group). The dashed line represents the control values from air-exposed mice. *p Value ≤0.05, **p value ≤0.01, ***p value ≤0.001.
In terms of linking PM components to changes in % PMNs, positive associations were observed for Mg, P, Cu, K, Ti, Sn and Cs, while negative associations were observed with Be, V, S, As, Se, Cd, Pb and Zn (Table 3). Endotoxin was also positively associated with % PMNs. To investigate the role of PM-bound endotoxin on lung inflammation, additional animals were challenged with purified endotoxin (E. coli 0127:B8) by aspiration. Aspiration with 0.1, 0.5, 1.0, 5.0 or 10.0 ng endotoxin/mouse (approximately 7, 20, 41, 205 and 410 Endotoxin Units (EUs)/mouse as measured by the Limulus lysate assay) resulted in 0%, 0%, 1%, 13% and 15% PMNs, respectively. Finally, no significant correlations were observed between trace element concentrations and total protein.
Super-coarse versus coarse versus fine PM
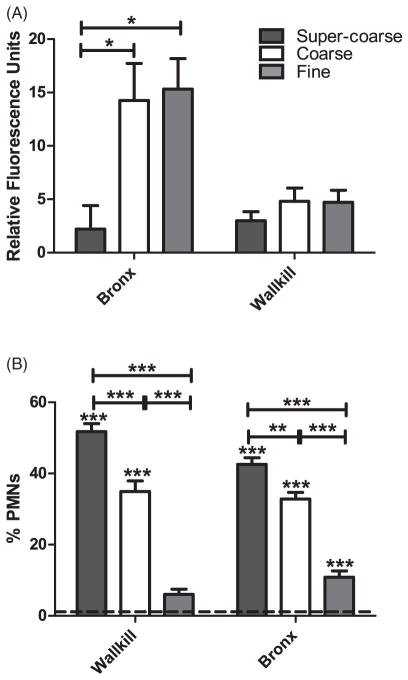
For a small subset of samples, super-coarse PM (PM>10 μm) was collected simultaneously with fine and coarse PM, during the summer season at the Wallkill and Bronx sites. This subset of samples was tested in the vascular endothelial (HPMEC-ST1.6R) cells and the mouse model. The effect of super-coarse PM on ROS production differed between sites. The ROS response to super-coarse PM was not significantly different compared to the other size fractions for Wallkill, but was significantly less potent than the coarse and fine PM collected in the Bronx (Figure 5A, note that the samples presented in Figure 5A were analyzed with a different model plate reader and the relative fluorescence units differ from the other reported ROS values). Unlike the in vitro results, the super-coarse PM from both sites elicited a significantly greater inflammatory response (% PMNs) than did the corresponding coarse and fine PM in the mouse model (Figure 5B).
Figure 5.
The effect of super-coarse PM (versus coarse and fine PM) on relative fluorescence in vascular endothelial cells (A) and % PMNs (B) in mice. Super-coarse PM was collected only at the Wallkill and Bronx locations during the summer. *p Value ≤0.05, **p value ≤0.01, ***p value ≤0.001.
Discussion
This study compared the relative in vitro and in vivo toxicity of three different PM size fractions (super-coarse, coarse, and fine PM) at three rural and two urban sites in the NYC metropolitan area during two seasons (summer and winter). The particles were collected using a high-volume cascade impactor and characterized by their mass, trace element and endotoxin concentrations. As demonstrated, ambient PM elicited a ROS response in both pulmonary airway epithelial and microvascular endothelial cells, as well as an inflammatory response in the mouse lung. These responses were dependent on the size of the particles as well as their locale (urban versus rural). Additionally, when assessed by the individual collection site, different ROS and inflammatory responses were observed. These data demonstrate that the sources and composition of ambient PM drive the ROS and inflammatory responses, suggesting that the use of PM mass concentration, as the primary metric for the selection of health policies for ambient PM, can be augmented by understanding PM speciation.
