Abstract
The hybrid mouse diversity panel (HMDP), a panel of 100 strains, has been employed in genome wide association studies (GWAS) to study complex traits in mice. Hearing is a complex trait and the CBA/CaJ mouse strain is a widely used model for age-related hearing loss (ARHI) and noise induced hearing loss (NIHL). The CBA/CaJ strain's youthful sensitivity to noise and limited age-related loss led us to attempt to identify additional strains segregating a similar phenotype for our panel. FVB/NJ is part of the HMDP and has been previously described as having a similar ARHI phenotype to CBA/CaJ. For these reasons, we have studied the FVB/NJ mouse for ARHI and NIHL phenotypes in hopes of incorporating its phenotype into HMDP studies. We demonstrate that FVB/NJ exhibits ARHI at an earlier age than CBA/CaJ and young FVB/NJ mice are vulnerable to NIHL up until 10 to 12 weeks. This suggests that FVB/NJ may be used as an additional genetic model for neural forms of progressive hearing loss and for the study of youthful sensitivity to noise.
Keywords: Hybrid mouse diversity panel, noise-induced hearing loss, age-related hearing impairment, auditory brainstem response, distortion product otoacoustic emissions, FVB/NJ mice
Introduction
The two most common forms of hearing loss in our society are age-related (ARHI) and noise-induced (NIHL). Age-related hearing loss is one of the most common sensory abnormalities in the world, affecting 30% of people by the age of 65 [National Institute on Deafness and Communication Disorders, 2010]. Noise-induced hearing loss is the most common preventable form of sensorineural hearing loss (SNHL) worldwide [Dobie, 2008] and approximately 500 million individuals are exposed to hazardous levels of noise in occupational and social environments [Sliwinska-Kowalska and Davis, 2012]. Of concern, adolescents and young adults are increasingly exposed to hazardous levels of noise during social activities [Smith et al., 2000; Sadhra et al., 2002; Serra et al., 2005; Vogel et al., 2007]. Several reports have shown that this age group reports symptoms of hearing loss [Niskar et al., 2001; Shargorodsky et al., 2010; Henderson et al., 2011] and a cohort of children and adolescents aged 6 to 19 were found to have audiometric evidence of NIHL [Maassen et al, 2001; Mercier et al., 2002]. In addition, an increasing number of teenagers and adolescents are using personal listening devices [Punch et al., 2011; Vogel et al., 2007] and listening to music at damaging levels [McNeill et al., 2010; Portnuff et al., 2011] with significant threshold shifts at high frequency and decreased distortion product otoacoustic emission (DPOAE) amplitudes [Sulaiman et al., 2013]. Moreover, studies in both humans and mice have shown evidence that the effects of noise exposure and aging combine, leading to a faster progression of hearing loss with age [Gates et al., 2000; Kujawa and Liberman, 2006].
Identifying susceptibility genes will ultimately inform treatment strategies for these two ailments. We are able to exploit the evidence that there are genetic and environmental factors influencing ARHI and NIHL [Friedman et al., 2009; White et al., 2009]. Recently, genome wide association studies (GWAS) have been employed to discover genes conferring susceptibility to complex traits in humans. We have recently identified variations in GRM7 associated with ARHI in a European cohort and validated our findings in a US cohort [Friedman et al., 2009; Newman et al., 2012]
Estimates from human twin studies suggest that the heritability of NIHL is approximately 36% [Johnson et al., 1992] and candidate gene studies using single nucleotide polymorphisms (SNPs) have identified a small number of potential susceptibility genes [Henderson et al., 1991; Fortunado et al., 2004; Helfer et al., 2005; Van Eyken et al., 2007; Price, 2007]. Unfortunately, many of these studies have low statistical power due to small sample sizes and lack replication. Finally, the combined risk from these alleles fails to account for the majority of the genetic risk. [Heinonen-Guzejev et al., 2005].
Although many insights have been gleaned for many human diseases utilizing GWAS and candidate gene studies, only a modest proportion of the genetic risk has been explained for most traits and the effects of environmental exposures such as noise are difficult to control [McCarthy et al., 2008]. It is for this reason that many researchers have turned to mouse models. For almost 50 years, the mouse has been an essential animal model for studies in hearing loss. Advances in mouse genetics including genome sequencing and high-density SNP maps provide a suitable system for the study of complex traits such as ARHI and NIHL. Strain variation in ARHI and NIHL susceptibility has been demonstrated in both in our laboratory [Niu et al., 2006] and in others [Van Laer et al., 2006; Konings et al., 2007; Konings et al., 2009a]. These data indicate that a significant component of these forms of hearing loss is heritable. Furthermore, several strain-specific loci for ARHI are also associated with NIHL susceptibility, confirming an overlap [Konings et al., 2009b].
Much of the progress in the genetics of hearing disorders in the mouse has come from the application of linkage analysis (i.e. QTL analysis) to identify naturally occurring single gene mutations (Mendelian traits) and the analysis of targeted gene deletions. Little attention has been directed towards the definition of the genetics of common hearing disorders. Classical genetic approaches have been used to identify several QTL that modulate ARHI and NIHL susceptibility [Johnson et al., 2006]. One of the most significant shortcomings of QTL analysis is the use of a limited resource (i.e. a segregating F2 population). Another limitation of this approach is the genomic resolution, typically on the order of 10 Mbp or greater. It is for these reasons that we have begun using a novel high-resolution association mapping strategy, the hybrid mouse diversity panel (HMDP), to study common forms of hearing loss in mice.
The HMDP is a panel that combines the use of 29 classical inbred and 71 recombinant inbred strains of mice for association studies [Bennett et al., 2010]. Power calculations have demonstrated that this panel is superior to traditional linkage analysis and is capable of detecting loci responsible for 10% of the overall variance. Several studies have successfully mapped candidate loci for complex traits using this panel [Farber et al., 2011; Park et al., 2011; Smolock et al., 2012; Davis et al., 2013]. Use of the HMDP for GWAS in mice can allow us to map genes at a much higher resolution than traditional analyses. A minimum requirement for success is the accurate phenotyping of the clinical traits of interest in mice that are distantly related.
