Abstract
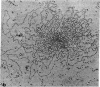
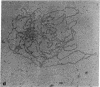
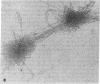
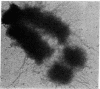
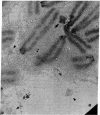
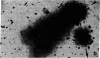
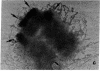
The Miller spreading procedure was applied to mouse metaphase spreads of methotrexate-resistant 3T3 cells that contain large numbers of minute chromosomes and dihydrofolate reductase genes. There is substantial variation in both size and numbers of minutes in individual cells, the smallest of which (estimated as 5 × 103 kilobase pairs) would be undetected by standard light microscopic analyses. Minute chromosomes are composed of nucleosomal chromatin, which is organized into typical higher order fibers that are folded to form rosette-like structures characteristic of normal chromosome organization. There is no evidence that the DNA in minutes is linear. Minutes exist singly and in pairs, and members of a pair are connected by higher order chromatin fibers, suggesting that they are topologically interlocked. They are often closely apposed to chromosomal telomeres or arms, a configuration that may be involved in their distribution at mitosis. In addition to typical minutes, which do not possess kinetochores, a small marker chromosome possessing all of the features of a centromere region is present in parental and resistant cells. An unusual feature of this cell line is the retention of resistance, minute chromosomes, and amplified dihydrofolate reductase genes; most methotrexate-resistant mouse cell lines with minute chromosomes lose these properties when grown in the absence of methotrexate.
Keywords: electron microscopy, amplification, chromosome organization
Full text
PDF

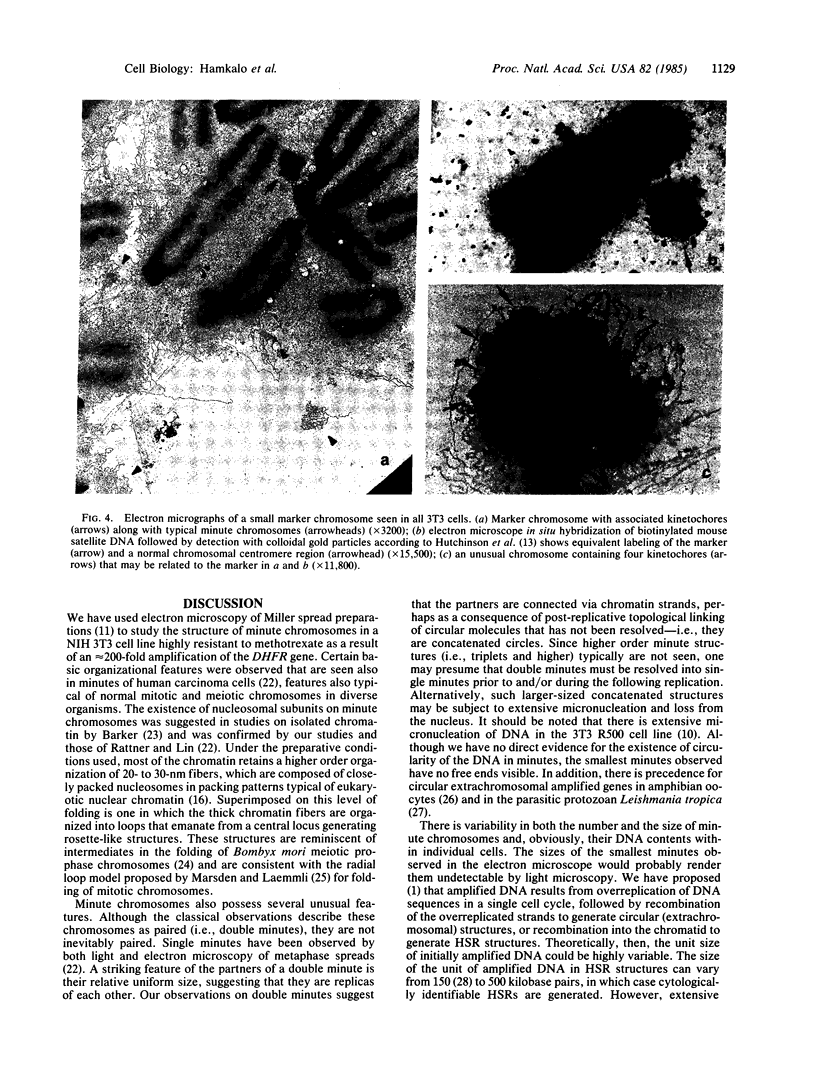

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balaban-Malenbaum G., Gilbert F. Double minute chromosomes and the homogeneously staining regions in chromosomes of a human neuroblastoma cell line. Science. 1977 Nov 18;198(4318):739–741. doi: 10.1126/science.71759. [DOI] [PubMed] [Google Scholar]
- Barker P. E., Stubblefield E. Ultrastructure of double minutes from a human tumor cell line. J Cell Biol. 1979 Dec;83(3):663–666. doi: 10.1083/jcb.83.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M., Coderre J. A., Santi D. V., Schimke R. T. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984 Sep;38(2):431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Spengler B. A. Metaphase chromosome anomaly: association with drug resistance and cell-specific products. Science. 1976 Jan 16;191(4223):185–187. doi: 10.1126/science.942798. [DOI] [PubMed] [Google Scholar]
- Bostock C. J., Clark E. M. Satellite DNA in large marker chromosomes of methotrexate-resistant mouse cells. Cell. 1980 Mar;19(3):709–715. doi: 10.1016/s0092-8674(80)80047-x. [DOI] [PubMed] [Google Scholar]
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. C., Beverley S. M., Schimke R. T. Relationship of amplified dihydrofolate reductase genes to double minute chromosomes in unstably resistant mouse fibroblast cell lines. Mol Cell Biol. 1981 Dec;1(12):1077–1083. doi: 10.1128/mcb.1.12.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse G. F., Simonsen C. C., McEwan R. N., Schimke R. T. Structure of amplified normal and variant dihydrofolate reductase genes in mouse sarcoma S180 cells. J Biol Chem. 1982 Jul 10;257(13):7887–7897. [PubMed] [Google Scholar]
- Dolnick B. J., Berenson R. J., Bertino J. R., Kaufman R. J., Nunberg J. H., Schimke R. T. Correlation of dihydrofolate reductase elevation with gene amplification in a homogeneously staining chromosomal region in L5178Y cells. J Cell Biol. 1979 Nov;83(2 Pt 1):394–402. doi: 10.1083/jcb.83.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Solenoidal model for superstructure in chromatin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fougere-Deschatrette C., Schimke R. T., Weil D., Weiss M. C. A study of chromosomal changes associated with amplified dihydrofolate reductase genes in rat hepatoma cells and their dedifferentiated variants. J Cell Biol. 1984 Aug;99(2):497–502. doi: 10.1083/jcb.99.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber D. A., Schimke R. T. Unstable amplification of an altered dihydrofolate reductase gene associated with double-minute chromosomes. Cell. 1981 Nov;26(3 Pt 1):355–362. doi: 10.1016/0092-8674(81)90204-x. [DOI] [PubMed] [Google Scholar]
