Abstract
Objective
To compare body composition parameters estimated by air displacement plethysmography (ADP) to dual x-ray absorptiometry (DXA) in body mass index (BMI) classifications that include extremely obese (BMI≥40.0kg/m2), and to examine if differences between analyses were influenced by BMI.
Design and Methods
Fat free mass (FFM,kg), fat mass (FM,kg) and body fat (BF,%) were analyzed with both technologies.
Results
All outcome measures of ADP and DXA were highly correlated (r≥0.95,P<0.001 for FFM, FM and BF), but Bland-Altman analyses revealed significant bias (P<0.01 for all). ADP estimated greater FFM and lower FM and BF (P<0.01 for all). BMI explained 27% of the variance in differences between FFM measurements (P<0.001), and 37% and 33% of the variances in differences between FM and BF measurements, respectively (P<0.001 for both). Within normal weight and overweight classifications, ADP estimated greater FFM and lower FM and BF (P<0.001 for all), but the opposite occurred within the extremely obese classification; ADP estimated lower FFM and greater FM and BF (P<0.05 for all).
Conclusions
Body composition analyses by the two technologies were strongly congruent, but systematically different and influenced by BMI. Caution should be taken when utilizing ADP to estimate body composition parameters over a wide range of BMI classifications that include extremely obese.
Keywords: body composition, air displacement plethysmograph, BOD POD, extreme obesity
Introduction
The dramatic increase in extreme obesity over the past decade 1, 2 has elevated the need to determine which body composition methods are valid and reliable in this population. Body composition analysis provides clinical and scientific information relevant to health risks associated with obesity that is more advantageous than body mass index (BMI) alone 3, 4. In addition, body composition analysis can determine the effectiveness of interventions used to induce changes in body composition compartments such as fat and muscle mass.
The gold standard of body composition analysis employs the four compartment model in which fat, mineral, water and protein are measured 5. However, the body composition technique commonly employed in research studies investigating extremely obese adults is dual x-ray absorptiometery (DXA) 6-12. The popularity of this method can be attributed to the ease of use and wide spread availability of DXA, as well as good reliability and reproducibility; the coefficient of variation (CV) is about 2 % across several studies with heterogeneous populations 13. Errors in estimation of body composition parameters are due to factors that impact assumed attenuation coefficients for fat and lean tissue, including differentiating bone from lean tissue, hydration status, tissue thickness and fat distribution 13. These are all issues that become more predominant as BMI increases to the extremely obese classification (BMI ≥ 40 kg/m2). For example, greater tissue thickness lends to a greater estimation of fat tissue 13, 14 so that fat mass (FM) and percentage of total body weight that is body fat (BF) are overestimated in obese adults. Further limitations of using DXA to measure body composition in the obese population include the scanner's field of view and the table's maximum weight capacity. Despite these particular disadvantages of using DXA for body composition analysis in extremely obese adults, as well as the method's lack of establishment as a gold standard, DXA continues to be a predominant method utilized in the literature.
One of the more promising technological advancements in body composition analysis that has potential utility for extremely obese adults is air displacement plethysmography (ADP). The BOD POD (an ADP unit by COSMED, California, USA) not only accommodates a wider range of body sizes than DXA, but is also non-invasive and is relatively rapid and easy to perform 5. The within subject CV for repeated measures of body composition parameters with BOD POD ranges between 2.0 to 3.3 % for adults tested on the same day15 or across several days 16, 17. ADP is a method based on the two compartment model and assumes a constant density of FM and fat free mass (FFM). The assumption of a constant FFM density may be violated in the extremely obese population and lend to error in estimations of body composition parameters using this method. In the literature thus far, ADP has been compared to other methods of body composition analysis for use in the higher classification of obesity by a limited amount of studies; one used the reference method hydrostatic weighing 18 while the other used a three compartment model 19. Of those studies that compared ADP to DXA_three previous studies included the higher classifications of obesity, with the maximum BMI only reaching 40 kg/m2 15, 17, 20.