Resistance to noise exposure and age-related changes are important phenotypes to understand and use in GWAS. Historically, CBA/CaJ mice have been extensively studied as a model of resistance to NIHL [Kujawa and Liberman, 2006; Li, 1992; Li and Borg, 1993] and ARHI [Li and Borg, 1991; Willott et al,. 1992; Frisina et al., 1997]. They also exhibit ARHI at a much older age than other strains of mice [Martin et al,. 2007; Zheng et al., 1999; Zhou et al,. 2006]. Of additional interest, a youthful sensitivity to NIHL has been demonstrated in CBA/CaJ mice. In an effort to identify mice with a phenotype similar to CBA/CaJ, we explored strains that have been grouped as “CBA-like.” FVB/NJ mice have been described as “CBA-like” in comparisons of hearing phenotypes via DPOAE [Martin et al., 2007] and auditory brain response (ABR) measurements [Zheng et al., 1999; Zhou et al,. 2006]. Yet, previous studies have not characterized NIHL in FVB/NJ mice. In this manuscript, we characterize FVB/NJ noise susceptibility and age-related hearing changes by ABR and DPOAE. We demonstrate that like CBA/CaJ, FVB/NJ mice exhibit susceptibility to NIHL at a young age, and age-related changes in auditory thresholds. CBA/CaJ has recently been added to the HMDP. Identifying additional strains, such as FVB/NJ, within the panel that are “CBA-like” will only enhance our ability to map the loci responsible for both the youthful sensitivity to noise and the strain variation in ARHI.
Methods
Animals
FVB/NJ mice were housed with ambient noise not exceeding that of normal air conditioning. Occasional periods of increased noise may have been present weekly during cage changing activity. To avoid any confounding variables related to sex, only female animals were used. Pre-exposure threshold levels were obtained via ABR from 4-5, 9-10, 12-13, 15-16, 20-22, 26, and 34 week old mice (n=4 to 17). Mice were purchased from The Jackson Labs (Bar Harbor, ME) or bred internally. The use of animals described in this study was performed in accordance with guidelines placed by the Institutional Animal Care and Use Committee (IACUC) at the House Research Institute and with principles stated in the Declaration of Helsinki. All procedures were approved by the IACUC and conformed to institutional standards.
Noise exposure and audiometric equipment
5, 10, 12, and 22 week old mice (n=4 to 13) were exposed to 10 kHz octave band noise (OBN) for 2 hours at 100 dB SPL using a method adapted from Kujawa and Liberman (2009). The OBN noise exposure was previously described [White et al., 2009]. During exposure, mice were housed in a circular ¼ inch wire-mesh exposure cage with four pie shaped compartments and were permitted to move about within the compartment. The cage was placed in a MAC-1 sound-proof chamber designed by Industrial Acoustics (IAC, Bronx, NY) and the sound chamber was lined with 1-in-thick acoustical soundproofing foam to minimize sound reflections. Noise recordings were played with a Fostex FT17H Tweeter Speaker built into the top of the sound chamber. Calibration of the damaging noise was done with a B&K sound level meter with a variation of 1.5 dB across the cage.
Stimuli were delivered by a custom acoustic system, consisting of two miniature speakers. Sound pressure was measured by an electret condenser microphone. A data acquisition board from National Instruments (National Instruments Corporation, Austin, Texas) was controlled by custom software and was used to generate the stimuli and to process the response. All tests were performed in a separate MAC-1 sound-proof chamber to eliminate both environmental and electrical noise. Testing involved the right ear only.
ABR and DPOAE measurements
Mice were anesthetized with an intraperitoneal injection of a mixture of ketamine (80 mg/kg body weight) and xylazine (16 mg/kg body weight). Body temperature was maintained and an artificial tear ointment was applied to the eyes. Mice recovered on a heating pad at body temperature.
Auditory signals were presented as tone pips with a rise and a fall time of 0.5 msec and a total duration of 5 msec at the frequencies 4, 8, 12, 16, 24, and 32 kHz. Tone pips were delivered below threshold and then increased in 5 dB increments until reaching 100 dB. Signals were presented at a rate of 30 per second. Responses were filtered with a 0.3 to 3 kHz passband and amplified 10,000 times. Up to 512 waveforms were averaged for each stimulus intensity. Stainless-steel electrodes were placed subcutaneously at the vertex of the head and the right mastoid, with a ground electrode at the base of the tail. Hearing threshold was determined by visual inspection of ABR waveforms and was defined as the minimum intensity at which wave 1 could be distinguished. Data was stored for offline analysis of peak to peak (p-p) values (from peak to trough) for wave 1 amplitudes.
DPOAEs were obtained as input/output (I-O) functions with 2f1- f2 as the primary measure. Primary tones were set at a ratio of f2/f1 = 1.2 with the f2 between 5.6 to 32 kHz, f2 level set 10 dB less than the f1 level, and L2 ranging from 20 to 70 dB. The DPOAEs were extracted after both waveform and spectral averaging. The noise floor was obtained by averaging 6 spectral points above and below the 2f1- f2. Threshold was defined as the L2 level needed to produce a DPOAE of 0 dB SPL, with a signal to noise ratio of at least 3 dB.
Determination of permanent threshold shifts
Pre-exposure threshold levels were obtained at least 4 days prior to noise exposure. These threshold levels were used as a baseline to determine threshold shifts after exposure to noise. Animals were assessed for noise damage three to four weeks after exposure. The permanent threshold shifts (PTS) for ABR and DPOAE were defined as the difference between pre-exposure and post-exposure thresholds at each tested frequency or L2 level, respectively.
Statistics
Comparisons between thresholds, amplitudes and PTS were performed using student's t tests (Welsh test). P-values less than 0.05 were considered significant.