Oxidative stress has been implicated as a possible mechanism to explain PM-related health effects, either directly by the innate oxidative potential of ambient particles or from a cellular response to the particles (Ghio et al., 2012; Mazzoli-Rocha et al., 2010). Oxidative stress occurs when there is an imbalance of antioxidants and ROS, such as superoxide anion, hydrogen peroxide and hydroxyl radicals. PM from various locations has been shown to induce ROS both in human lung epithelial cells (Shi et al., 2006) and in cell-free models (Cho et al., 2005; Ntziachristos et al., 2007; Shen & Anastasio, 2011). In a study conducted in Rubidoux, California, the ultrafine size range was observed to elicit the greatest ROS response (Venkatachari et al., 2005). As our study did not directly examine this size fraction (i.e. the ultrafine PM was collected as part of the PM2.5 fraction), it is likely that our fine PM ROS response contained the response of PM2.5-0.1 as well as ultrafine PM (<0.1 μm). We also sought to investigate the ROS response of PM with an aerodynamic diameter greater than 10 μm, which we have called super-coarse PM. Unlike in the Rubidoux study, our super-coarse PM samples generated a lower ROS response than that of coarse and fine PM (Figure 5A). Interestingly, this difference was much more evident in the urban Bronx sample. When these samples were aspirated into mice, however, the super-coarse PM from both the rural and urban sites elicited a statistically significant greater inflammatory response than both the coarse and fine PM (Figure 5B), although the biological significance of the difference between the response to super-coarse and coarse PM is unclear.
The current study clearly demonstrates that the PM collection site significantly affects both ROS and inflammatory responses. The observed differences occurred for PM collected at rural versus urban locales as well as among the five individual collection sites within the urban and rural locales. Because the five locations were geographically separated within the NYC metropolitan area, the spatial-dependent variability in responses was not surprising. Although it could be hypothesized from previous studies that urban PM would be more toxic than rural PM, the variability in response between the Manhattan and Bronx urban PM samples suggests that the chemical composition of urban PM is partially driven by local sources that may be major contributors to the observed effects.
There was more variability in ROS response from the two urban locations compared with the three rural locations. It is possible that the local sources of ambient PM are more variable between the two urban locations, in which sources such as daily traffic combustion emissions, resuspended road dust (e.g. brake wear particles), and residual oil burning for dual purposes (space and hot water heating), as well as meteorological conditions such as wind direction, could influence the spatial variability of the air mass being collected. PM in rural locations, however, might originate from less diverse sources and therefore would be less influenced by weather conditions. In one study looking at intra-urban variability of PM10 and PM2.5 collected in Beirut, researchers found that PM mass concentrations were relatively homogenous, but the individual components were much more varied (Massoud et al., 2011). The daily variability in the ROS response for an urban NY collection site is highlighted in Figure 2, in which the ROS response to Bronx winter samples are broken down by individual sampling periods. An interesting trend is observed for the fine and coarse PM fractions, in which a decrease in ROS response during sampling periods 6–8 (Monday through Monday) was seen, suggesting that a temporal change in the source of ambient PM for both size fractions contributed to this varying ROS response.
Surprisingly, no seasonal differences were observed in this study. It has been hypothesized that seasonal differences in PM toxicity might be linked to changes in the composition of PM, as well as variations in the ratio of size fraction distributions (Becker et al., 2005; Plummer et al., 2012). Previous work has demonstrated that seasonal variability contributes to changes in toxicity (Cheung et al., 2012). One such study looked at the acute toxic effects of PM10 and PM2.5 in the mouse lung using summer and winter samples and found that both size and season affected the biological outcomes (Farina et al., 2011). The lack of seasonal differences in our study suggests that particle composition may not change significantly for the NY metropolitan area from one season to the next. For example, dual-use boilers are commonly used in NYC for space heating in winter and domestic hot water heating in all seasons, thus resulting in the potential for high levels of Ni and V year-round. It is also possible that while particle composition may change between seasons, the overall particle toxicity of summer and winter PM samples may have remained the same in our in vitro and in vivo bioassays. Indeed, while changes were observed in metal concentrations between seasons for various elements, significant correlations between ROS response and trace element concentration were still observed for S, V, Co, Ni, Cu, Zn, Sb and Cs during both seasons (data not shown). During winter but not summer, positive correlations between ROS and potassium (K) and calcium (Ca) were observed.