The primary aim of this study was to compare the absolute and proportionate amounts of FM and FFM estimated by ADP to the reference method, DXA, in a group that included extremely obese adults. We hypothesized that these body composition parameters estimated using the ADP methodology would not significantly differ from DXA analysis across the spectrum of BMI classifications ranging from normal weight (BMI: 18 – 24.9 kg/m2) to extremely obese.
Methods and Procedures
Men and women greater than 18 years of age were recruited from 2007-2010. An additional 23 subjects from an ancillary study examining body composition and resting energy expenditure responses to bariatric surgeries also had pre-operative data included in the following analysis. All participants provided written informed consent approved by the Institutional Review Board of the University of Pittsburgh, and completed testing at the Endocrinology and Metabolism Research Center at the University of Pittsburgh.
Body Composition Assessment
Participants were asked not to exercise the day of their appointment, and to not drink or eat anything other than water for three to four hours prior to their appointment. Upon arrival, subjects were asked to void, and women completed a urine pregnancy test to exclude those who were pregnant. Subjects removed all excess clothing and accessories, except for swimsuits, compression shorts, or other close fitting undergarments. Height (cm) and total body weight (BW, kg) were measured on a calibrated, digital scale (Tanita Corporation of America, Inc., Illinois, USA) to calculate BMI (kg/m2). No participants were excluded due to exceeding the maximum weight capacity or field of view of the DXA. BW (kg), FFM (kg), FM (kg), and BF (%) were determined by both methods of body composition analysis. Each participant completed a test with the ADP unit and a total body DXA scan on the same day, and within the same hour. The testing order was completed in a random order.
ADP
BF was calculated from the output of the BOD POD software. The estimated BF from the software was not used for the analysis. The BOD POD was calibrated prior to each test. Subjects were provided a swim cap to wear over their head with their hair tucked in. BW was determined with the equipment's scale. After completing three tests, the software (version 2.14) predicted the following variables in all subjects: thoracic gas volume, FFM, FM and BF.
DXA
All measurements were made with Lunar Densitometry model (General Electric Medical Systems Lunar, Wisconsin, USA) and analyzed with the accompanying software (enCORE 2003, version 8.50). The equipment was calibrated at the beginning of each test day, and also calibrated quarterly with phantom spine scans. In addition to the clothing worn for the assessments, subjects were offered a hospital gown. All subjects had a total body scan completed while lying motionless in the supine position. BW was calculated as the sum of bone mineral content, lean tissue and FM. Although DXA provides estimates of three compartments of body composition, the current study focused on the estimates of two compartments (fat and fat free content) to compare to ADP analyses. FFM was calculated as bone mineral content plus lean tissue. BF was calculated by dividing FM by BW and multiplying by 100.
Statistical Analysis
The Statistical Package for Social Science (SPSS 17.0, SPSS Inc., Illinois, USA) was used for the following analyses with significance level set at P<0.05. According to the Shapiro-Wilk test, the assumption of normality was not met by any of the body composition parameters among the total sample. For descriptive purposes, frequencies (n) and percentages (%) of categorical data and medians with 25th and 75th percentiles of continuous variables were presented. Body composition parameters measured by ADP were compared to those estimated by DXA with Wilcoxon signed rank tests in the total sample and among each BMI classification group. Spearman's rank correlation coefficients were used to evaluate the strength of the relationships between the methods’ estimation of FFM, FM and BF. Bland-Altman plots 21 were also used to determine agreement between outcome measures between methods.
Multiple linear regression was used to examine if BMI contributed to the differences between each method's determination of body composition variables (FFM, FM and BF). Differences were calculated by subtracting the estimates of body composition parameters of the reference method from the ADP method. Demographic data (age, sex, race) were used to adjust for potential confounding in regression analyses, as was the difference in BW determined by ADP from DXA. Two participants’ data were identified as points with high residual and leverage; removing their data was believed to substantially change the estimate of coefficients and therefore their data were eliminated from the dataset. The participants were a 25 year old, Hispanic female with a BMI of 19.3 kg/m2 and a 23 year old Caucasian female with a BMI of 17.7 kg/m2. All other assumptions of the multiple linear regression models were not violated.