Results
FVB/NJ mice demonstrate age-related changes in hearing
Zheng et al., in 1999, characterized ABR thresholds in young and old classically inbred strains of mice. In their study, they demonstrated that CBA/CaJ mice maintain stable threshold sensitivity as they age [Henry, 1982; Jimenez et al., 1999]. The FVB/NJ strain was not detailed in that study but has long been considered phenotypically similar to CBA/CaJ. To explore this in the hopes of identifying an identical phenotype for genetic study, we sought to characterize the hearing of FVB/NJ mice with age utilizing ABR. Martin et al., in 2007, conducted a comparison of DPOAE in 28 inbred strains of mice, including FVB/NJ. In their study, DP-grams for FVB/NJ mice were identical to those of CBA/CaJ demonstrating stable outer hair cell function over time (5 months being the oldest). Their conclusion supported the notion that FVB/NJ was in fact a very similar strain with regard to ARHI. In addition, in a study by Jimenez et al. CBA/CaJ mice have been shown to display stable DPOAEs for up to 15 months of age [Jimenez et al., 1999].
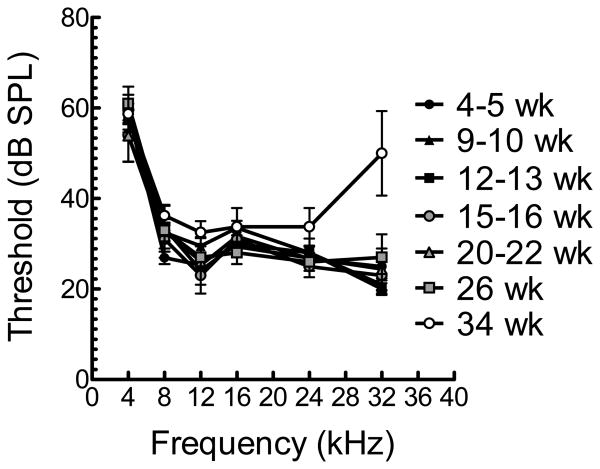
To characterize hearing with age in FVB/NJ mice, we performed ABR at several ages ranging from 5 weeks to 34 weeks (Figure 1). Unlike CBA/CaJ, 34 week-old FVB/NJ mice demonstrated statistically significantly elevated thresholds at 32kHz (p < 0.027). These data suggest that, unlike the stability of OHC function (DPOAE) demonstrated by Martin et al. in FVB/NJ mice [Martin et al., 2007], there may be a loss of cochlear neurons accounting for age-related changes that might serve as a good genetic model for neural presbycusis. We sought to test this notion by looking at wave I p-p amplitudes over time.
Figure 1.
Mean ABR thresholds (± SD) for mice of various ages from 5 to 34 weeks. Mice at 34 weeks demonstrate significantly elevated thresholds at 32 kHz (p < 0.027).
Suprathreshold ABR in FVB/NJ mice supports the notion that ARHI in FVB/NJ mice results from cochlear neuronal loss
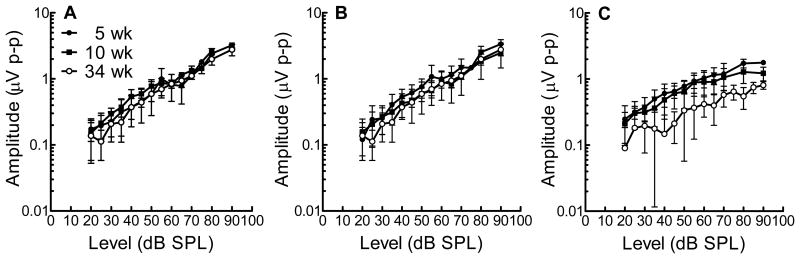
We performed suprathreshold ABR measurements of p-p wave I amplitudes across a select number of time points (Figure 2 a, b, c). Although measurements for 8 kHz and 16 kHz are similar between 5, 10, and 34 week-old mice, p-p wave I amplitudes at 32 kHz are significantly reduced when comparing the youngest mice (5 week-old) to the oldest mice (34 week-old) (Figure 2c). These data, in light of the DPOAE data described previously [Jimenez et al., 1999; Martin et al., 2007] support the notion that the age-related changes in the FVB/NJ mice at 34 weeks are the result of loss of cochlear neurons.
Figure 2.
ABR wave 1 amplitudes for 5, 10, 34 week old mice (mean ± SD) show that suprathreshold amplitudes at (A) 8kHz and (B) 16kHz do not show a difference in threshold values, but at (C) 32kHz, there is a significant difference decrease in suprathreshold values for old mice (34 weeks) in comparison to 5 week old mice.
FVB/NJ mice are vulnerable to NIHL at an early age
As previously described, permanent thresholds shifts after noise exposure are measured at approximately three to four weeks post exposure when they are at a steady state [Miller et al., 1963]. To further characterize the potential similarities between CBA/CaJ and FVB/NJ we evaluated vulnerability to NIHL with age.