Conflicting results have been observed in studies looking into the toxicity of coarse versus fine PM in cell and animal assays as well as in epidemiological studies. In a previous epidemiological study, investigators examined the acute exposure to coarse particles in 15 Californian counties, and determined that coarse PM was associated with increased mortality (Malig & Ostro, 2009; Zanobetti & Schwartz, 2009). Additionally, while a number of studies have associated coarse PM with pro-inflammatory effects in the lungs (Schins et al., 2004; Wegesser & Last, 2008), pulmonary inflammation (Graff et al., 2009), respiratory disease (Host et al., 2008), cardiovascular mortality (Malig & Ostro, 2009; Mar et al., 2000; Ostro et al., 2000; Wilson et al., 2007), cardiopulmonary complications (Monn & Becker, 1999; Svendsen et al., 2007), and decreased heart rate variability (Chang et al., 2007; Gong et al., 2004; Lipsett et al., 2006), some epidemiological studies have not been able to make a clear association between coarse particles and adverse health effects (Peng et al., 2008; Puett et al., 2009). Many of the contradictions from epidemiological work on coarse particles arise from findings that are ambiguous due to relatively low exposure levels and the examination of only single cities or regions (Malig & Ostro, 2009; Perez et al., 2009). In addition, many epidemiology studies have calculated “coarse” PM by the less than optimal technique of subtracting PM2.5 from PM10. The use of cascade impactor technology in the present and other toxicology studies provides a basis for a more precise examination of the relative contributions of coarse and fine PM, as well as of the chemical components within each size fraction. Indeed, in a recent study, Tong et al. (2010) observed significantly greater pulmonary inflammation in mice instilled with coarse PM than with fine or ultrafine PM, whereas ultrafine PM produced greater cardiovascular effects.
Interestingly, the present study demonstrated a lack of correlation between the in vitro ROS response and the in vivo pulmonary inflammation response. In particular, the induction of pulmonary inflammation in mice (i.e. PMNs in lavage fluid) was dominated by coarse particles at all five sampling sites (both urban and rural), whereas only urban fine PM produced significant increases in ROS in the cell bioassays (Figure 4). This lack of correlation between the in vitro and in vivo responses to PM suggests that the proposed role of oxidative stress in the adverse pulmonary effects of inhaled ambient PM may be incorrect. Alternatively, this lack of correlation suggests that a different mechanism underlies the induction of lung inflammation by ambient PM. Thus, lung parameters other than those examined here could be linked to PM induction of oxidative stress.
In the current study, the contribution of individual trace elements to the ROS response, overall as well as between locations, seasons, locale and size fraction, was examined. Previous studies have suggested that certain metals, namely transition metals such as Zn (Jalava et al., 2009; See et al., 2007), V (Happo et al., 2008; See et al., 2007), Mn (Boogaard et al., 2012; See et al., 2007), and Cr (Boogaard et al., 2012) contribute significantly to the toxicity of PM. In the present study, P, S, V, Co, Ni, Zn, Cu, Be and Sb were most correlated with the overall ROS response, whereas there were negative correlations for Mg and Ca but not Cu, each of which has been used as a marker of resuspended road dust. This finding was contradictory to the results obtained by Happo et al. (2008), in which soil markers were positively associated with inflammation. Sb, which was found to be enriched in the urban locations, was highly correlated with ROS; Sb can originate from brake pad wear, but is also released into the environment from municipal solid waste, sewage waste incineration and fossil fuel combustion (Canepari et al., 2010). Interestingly, Fe, which has been found to be highly correlated with ROS responses (Boogaard et al., 2012; Jeng, 2010; Ntziachristos et al., 2007) was not found to correlate with ROS responses in the present study. Contrasting results could be due, in part, to different ratios of the elements present in the PM samples collected at different locations. Additionally, our study sought to compare endothelial and epithelial cell responses to trace metals concentrations, but the epithelial cell response was much lower at the 50 μg/mL PM treatment level and would not permit inter-cellular comparisons or correlations with trace elements.