Results
Subjects
Subject characteristics are presented in Table 1 for the total sample and by BMI classification. Of the 109 participants who completed the study, 98 were non-Hispanic whites, 6 were non-Hispanic blacks, 2 were Hispanic and 3 were of other racial background. The BMI range for the normal weight group was 17.8-24.9 kg/m2, 25.0-29.8 kg/m2 for the overweight group, 30.3-39.2 kg/m2 for the obese group, and 41.1-51.5 kg/m2 for the extremely obese group.
Table 1.
Medians (quartiles) presented for subject characteristics among total sample and by body mass index (BMI) classification groups.
| Total (n=109) | Normal Weight (n=55) | Overweight (n=24) | Obese (n=16) | Extreme Obese (n=14) | |
|---|---|---|---|---|---|
| Age, yrs | 34 (23,48) | 26 (22,36) | 37 (27,47) | 51 (38,56) | 48 (35,54) |
| Caucasian, n (%) | 98 (89.9) | 50 (90.9) | 21 (87.5) | 14 (87.5) | 13 (92.9) |
| Female, n (%) | 83 (76.1) | 43 (78.2) | 12 (50.0) | 15 (93.8) | 13 (92.9) |
| BMI, kg/m2 | 24.9 (22.2,30.9) | 22.3 (20.2,23.8) | 27.8 (25.6,29.2) | 33.6 (31.3,36.7) | 42.4 (41.5,43.4) |
Comparison of FFM estimations by ADP and DXA
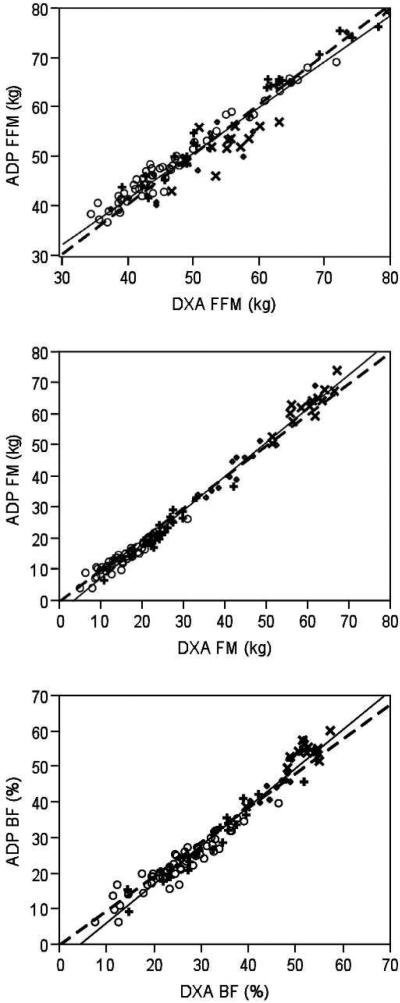
There was strong agreement between the two methods’ estimates of FFM (r=0.95, P<0.001; Figure 1A) with ADP estimating higher FFM compare to DXA (P<0.001; Table 2). The Bland-Altman analysis detected significant bias (P=0.002; Figure 2A). When stratified by BMI groups, ADP continued to estimate significantly higher FFM than DXA in only the normal weight and overweight participants (P≤0.001 for both groups), but significantly lower FFM than DXA in extreme obese participants (P<0.05; Table 3). There was no difference between the methods’ estimates of FFM in the obese group (P>0.05; Table 3).
Figure 1.
Relationship between body composition parameters estimated by air displacement plethysmography (ADP) and dual x-ray absorptiometry (DXA) across BMI values of adults (○normal weight; +overweight; ◇obese; ×extremely obese), including fat free mass (FFM, kg; A), fat mass (FM, kg; B), and body fat (BF, %; C). Solid line represents line of fit. Dashed line represents line of fit forced through an intercept of 0.
Table 2.