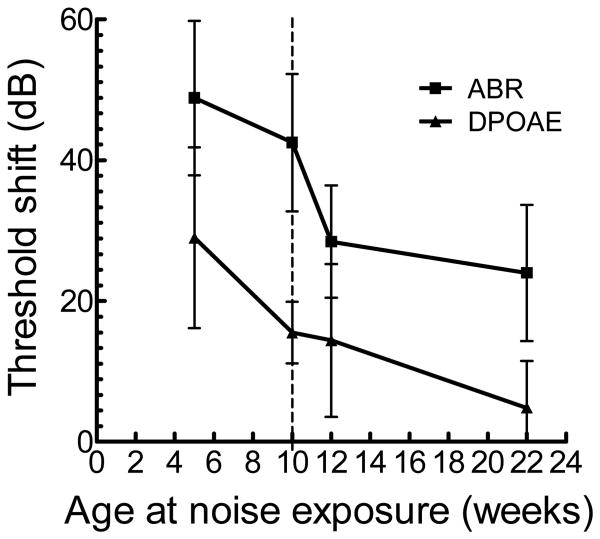
In brief, age at the time of noise exposure was systematically varied at 5, 10, 12, and 22 weeks. As noted in a previous study [Kujawa and Liberman, 2006], the largest threshold shifts were noted at 16 kHz and the age-dependent vulnerability can be seen in Figure 3. The youngest mice (5 weeks) demonstrate the greatest vulnerability to NIHL with PTS of approximately 50 dB for ABR and 30 dB for DPOAE suggesting a combined injury with the larger component of the injury occurring in the cochlear neurons. Further analysis of the data shows resistance to noise occurring at approximately 12 weeks when measuring ABR and roughly 10 weeks for the DPOAEs. The older mice (22 weeks) have a minimal PTS by DPOAE; however, in contrast, the PTS remains relatively high when looking at the ABR. These data confirm a greater vulnerability to NIHL in the younger animals, and also suggest that the OHC are both the earliest site of resistance with aging and more resistant to noise than the cochlear neurons at all ages.
Figure 3.
Permanent threshold shifts (PTS) for ABR and DPOAE at 16kHz for mice exposed to noise during various ages (mean ± SD). The vertical dashed line denotes the age of exposure (10 weeks) at which the PTS for DPOAE has dropped significantly (p = 0.0099) without a corresponding change in PTS for ABR (p > 0.05), and at 12 weeks, PTS for ABR has diminished significantly as well (p = 0.000902).
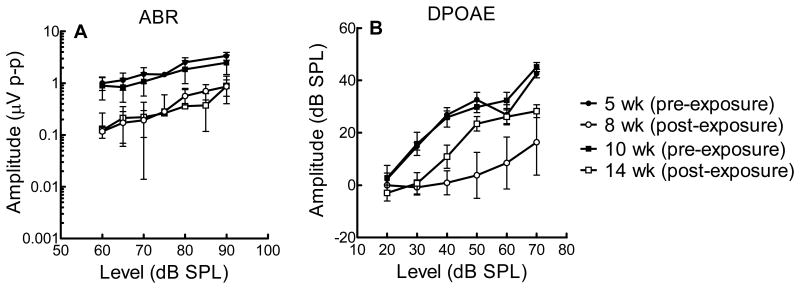
To study the effect of NIHL on outer hair cells and the cochlear nerve further, we looked at suprathreshold responses (DPOAE and ABR) to a 16 kHz stimulus in mice exposed to noise at 5 and 10 weeks (Figure 4). As anticipated, the post-exposure amplitudes were diminished in both groups of mice. On closer observation, the cochlear neurons do not show differences in sensitivity to noise between the groups. On the other hand, the DPOAE I-O functions demonstrate greater OHC resistance in the older mice (Figure 4). These data are congruent with the findings shown in Figure 3 and reinforce the notion that the OHCs experience a decreased sensitivity to noise at an earlier age than cochlear neurons.
Figure 4.
(A) ABR wave 1 and (B) DPOAE amplitudes (I-O functions) for mice at age of exposure and post-exposure for 16kHz (mean ± SD). Mice exposed at 5 weeks demonstrate a dramatic decrease in DPOAE amplitude after noise exposure in comparison to mice exposed at 10 weeks.
Discussion
Early neural presbycusis and noise susceptibility
CBA/CaJ is a well-characterized model for delayed onset ARHI and exhibits stable ABR thresholds until later in life at 39 weeks of age at least [Zheng et al., 1999; Yoshida et al., 2000; May et al., 2002]. Although FVB/NJ has been previously thought to be similar to CBA/CaJ, our results indicate that FVB/NJ exhibits high frequency ARHI at a significantly younger age (Figure 1) than CBA/CaJ [Li, 1992; May et al., 2006]. Using Schuknecht's models of presbycusis as a framework to understand ARHI, neural presbycusis describes neuronal degeneration that occurs sooner and at a greater degree than loss of hair cells [Schuknecht and Gacek, 1993]. A few models have been reported, including knockouts of the nicotinic acetylcholine receptor beta 2 subunit [Boa et al., 2005] and SOD1 [Keithley et al., 2005]. FVB/NJ exhibits neural presbycusis, with high frequency loss at 32kHz starting at 34 weeks as demonstrated by decreased ABR wave 1 amplitudes (Figure 2). Thus, FVB/NJ exhibits an increase in ABR thresholds at an earlier age than CBA/CaJ and can be used as a model for earlier onset neural presbycusis.
In C57BL/6J mice, the Ahl locus has also been found to confer genetic susceptibility to noise damage within the cochlea [Harding et al,. 2005]. Increased vulnerability to NIHL has been attributed to the Dystrophin gene [Chen et al., 2012], Vasodilator-stimulated phosphoprotein [Schick et al., 2004], and Heat shock factor 1 genes [Sugahara et al., 2003; Farber et al., 2011]. In addition to Cdh23Ahl, Cdh23v transgenic mice with knockouts of the genes Cp, GPx1, Pmca2, Sod1, and Trpv4 show vulnerability to noise damage [Ohlemiller, 2006].
Yet, when studying the effect of noise on hearing loss, age has also been shown to interact with noise, influencing the degree of damage. Resistance to NIHL with age has been previously reported in CBA/CaJ [Nemoto et al., 2004; Kujawa and Liberman, 2006] and CBA/Ca mice [Li and Borg, 1993; Li et al., 1993] and evidence suggests that mice are much more susceptible to NIHL at younger ages [Henry, 1982; Ohlemiller et al., 2000; Saunders and Hirsch, 1976]. FVB/NJ also exhibits resistance with age, with decreased PTS for both ABR and DPOAE measurements in older mice (Figure 2).