Various studies have examined the response of endotoxin and inflammatory markers. Schins et al. (2004) found that despite different locations for sampling PM, coarse PM elicited a greater response than fine PM using inflammatory markers from lavage fluid in rat lungs. It was suggested that the higher endotoxin content in this size fraction might account for their findings; similar relationships between endotoxin and inflammatory responses have been reported (Becker et al., 1996, 2003; Gilmour et al., 2007; Steenhof et al., 2011) and this relationship seems to be supported by our work. Contrasting results from a study done in California, however, showed that endotoxin was not related to pro-inflammatory responses of coarse PM, but rather that insoluble components found in the PM mixture were responsible (Wegesser & Last, 2008). This observation was made by using heat treatment of PM samples to inactivate the endotoxin. In our study, BAL levels of % PMNs were increased in a dose-dependent manner in additional mice treated with a range of doses of endotoxin. Importantly, the inflammation produced by even ~400 EUs/mouse was much less than that produced by the extracted PM samples used in the coarse PM aspiration experiments, thus suggesting that endotoxin is not responsible for the observed effects and may co-correlate with another PM component.
Limitations of the present study include a lack of organic and ionic composition data for the PM samples obtained, as it has been shown in a previous study that volatile organic constituents correlate with inflammatory responses (Wang et al., 2012). Additionally, the use of a high-volume cascade impactor for sample collection necessitated extraction from samples that were deposited on PUF substrates. By mass, the extraction efficiency of this method was approximately 60% for the final filter (fine PM) and 80% for the PUF substrates (coarse PM). Moreover, the aqueous extraction and lyophilization methods would not be optimal for bioassay tests of non-polar components, such as elemental carbon, or semi-volatile and volatile components. Thus, additional research is needed to investigate the role of non-polar and volatile components in PM toxicity. Additionally, these results apply to PM samples collected in the metropolitan NYC area and should not be extrapolated to urban and rural PM present in other regions of the US and elsewhere.
Conclusions
In summary, this study has shown that the size fraction, locale (urban versus rural) and collection site of PM samples in the NYC metropolitan area affect the ROS response in pulmonary endothelial and epithelial cells, as well as the pulmonary inflammatory response to PM aspiration in a mouse model. Fine PM greatly affected the in vitro responses, whereas coarse PM affected in vivo responses. The responses generated from this study were dependent on the chemical composition of PM, which support previous studies that suggest mass concentration alone may not be sufficient for evaluating the toxic responses to PM exposure.
Acknowledgments
The authors would like to acknowledge support by EPA (RD-83374201), the Health Effects Institute, an NIEHS T32 grant (ES007324) and the Facility Cores of NYU School of Medicine’s NIEHS Center of Excellence (P30 ES000260) and the Exposure Assessment Facility Core of Columbia’s NIEHS Center (P30 ES009089).
Footnotes
Declaration of interest
The authors report no declaration of interest.