Comparison between body composition values estimated by air displacement plethysmography (ADP) and dual x-ray absorptiometry (DXA) for total sample (n=109). Medians (quartiles) are presented. P values presented from Wilcoxon Signed Ranks analysis.
| ADP | DXA | P value | |
|---|---|---|---|
| Body weight, kg | 73.0 (61.5,89.7) | 73.2 (61.4,89.9) | 0.010 |
| Fat free mass, kg | 49.4 (44.0,56.6) | 48.6 (42.8,58.0) | 0.001 |
| Fat mass, kg | 18.8 (13.0,35.0) | 21.6 (14.6,36.1) | 0.000 |
| Body fat, % | 27.0 (20.1,41.8) | 31.1 (23.3,44.0) | 0.000 |
Figure 2.
Agreement between fat free mass (FFM, kg; A), fat mass (FM, kg; B), and body fat (BF, %; C) estimated by air displacement plethysmography and dual x-ray absorptiometry. Solid line represents the difference of ADP from DXA. Dashed lines represent the confidence intervals. The BMI of the participants are represented with the following symbols: ○normal weight, +overweight, ◇obese and ×extremely obese.
Table 3.
Comparison between body composition parameters estimated by air displacement plethysmography (ADP) and dual x-ray absorptiometry (DXA) among body mass index classification groups. Data presented in medians and percentiles. P values presented from Wilcoxon Signed Ranks analysis.
| Normal Weight (n=55) | Overweight (n=24) | Obese (n=16) | Extreme Obese (n=14) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADP | DXA | P value | ADP | DXA | P value | ADP | DXA | P value | ADP | DXA | P value | |
| Body weight, kg | 61.5 (55.6,66.8) | 61.5 (55.8,66.6) | .001 | 79.0 (73.9,87.2) | 79.3 (73.4,87.7) | .016 | 93.8 (82.2,98.2) | 92.0 (82.2,98.5) | .215 | 115.7 (109.0,123.8) | 115.7 (109.1,123.0) | .470 |
| Fat free mass, kg | 46.3 (42.4,52.9) | 42.9 (41.0,52.0) | .000 | 56.5 (49.1,65.5) | 55.4 (47.5,63.3) | .001 | 49.1 (41.8,54.7) | 48.5 (43.9,53.4) | .836 | 53.5 (51.0,56.2) | 55.4 (52.2,58.8) | .022 |
| Fat mass, kg | 13.2 (10.5,16.9) | 14.9 (11.3,19.3) | .000 | 21.3 (18.7,26.6) | 23.8 (21.3,27.1) | .000 | 42.6 (34.6,49.3) | 42.0 (35.8,47.9) | .469 | 62.8 (50.1,66.0) | 60.7 (55.8,63.5) | .004 |
| Body Fat, % | 21.6 (18.4,27.2) | 24.9 (19.4,31.1) | .000 | 26.6 (20.9,34.1) | 30.5 (23.9,35.7) | .000 | 46.2 (41.7,52.5) | 48.0 (43.8,49.1) | .796 | 54.6 (52.6,55.7) | 51.9 (50.1,51.9) | .009 |
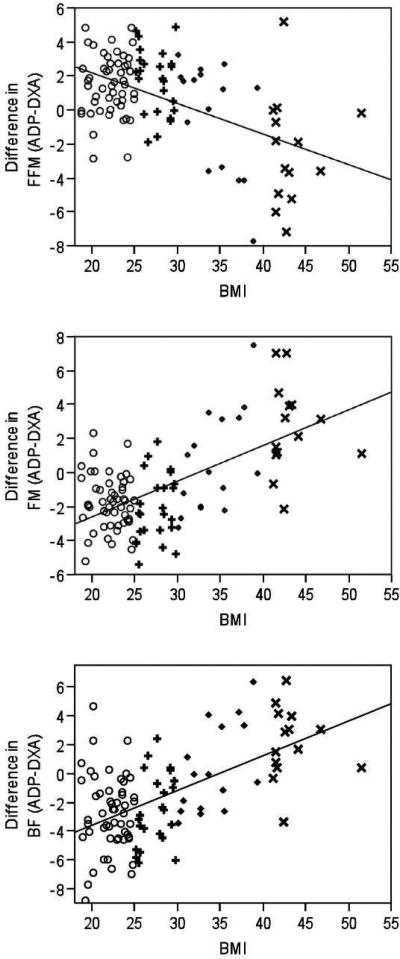
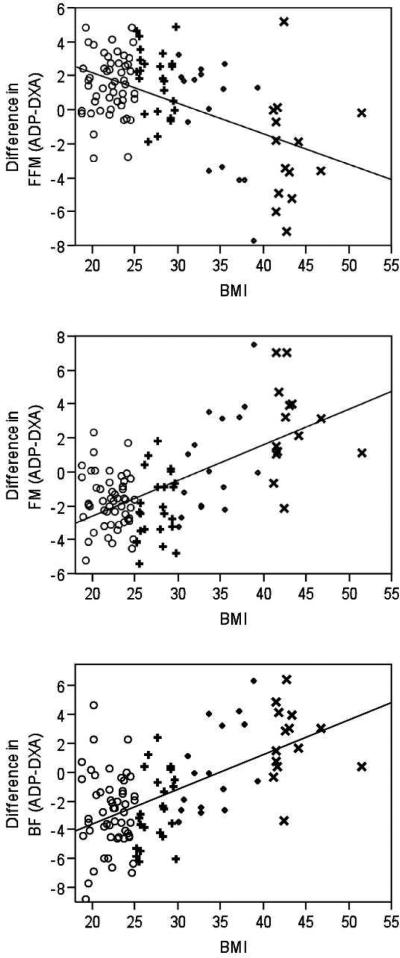