Age influences the effects of noise exposure
In 2006, Kujawa and Liberman showed that young CBA/CaJ mice were much more sensitive to high-level noise damage than older mice after exposure to 100dB SPL OBN for 2 hours. Permanent threshold shifts were observed to decrease extensively between 8 and 16 weeks of exposure for both ABR and DPOAE. In a study by Henry (1983), CBA/J mice also showed dramatically decreased sensitivity to NIHL after 16 weeks [Henry, 1983]. To investigate this large change in sensitivity with age between 8 and 16 weeks in FVB/NJ, we looked at noise exposed mice at 5, 10, 12 and 22 weeks. Similar to Kujawa and Liberman's findings, our results show relatively high threshold shifts at 16 kHz for both ABR and DPOAE at 5 weeks of exposure, approximately 50 and 30 dB, respectively, and lower values for exposure at 22 weeks (Figure 3). Looking at exposure at 10 and 12 weeks, the PTS interestingly drops first for DPOAE at 10 weeks exposure (Figure 4), followed by a decrease in PTS for ABR at 12 weeks exposure (Figure 3). These results suggest that the OHCs become more resistant to noise damage at a younger age than the inner hair cells (IHCs) and cochlear nerve.
Based upon the present findings that the DPOAE PTS decreases before the ABR, one may infer that the IHCs could exhibit greater or earlier morphological changes than the OHCs secondary to noise exposure at these ages. However, noise exposure has been shown to cause OHC loss, followed by IHC and spiral ganglion cell loss [Ou et al., 2000; Wang et al., 2002]. After exposure to noise (2 hours of broad band noise at 106 dB), male CBA/CaJ mice at 10 to 12 weeks of age show greater degrees of OHC loss than IHC loss towards the basal end of the cochlea [Hirose and Liberman, 2003]. Similar findings are reported in 12 week old CBA/J mice [Chen et al., 2012].
On the other hand, our results are consistent with the notion that the cochlear nerve is relatively more sensitive than the OHCs at younger ages. This agrees with a study which showed that 16 week old CBA/CaJ mice exposed to 2 hr OBN at 100 dB displayed complete recovery of DPOAEs by 8 weeks and ABRs which showed only modest recovery, especially at suprathreshold values [Kujawa and Liberman, 2009]. Although our study uses mice exposed to traumatic levels of noise, moderate noise exposure in CBA/CaJ mice has recently been shown to damage the cochlear nerve at a dramatically greater degree than OHCs. In CBA/CaJ mice from 4 to 144 weeks of age, exposure to moderate levels of noise (between 50 to 60 dB >95% of time and less than 80 dB >99% of the time) caused shown IHC synaptic loss prior to changes in OHC thresholds and hair cell counts [Maison et al., 2013]. This evidence suggests that noise induced damage may have its greatest affect on the cochlear nerve across a range of ages.
FVB/NJ, as part of the HMDP, adds to our capacity to map hearing loss loci via GWAS
In recent years, mouse GWAS have led to the discovery of many genes associated with a variety of complex traits in humans. Successful use of GWAS for hearing disorders rests on the basis that the genes controlling the hearing phenotype in mice are similar to those in humans and that the genetic and phenotypic variation in mouse strains can be harnessed to study the variation in human populations. In the past, some GWAS have been challenged as lacking enough power to detect genes that may have small contributions to complex phenotypes [Pletcher et al. 2004, de Bakker et al. 2005, Payseur and Place 2007]. A solution to this constraint, the HMDP, was created as a resource for GWAS and has the power to map complex traits with relatively small variances in phenotype (70% power to detect effect sizes < 10%) [Bennett, 2010]. The HMDP includes FVB/NJ and has been successfully used to find candidate loci for several complex traits in the mouse including fear conditioning [Park et al., 2011], bone morphogenic density [Farber et al., 2011], heart rate [Smolock et al., 2012], and blood cell traits [Davis et al., 2013]. The identification of FVB/NJ's phenotypic similarity to CBA/CaJ, while having distant relatedness, provides an additional useful strain for the study of susceptibilities to both ARHI and NIHL in mice.
Acknowledgments
The authors would like to acknowledge Dr. M. Charles Liberman for providing hardware specifications and software for audiometric testing. In addition, we thank John Wygonski for building and testing the acoustic sound delivery apparatus, Ping Luo for engineering support, Cory White for his assistance with statistics and Aldo Castillo for technical assistance with data collection.