References
- Becker S, Dailey LA, Soukup JM, et al. Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environ Health Perspect. 2005;113:1032–8. doi: 10.1289/ehp.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Soukup JM, Gilmour MI, Devlin RB. Stimulation of human and rat alveolar macrophages by urban air particulates: effects on oxidant radical generation and cytokine production. Toxicol Appl Pharmacol. 1996;141:637–48. doi: 10.1006/taap.1996.0330. [DOI] [PubMed] [Google Scholar]
- Becker S, Soukup JM, Sioutas C, Cassee FR. Response of human alveolar macrophages to ultrafine, fine, and coarse urban air pollution particles. Experiment Lung Res. 2003;29:29–44. doi: 10.1080/01902140303762. [DOI] [PubMed] [Google Scholar]
- Boogaard H, Janssen NA, Fischer PH, et al. Contrasts in oxidative potential and other particulate matter characteristics collected near major streets and background locations. Environ Health Perspect. 2012;120:185–91. doi: 10.1289/ehp.1103667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunekreef B, Forsberg B. Epidemiological evidence of effects of coarse airborne particles on health. Eur Respir J. 2005;26:309–18. doi: 10.1183/09031936.05.00001805. [DOI] [PubMed] [Google Scholar]
- Canepari S, Marconi E, Astolfi ML, Perrino C. Relevance of Sb(III), Sb(V), and Sb-containing nano-particles in urban atmospheric particulate matter. Analyt Bioanalyt Chem. 2010;397:2533–42. doi: 10.1007/s00216-010-3818-1. [DOI] [PubMed] [Google Scholar]
- Chang L, Tang C, Pan Y, Chan C. Association of heart rate variability of the elderly with personal exposure to PM1, PM1–2.5, PM2.5–10. Bull Environ Contam Toxicol. 2007;79:552–6. doi: 10.1007/s00128-007-9233-4. [DOI] [PubMed] [Google Scholar]
- Chen R, Li Y, Ma Y, et al. Coarse particles and mortality in three Chinese cities: the China Air Pollution and Health Effects Study (CAPES) Sci Total Environ. 2011;409:4934–8. doi: 10.1016/j.scitotenv.2011.08.058. [DOI] [PubMed] [Google Scholar]
- Cheung K, Shafer MM, Schauer JJ, Sioutas C. Diurnal trends in oxidative potential of coarse particulate matter in the los angeles basin and their relation to sources and chemical composition. Environ Sci Technol. 2012;46:3779–87. doi: 10.1021/es204211v. [DOI] [PubMed] [Google Scholar]
- Cho AK, Sioutas C, Miguel AH, et al. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ Res. 2005;99:40–7. doi: 10.1016/j.envres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–9. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Farina F, Sancini G, Mantecca P, et al. The acute toxic effects of particulate matter in mouse lung are related to size and season of collection. Toxicol Lett. 2011;202:209–17. doi: 10.1016/j.toxlet.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Carraway MS, Madden MC. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health B Crit Rev. 2012;15:1–21. doi: 10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]
- Gilmour MI, McGee J, Duvall RM, et al. Comparative toxicity of size-fractionated airborne particulate matter obtained from different cities in the United States. Inhal Toxicol. 2007;19:7–16. doi: 10.1080/08958370701490379. [DOI] [PubMed] [Google Scholar]
- Gong H, Linn WS, Terrell SL, et al. Altered heart-rate variability in asthmatic and healthy volunteers exposed to concentrated ambient coarse particles. Inhal Toxicol. 2004;16:335–43. doi: 10.1080/08958370490439470. [DOI] [PubMed] [Google Scholar]
- Graff DW, Cascio WE, Rappold A, et al. Exposure to concentrated coarse air pollution particles causes mild cardiopulmonary effects in healthy young adults. Environ Health Perspect. 2009;117:1089–93. doi: 10.1289/ehp0900558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halatek T, Stepnik M, Stetkiewicz J, et al. The inflammatory response in lungs of rats exposed on the airborne particles collected during different seasons in four European cities. J Environ Sci Health, Part A. 2011;46:1469–81. doi: 10.1080/10978526.2011.609064. [DOI] [PubMed] [Google Scholar]
- Happo MS, Hirvonen MR, Halinen AI, et al. Chemical compositions responsible for inflammation and tissue damage in the mouse lung by coarse and fine particulate samples from contrasting air pollution in Europe. Inhal Toxicol. 2008;20:1215–31. doi: 10.1080/08958370802147282. [DOI] [PubMed] [Google Scholar]