- Hsu T. C., Cooper J. E., Mace M. L., Jr, Brinkley B. R. Arrangement of centromeres in mouse cells. Chromosoma. 1971;34(1):73–87. doi: 10.1007/BF00285517. [DOI] [PubMed] [Google Scholar]
- Hutchison N. J., Langer-Safer P. R., Ward D. C., Hamkalo B. A. In situ hybridization at the electron microscope level: hybrid detection by autoradiography and colloidal gold. J Cell Biol. 1982 Nov;95(2 Pt 1):609–618. doi: 10.1083/jcb.95.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Brown P. C., Schimke R. T. Loss and stabilization of amplified dihydrofolate reductase genes in mouse sarcoma S-180 cell lines. Mol Cell Biol. 1981 Dec;1(12):1084–1093. doi: 10.1128/mcb.1.12.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levan A., Levan G. Have double minutes functioning centromeres? Hereditas. 1978;88(1):81–92. doi: 10.1111/j.1601-5223.1978.tb01606.x. [DOI] [PubMed] [Google Scholar]
- Marsden M. P., Laemmli U. K. Metaphase chromosome structure: evidence for a radial loop model. Cell. 1979 Aug;17(4):849–858. doi: 10.1016/0092-8674(79)90325-8. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. D., Heintz N. H., White W. C., Rothman S. M., Hamlin J. L. Methotrexate-resistant Chinese hamster ovary cells have amplified a 135-kilobase-pair region that includes the dihydrofolate reductase gene. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6043–6047. doi: 10.1073/pnas.78.10.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller O. L., Jr, Beatty B. R. Extrachromosomal nucleolar genes in amphibian oocytes. Genetics. 1969;61(1 Suppl):133–143. [PubMed] [Google Scholar]
- Miller O. L., Jr, Beatty B. R. Visualization of nucleolar genes. Science. 1969 May 23;164(3882):955–957. doi: 10.1126/science.164.3882.955. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Schimke R. T., Urlaub G., Chasin L. A. Amplified dihydrofolate reductase genes are localized to a homogeneously staining region of a single chromosome in a methotrexate-resistant Chinese hamster ovary cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5553–5556. doi: 10.1073/pnas.75.11.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn L. A., Moore G. E., Morgan R. T., Woods L. K. Cell lines from human colon carcinoma with unusual cell products, double minutes, and homogeneously staining regions. Cancer Res. 1979 Dec;39(12):4914–4924. [PubMed] [Google Scholar]
- Rattner J. B., Goldsmith M., Hamkalo B. A. Chromatin organization during meiotic prophase of Bombyx mori. Chromosoma. 1980;79(2):215–224. doi: 10.1007/BF01175187. [DOI] [PubMed] [Google Scholar]
- Rattner J. B., Hamkalo B. A. Higher order structure in metaphase chromosomes. I. The 250 A fiber. Chromosoma. 1978 Dec 6;69(3):363–372. doi: 10.1007/BF00332139. [DOI] [PubMed] [Google Scholar]
- Rattner J. B., Hamkalo B. A. Nucleosome packing in interphase chromatin. J Cell Biol. 1979 May;81(2):453–457. doi: 10.1083/jcb.81.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner J. B., Lin C. C. Ultrastructural organization of double minute chromosomes and HSR regions in human colon carcinoma cells. Cytogenet Cell Genet. 1984;38(3):176–181. doi: 10.1159/000132056. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Brown P. C., Kaufman R. J., McGrogan M., Slate D. L. Chromosomal and extrachromosomal localization of amplified dihydrofolate reductase genes in cultured mammalian cells. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):785–797. doi: 10.1101/sqb.1981.045.01.097. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]