Funding: This study was supported by NIH NIDCD grant R01 DC010856-01 (RAF) and NIH NIDCD Engineering Core grant P30 DC006276-11.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- Bao J, Lei D, Du Y, Ohlemiller KK, Beaudet AL, Role LW. Requirement of nicotinic acetylcholine receptor subunit b2 in the maintenance of spiral ganglion neuron during aging. J Neurosci. 2005;25:3041–3045. doi: 10.1523/JNEUROSCI.5277-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BJ, Farber CR, Orozco L, Kang HM, Ghazalpour A, Siemers N, Neubauer M, Neuhaus I, Yordanova R, Guan B, Truong A, Yang WP, He A, Kayne P, Gargalovic P, Kirchgessner T, Pan C, Castellani LW, Kostem E, Furlotte N, Drake TA, Eskin E, Lusis AJ. A high-resolution association mapping panel for the dissection of complex traits in mice. Genome Res. 2010;20(2):281–290. doi: 10.1101/gr.099234.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FQ, Zheng HW, Hill K, Sha SH. Traumatic noise activates Rho-family GTPases through transient cellular energy depletion. J Neurosci. 2012;32(36):12421–30. doi: 10.1523/JNEUROSCI.6381-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RC, van Nas A, Bennett B, Orozco L, Pan C, Rau CD, Eskin E, Lusis AJ. Genome-wide association mapping of blood cell traits in mice. Mamm Genome. 2013;24:105–118. doi: 10.1007/s00335-013-9448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37(11):1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- DeStefano AL, Gates GA, Heard-Costa N, Myers RH, Baldwin CT. Genomewide linkage analysis to presbycusis in the Framingham Heart Study. Arch Otolaryngol Head Neck Surg. 2003;129(3):285–289. doi: 10.1001/archotol.129.3.285. [DOI] [PubMed] [Google Scholar]
- Dobie RA. The burdens of age-related and occupational noise-induced hearing loss in the United States. Ear Hear. 2008;24(4):565–577. doi: 10.1097/AUD.0b013e31817349ec. [DOI] [PubMed] [Google Scholar]
- Farber CR, Bennett BJ, Orozco L, Zou W, Lira A, Kostem E, Kang HM, Furlotte N, Berberyan A, Ghazalpour A, Suwanwela J, Drake TA, Eskin E, Wang QT, Teitelbaum SL, Lusis AJ. Mouse genome-wide association and systems genetics identify Asxl2 as a regulator of bone mineral density and osteoclastogenesis. PLoS Genet. 2011;7(4):e1002038. doi: 10.1371/journal.pgen.1002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, Eskin E. Genome-wide association studies in mice. Nature Reviews Genetics. 2012;13(11):807–817. doi: 10.1038/nrg3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina RD, Walton JP, Lynch-Armour MA, Klotz DA. Efferent projections of a physiologically characterized region of the inferior colliculus of the young adult CBA mouse. J Acoust Soc Am. 1997;101(5 Pt 1):2741–2753. doi: 10.1121/1.418562. [DOI] [PubMed] [Google Scholar]
- Gates GA, Couropmitree NN, Myers RH. Genetic associations in age-related hearing thresholds. Arch Otolaryngol Head Neck Surg. 1999;125(6):654–659. doi: 10.1001/archotol.125.6.654. [DOI] [PubMed] [Google Scholar]
- Gates GA, Schmid P, Kujawa SG, Nam B, D'Agostino R. Longitudinal threshold changes in older men with audiometric notches. Hear Res. 2000;141(1-2):220–228. doi: 10.1016/s0378-5955(99)00223-3. [DOI] [PubMed] [Google Scholar]
- Harding GW, Bohne BA, Vos JD. The effect of an age-related hearing loss gene (Ahl) on noise-induced hearing loss and cochlear damage from low-frequency noise. Hear Res. 2005;204:90–100. doi: 10.1016/j.heares.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Heinonen-Guzejev M, Vuorinen HS, Mussalo-Rauhamaa H, Heikkilä K, Koskenvuo M, Kaprio J. Genetic component of noise sensitivity. Twin Res Hum Genet. 2005;8(3):245–249. doi: 10.1375/1832427054253112. [DOI] [PubMed] [Google Scholar]
- Helfer TM, Jordan NN, Lee RB. Postdeployment hearing loss in U.S. Army soldiers seen at audiology clinics from April 1, 2003, through March 31, 2004. Am J Audiol. 2005;14(2):161–8. doi: 10.1044/1059-0889(2005/018). [DOI] [PubMed] [Google Scholar]
- Henderson E, Testa MA, Hartnick C. Prevalence of noise-induced hearing-threshold shifts and hearing loss among US youths. Pediatrics. 2011;127:e39–e46. doi: 10.1542/peds.2010-0926. [DOI] [PubMed] [Google Scholar]
- Henry KR. Influence of genotype and age on noise-induced auditory losses. Behav Genet. 1982;12:563–573. doi: 10.1007/BF01070410. [DOI] [PubMed] [Google Scholar]
- Henry KR. Lifelong susceptibility to acoustic trauma: changing patterns of cochlear damage over the life span of the mouse. Audiology. 1983;22:372–383. doi: 10.3109/00206098309072797. [DOI] [PubMed] [Google Scholar]
- Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J Assoc Res Otolaryngol. 2003;4(3):339–352. doi: 10.1007/s10162-002-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez AM, Stagner BB, Martin GK, Lonsbury-Martin BL. Age-related loss of distortion product otoacoustic emissions in four mouse strains. Hear Res. 1999;138(1-2):91–105. doi: 10.1016/s0378-5955(99)00154-9. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Noben-Trauth K. Strain background effects and genetic modifiers of hearing in mice. Brain Res. 2006;1091(1):79–88. doi: 10.1016/j.brainres.2006.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE, DeFries JC, Markel PD. Mapping quantitative trait loci for behavioral traits in the mouse. Behav Genet. 1992;22(6):635–53. doi: 10.1007/BF01066635. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Wang X, Fishcel-Ghodsian N, Johnson KR. Cu/Zn superoxide dismutase and age-related hearing loss. Hear Res. 2005;209:76–85. doi: 10.1016/j.heares.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings A, Van Laer L, Pawelczyk M, Carlsson PI, Bondeson ML, Rajkowska E, Dudarewicz A, Vandevelde A, Fransen E, Huyghe J, Borg E, Sliwinska-Kowalska M, Van Camp G. Association between variations in CAT and noise-induced hearing loss in two independent noise-exposed populations. Hum Mol Genet. 2007;16(15):1872–1883. doi: 10.1093/hmg/ddm135. [DOI] [PubMed] [Google Scholar]