- Host S, Larrieu S, Pascal L, et al. Short-term associations between fine and coarse particles and hospital admissions for cardiorespiratory diseases in six French cities. Occup Environ Med. 2008;65:544–51. doi: 10.1136/oem.2007.036194. [DOI] [PubMed] [Google Scholar]
- Jalava PI, Hirvonen MR, Sillanpaa M, et al. Associations of urban air particulate composition with inflammatory and cytotoxic responses in RAW 246.7 cell line. Inhal Toxicol. 2009;21:994–1006. doi: 10.1080/08958370802695710. [DOI] [PubMed] [Google Scholar]
- Jeng HA. Chemical composition of ambient particulate matter and redox activity. Environ Monit Assess. 2010;169:597–606. doi: 10.1007/s10661-009-1199-8. [DOI] [PubMed] [Google Scholar]
- Kinney PL, Thurston GD. Field evaluation of instrument performance: statistical considerations. Appl Occup Environ Hygiene. 1993;8:267–71. [Google Scholar]
- Lin M, Stieb DM, Chen Y. Coarse particulate matter and hospitalization for respiratory infections in children younger than 15 years in Toronto: a case-crossover analysis. Pediatrics. 2005;116:e235–40. doi: 10.1542/peds.2004-2012. [DOI] [PubMed] [Google Scholar]
- Lipsett MJ, Tsai FC, Roger L, et al. Coarse particles and heart rate variability among older adults with coronary artery disease in the Coachella Valley, California. Environ Health Perspect. 2006;114:1215–20. doi: 10.1289/ehp.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malig BJ, Ostro BD. Coarse particles and mortality: evidence from a multi-city study in California. Occup Environ Med. 2009;66:832–9. doi: 10.1136/oem.2008.045393. [DOI] [PubMed] [Google Scholar]
- Mar TF, Norris GA, Koenig JQ, Larson TV. Associations between air pollution and mortality in Phoenix, 1995–1997. Environ Health Perspect. 2000;108:347–53. doi: 10.1289/ehp.00108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoud R, Shihadeh AL, Roumie M, et al. Intraurban variability of PM10 and PM2.5 in an Eastern Mediterranean city. Atmospheric Res. 2011;101:893–901. [Google Scholar]
- Mazzoli-Rocha F, Fernandes S, Einicker-Lamas M, Zin WA. Roles of oxidative stress in signaling and inflammation induced by particulate matter. Cell Biol Toxicol. 2010;26:481–98. doi: 10.1007/s10565-010-9158-2. [DOI] [PubMed] [Google Scholar]
- Monn C, Becker S. Cytotoxicity and induction of proinflammatory cytokines from human monocytes exposed to fine (PM2.5) and coarse particles (PM10–2.5) in outdoor and indoor air. Toxicol Appl Pharmacol. 1999;155:245–52. doi: 10.1006/taap.1998.8591. [DOI] [PubMed] [Google Scholar]
- Ntziachristos L, Froines JR, Cho AK, Sioutas C. Relationship between redox activity and chemical speciation of size-fractionated particulate matter. Part Fibre Toxicol. 2007;4:5. doi: 10.1186/1743-8977-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro BD, Broadwin R, Lipsett MJ. Coarse and fine particles and daily mortality in the Coachella Valley, California: a follow-up study. J Exposure Anal Environ Epidemiol. 2000;10:412–19. doi: 10.1038/sj.jea.7500094. [DOI] [PubMed] [Google Scholar]
- Peng RD, Chang HH, Bell ML, et al. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among medicare patients. JAMA. 2008;299:2172–9. doi: 10.1001/jama.299.18.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng RD, Dominici F, Pastor-Barriuso R, et al. Seasonal analyses of air pollution and mortality in 100 US cities. Am J Epidemiol. 2005;161:585–94. doi: 10.1093/aje/kwi075. [DOI] [PubMed] [Google Scholar]
- Perez L, Medina-Ramon M, Kunzli N, et al. Size fractionate particulate matter, vehicle traffic, and case-specific daily mortality in Barcelona, Spain. Environ Sci Technol. 2009;43:4707–14. doi: 10.1021/es8031488. [DOI] [PubMed] [Google Scholar]
- Peters A, Liu E, Verrier RL, et al. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11:11–17. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Plummer LE, Ham W, Kleeman MJ, et al. Influence of season and location on pulmonary response to California’s San Joaquin Valley airborne particulate matter. J Toxicol Environ Health A. 2012;75:253–71. doi: 10.1080/15287394.2012.640102. [DOI] [PubMed] [Google Scholar]
- Pope CA, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–41. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–7. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Puett RC, Hard JE, Yanosky JD, et al. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ Health Study. Environ Health Perspect. 2009;117:1697–701. doi: 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao GV, Tinkle S, Weissman DN, et al. Efficacy of a technique for exposing the mouse lung to particles aspirated from the pharynx. J Toxicol Environ Health A. 2003;66:1441–52. doi: 10.1080/15287390306417. [DOI] [PubMed] [Google Scholar]
- Sarnat SE, Klein M, Sarnat JA, et al. An examination of exposure measurement error from air pollutant spatial variability in time-series studies. J Expo Sci Environ Epidemiol. 2010;20:135–46. doi: 10.1038/jes.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schins RPF, Lightbody JH, Borm PJA, et al. Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol Appl Pharmacol. 2004;195:1–11. doi: 10.1016/j.taap.2003.10.002. [DOI] [PubMed] [Google Scholar]
- See SW, Wang YH, Balasubramanian R. Contrasting reactive oxygen species and transition metal concentrations in combustion aerosols. Environ Res. 2007;103:317–24. doi: 10.1016/j.envres.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Shen H, Anastasio C. Formation of hydroxyl radical from San Joaquin Valley particles extracted in a cell-free surrogate lung fluid. Atmos Chem Phys. 2011;11:9671–82. doi: 10.5194/acp-11-9671-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, Duffin R, Borm PJ, et al. Hydroxyl-radical-dependent DNA damage by ambient particulate matter from contrasting sampling locations. Environ Res. 2006;101:18–24. doi: 10.1016/j.envres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Shridhar V, Khillare PS, Agarwal T, Ray S. Metallic species in ambient particulate matter at rural and urban location of Delhi. J Hazard Mater. 2010;175:600–7. doi: 10.1016/j.jhazmat.2009.10.047. [DOI] [PubMed] [Google Scholar]
- Steenhof M, Gosens I, Strak M, et al. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential – the RAPTES project. Particle Fibre Toxicol. 2011;8:26. doi: 10.1186/1743-8977-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanie V, Armelle B, Laurent M, et al. Role of size and composition of traffic and agricultural aerosols in the molecular responses triggered in airway epithelial cells. Inhal Toxicol. 2011;23:627–40. doi: 10.3109/08958378.2011.599445. [DOI] [PubMed] [Google Scholar]
- Svendsen ER, Yeatts KB, Peden D, et al. Circulating neutrophil CD14 expression and the inverse association of ambient particulate matter on lung function in asthmatic children. Ann Allergy Asthma Immunol. 2007;99:244–53. doi: 10.1016/S1081-1206(10)60660-6. [DOI] [PubMed] [Google Scholar]
- Tong H, Cheng WY, Samet JM, et al. Differential cardiopulmonary effects of size-fractionated ambient particulate matter in mice. Cardiovasc Toxicol. 2010;10:259–67. doi: 10.1007/s12012-010-9082-y. [DOI] [PubMed] [Google Scholar]
- Turner MC, Krewski D, Pope CA, et al. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never-smokers. Am J Respir Crit Care Med. 2011;184:1374–81. doi: 10.1164/rccm.201106-1011OC. [DOI] [PubMed] [Google Scholar]
- Venkatachari P, Hopke PK, Grover BD, Eatough DJ. Measurement of particle-bound reactive oxygen species in rubidoux aerosols. J Atmos Chem. 2005;52:325–6. [Google Scholar]
- Wang F, Li C, Liu W, Jin Y. Effect of exposure to volatile organic compounds (VOCs) on airway inflammatory response in mice. J Toxicol Sci. 2012;37:739–48. doi: 10.2131/jts.37.739. [DOI] [PubMed] [Google Scholar]
- Wegesser TC, Last JA. Lung response to coarse PM: bioassay in mice. Toxicol Appl Pharmacol. 2008;230:159–66. doi: 10.1016/j.taap.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WE, Mar TF, Koenig JQ. Influence of exposure error and effect modification by socioeconomic status on the association of acute cardiovascular mortality with particulate matter in Phoenix. J Exposure Sci Environ Epidemiol. 2007;17:S11–19. doi: 10.1038/sj.jes.7500620. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect. 2009;117:898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]