- Konings A, Van Laer L, Michel S, Pawelczyk M, Carlsson PI, Bondeson ML, Rajkowska E, Dudarewicz A, Vandevelde A, Fransen E, Huyghe J, Borg E, Sliwinska-Kowalska M, Van Camp G. Variations in HSP70 genes associated with noise-induced hearing loss in two independent populations. Eur J Hum Genet. 2009a;17(3):329–35. doi: 10.1038/ejhg.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings A, Van Laer L, Wiktorek-Smagur A, Rajkowska E, Pawelczyk M, Carlsson PI, Bondeson ML, Dudarewicz A, Vandevelde A, Fransen E, Huyghe J, Borg E, Sliwinska-Kowalska M, Van Camp G. Candidate gene association study for noise-induced hearing loss in two independent noise-exposed populations. Ann Hum Genet. 2009b;73(2):215–24. doi: 10.1111/j.1469-1809.2008.00499.x. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26(7):2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS. Influence of genotype and age on acute acoustic trauma and recovery in CBA/Ca and C57BL/6J mice. Acta Oto-Laryngol. 1992;112:956–967. doi: 10.3109/00016489209137496. [DOI] [PubMed] [Google Scholar]
- Li HS, Borg E. Age-related loss of auditory sensitivity in two mouse genotypes. Acta otolaryngol. 1991;111:827–834. doi: 10.3109/00016489109138418. [DOI] [PubMed] [Google Scholar]
- Li HS, Borg E. Auditory degeneration after acoustic trauma in two genotypes of mice. Hear Res. 1993;68(1):19–27. doi: 10.1016/0378-5955(93)90060-e. [DOI] [PubMed] [Google Scholar]
- Li HS, Hultcrantz M, Borg E. Influence of age on noise-induced permanent threshold shifts in CBA/Ca and C57BL/6J mice. Audiology3. 1993;2:195–204. doi: 10.3109/00206099309072935. [DOI] [PubMed] [Google Scholar]
- Maassen M, Babisch W, Bachmann KD, Ising H, Lehnert G, Plath P, Plinkert P, Rebentisch E, Schuschke G, Spreng M, Stange G, Struwe V, Zenner HP. Ear damage caused by leisure noise. Noise Health. 2001;4:1–16. [PubMed] [Google Scholar]
- Maison SF, Usubuchi H, Liberman MC. Efferent feedback minimizes cochlear neuropathy from moderate noise exposure. J Neurosci. 2013;33(13):5542–5552. doi: 10.1523/JNEUROSCI.5027-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GK, Vazquez AE, Jimenez AM, Stagner BB, Howard MA, Lonsbury-Martin BL. Comparison of distortion product otoacoustic emissions in 28 inbred strains of mice. Hear Res. 2007;234(1-2):59–72. doi: 10.1016/j.heares.2007.09.002. [DOI] [PubMed] [Google Scholar]
- May BJ, Kimar S, Prosen CA. Auditory filter shapes of CBA/CaJ mice: behavioral assessments. J Acoust Soc Am. 2006;120(1):321–330. doi: 10.1121/1.2203593. [DOI] [PubMed] [Google Scholar]
- May BJ, Prosen CA, Weiss D, Vetter D. Behavioral investigation of some possible effects of the central olivocochlear pathways in transgenic mice. Hear Res. 2002;171:142–157. doi: 10.1016/s0378-5955(02)00495-1. [DOI] [PubMed] [Google Scholar]
- McCarthy MI, Goncalo RA, Lon RC, Goldstein DB, Little J, Ioannidis JPA, Hirschhorn JN. Genome-wide association studies for complex traits: consensus uncertainty and challenges. Nat Rev Genet. 2008;9:356–369. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- McNeill K, Keith SE, Feder K, Konkle AT, Michaud DS. MP3 player listening habits of 17 to 23 year old university students. J Acoust Soc Am. 128:646–653. doi: 10.1121/1.3458853. [DOI] [PubMed] [Google Scholar]
- Mercier V. Is Electronically Amplified Music too Loud? What do Young People Think? Noise Health 2010. 2002;4:47–55. [PubMed] [Google Scholar]
- Miller JD, Watson CS. Deafening effects of noise on the cat. Acta Otolaryngol Suppl. 1963;176:1–91. [Google Scholar]
- Nemoto M, Morita Y, Mishima Y, Takahashi S, Nomura T, Ushiki T, Shiroishi T, Kikkawa Y, Yonekawa H, Kominami R. Ahl3, a third locus on mouse chromosome 17 affecting age-related hearing loss. Biochem Biophys Res Commun. 2004;324:1283–1288. doi: 10.1016/j.bbrc.2004.09.186. [DOI] [PubMed] [Google Scholar]
- National Institute on Deafness and Communication Disorders. Quick Statistics [NIDCD Health Information] 2010 Jun 16; Retrived on October 23, 2013 from http://www.nidcd.nih.gov/health/statistics/Pages/quick.aspx.
- Niu H, Makmura L, Shen T, Sheth SS, Blair K, Friedman RA. Identification of two major loci that suppress hearing loss and cochlear dysmorphogenesis in Eya1bor/bor mice. Genomics. 2006;88(3):302–8. doi: 10.1016/j.ygeno.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Niskar AS, Kieszak SM, Holmes AE, Esteban E, Rubin C, Brody DJ. Estimated prevalence of noise-induced hearing threshold shifts among children 6 to 19 years of age: the Third National Health and Nutrition Examination Survey, 1988–1994, United States. Pediatrics. 2001;108:40–43. doi: 10.1542/peds.108.1.40. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006;1091(1):89–102. doi: 10.1016/j.brainres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Heidbreder AF. Vulnerability to noise-induced hearing loss in ‘middle-aged’ and young adult mice: a dose-response approach in CBA, C57BL, and BALB inbred strains. Hear Res. 2000;149(1-2):239–247. doi: 10.1016/s0378-5955(00)00191-x. [DOI] [PubMed] [Google Scholar]
- Ou HC, Bohne BA, Harding GW. Noise damage in the C57BL/CBA mouse cochlea. Hear Res. 2000;145:111–122. doi: 10.1016/s0378-5955(00)00081-2. [DOI] [PubMed] [Google Scholar]
- Park CC, Gale GD, de Jong S, Ghazalpour A, Bennett BJ, Farber CR, Langfelder P, Lin A, Khan AH, Eskin E, Horvath S, Lusis AJ, Ophoff RA, Smith DJ. Gene networks associated with conditional fear in mice identified using a systems genetics approach. BMC Syst Biol. 2011;5:43. doi: 10.1186/1752-0509-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payseur BA, Place M. Prospects for association mapping in classical inbred mouse strains. Genetics. 2007;175(4):1999–2008. doi: 10.1534/genetics.106.067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletcher MT, McClurg P, Batalov S, Su AI, Barnes SW, Lagler E, Korstanje R, Wang X, Nusskern D, Bogue MA, Mural RJ, Paigen B, Wiltshire T. Use of a dense single nucleotide polymorphism map for in silico mapping in the mouse. PLoS Biol. 2004;2(12):e393. doi: 10.1371/journal.pbio.0020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnuff CD, Fligor BJ, Arehart KH. Teenage use of portable listening devices: a hazard to hearing? J Am Acad Audiol. 2011;22:663–677. doi: 10.3766/jaaa.22.10.5. [DOI] [PubMed] [Google Scholar]
- Punch JL, Elfenbein JL, James RR. Targeting hearing health messages for users of personal listening devices. Am J Audiol. 2011;20(1):69–82. doi: 10.1044/1059-0889(2011/10-0039). [DOI] [PubMed] [Google Scholar]
- Sadhra S, Jackson CA, Ryder T, Brown MJ. Noise exposure and hearing loss among student employees working in university entertainment venues. Ann Occup Hyg. 2002;46:455–463. [PubMed] [Google Scholar]
- Saunders JC, Hirsch KA. Changes in cochlear microphonic sensitivity after priming C57BL/6j mice at various ages for audiogenic seizures. J Comp Physiol Psychol. 1976;90(2):212–220. doi: 10.1037/h0077198. [DOI] [PubMed] [Google Scholar]
- Schick B, Praetorius M, Eigenthaler M, Jung V, Müller M, Walter U, Knipper M. Increased noise sensitivity and altered inner ear MENA distribution in VASP-/- mice. Cell Tissue Res. 2004;318(3):493–502. doi: 10.1007/s00441-004-0964-9. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- Serra MR, Biassoni EC, Richter U, Minoldo G, Franco G, Abraham S, Carignani JA, Joekes S, Yacci MR. Recreational noise exposure and its effects on the hearing of adolescents. Part I: An interdisciplinary long-term study. Int J Audiol. 2005;44:65–73. doi: 10.1080/14992020400030010. [DOI] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan SG, Curhan GC, Eavey R. Change in prevalence of hearing loss in US adolescents. JAMA. 2010;304:772–778. doi: 10.1001/jama.2010.1124. [DOI] [PubMed] [Google Scholar]
- Smith PA, Davis A, Ferguson M, Lutman ME. The prevalence and type of social noise exposure in young adults in England. Noise Health. 2000;2:41–56. [PubMed] [Google Scholar]
- Smolock EM, Ilyushkina IA, Ghazalpour A, Gerloff J, Murashev AN, Lusis AJ, Korshunov VA. Genetic locus on mouse chromosome 7 controls elevated heart rate. Physiol Genomics. 2012;3;44:689–98. doi: 10.1152/physiolgenomics.00041.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman aH, Husain R, Seluakumaran K. Evaluation of early hearing damage in personal listening device users using extended high-frequency audiometry and otoacoustic emissions. Eur Arch Otorhinolaryngol. doi: 10.1007/s00405-013-2612-z. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Inouye S, Izu H, Katoh Y, Katsuki K, Takemoto T, Shimogori H, Yamashita H, Nakai A. Heat shock transcription factor HSF1 is required for survival of sensory hair cells against acoustic overexposure. Hear Res. 2003;182(1-2):88–96. doi: 10.1016/s0378-5955(03)00180-1. [DOI] [PubMed] [Google Scholar]
- Sliwinska-Kowalska M, Davis A. Noise-induced hearing loss. Noise Health. 2013;14(61):274–280. doi: 10.4103/1463-1741.104893. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;1;3:248–68. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CH, Ohmen JD, Sheth S, Zebboudj AF, McHugh RK, Hoffman LF, Lusis AJ, Davis RC, Friedman RA. Genome-wide screening for genetic loci associated with noise-induced hearing loss. Mamm Genome. 2009;20(4):207–213. doi: 10.1007/s00335-009-9178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Bross LS, McFadden SL. Morphology of the dorsal cochlear nucleus in C57BL/6J and CBA/J mice across the life span. J Comp Neurol. 1992;321(4):666–678. doi: 10.1002/cne.903210412. [DOI] [PubMed] [Google Scholar]
- Wong K, Bumpstead S, Van Der Weyden L, Reinholdt LG, Wilming LG, Adams DJ, Keane TM. Sequencing and characterization of the FVB/NJ mouse genome. Genome Biology. 2012;13:R72. doi: 10.1186/gb-2012-13-8-r72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eyken E, Van Laer L, Fransen E, Topsakal V, Hendrickx JJ, Demeester K, Van de Heyning P, Mäki-Torkko E, Hannula S, Sorri M, Jensen M, Parving A, Bille M, Baur M, Pfister M, Bonaconsa A, Mazzoli M, Orzan E, Espeso A, Stephens D, Verbruggen K, Huyghe J, Dhooge I, Huygen P, Kremer H, Cremers C, Kunst S, Manninen M, Pyykkö I, Rajkowska E, Pawelczyk M, Sliwinska-Kowalska M, Steffens M, Wienker T, Van Camp G. The contribution of GJB2 (Connexin 26) 35delG to age-related hearing impairment and noise-induced hearing loss. Otol Neurotol. 2007;28(7):970–5. doi: 10.197/MAO.0b013e3180dca1b9. [DOI] [PubMed] [Google Scholar]
- Vogel I, Brug J, Van der Ploeg CP, Raat H. Young people's exposure to loud music: a summary of the literature. Am J Prev Med. 2007;33:124–133. doi: 10.1016/j.amepre.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Hequembourg SJ, Atencio CA, Rosowski JJ, Liberman MC. Acoustic injury in mice: 129/SvEv is exceptionally resistant to noise-induced hearing loss. Hear Res. 2000;141:97–106. doi: 10.1016/s0378-5955(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130(1-2):94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Jen PH, Seburn KL, Frankel WN, Zheng QY. Auditory brainstem responses in 10 inbred strains of mice. Brain Res. 2006;1091(1):16–26. doi: 10.1016/j.brainres.2006.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]