The difference in the estimation of FFM by ADP compared to DXA had a large, negative correlation with BMI (r=−0.52, P<0.001; Figure 3A). BMI explained 37% of the proportion of variability in the difference of FFM estimated by both methods. For every 5 kg/m2 increase in BMI, there was a 2.59 kg decrease in the difference between FFM estimated by ADP and DXA (P<0.001). This suggests that as BMI increases, ADP underestimates FFM compared to DXA. In addition, Figure 3A indicates that the estimate of FFM by ADP in normal weight subjects is greater than the FFM estimate by DXA, but as BMI increases to the obese classifications, ADP estimates a lower FFM compared to DXA. When examining for possible covariates in the regression model, the difference in BW (P <0.001) and the male gender (P<0.01) were significant predictors of the difference in estimation of FFM, whereas age (P=0.48) and race (P=0.51) were not. BMI remained significantly associated with the difference between estimates of FFM when difference in BW and sex were held constant (P<0.001).
Figure 3.
Difference in estimations of body composition analysis by air displacement plethysmography (ADP) compared to dual x-ray absorptiometry (DXA) across BMI values of adults (○normal weight; +overweight; ◇obese; ×extremely obese), including fat free mass (FFM, kg; A), fat mass (FM, kg; B), and body fat (BF, %; C). Solid line represents line of fit.
Comparisons of Fat Content Estimations by ADP and DXA
There was strong agreement between the two methods’ estimations of fat content regardless of the unit of expression (kg for FM or % for BF) (FM: r=0.99, P <0.001, Figure 1B; BF: r=0.98, P <0.001, Figure 1C), although ADP provided significantly lower estimates of fat content (P<0.001 for both FM and BF; Table 2). Bland-Altman analysis detected a significant bias in both FM (P<0.001, Figure 2B) and BF (P<0.0001, Figure 2C) estimated by ADP compared to DXA. When stratified by BMI groups, ADP continued to estimate significantly lower fat content (expressed as either FM or BF) than DXA in normal weight and overweight participants (P≤0.001 for both groups; Table 3), but estimated higher fat content in the extreme obese group (P <0.01 for both FM and BF; Table 3). There were no significant differences in fat content estimated by the two methods in the obese group of class 1 and 2 participants (FM: P =0.47; BF: P =0.80; Table 3).
The difference in FM estimated by ADP compared to DXA had a large, positive correlation with BMI (r=0.61, P <0.001). BMI explained 37% of the difference between estimates of FM provided by ADP and DXA. A 5 kg/m2 increase in BMI was associated with a 3.03 kg increase in the difference in FM as estimated by ADP compared to DXA (P<0.001). This suggests that as BMI increases, ADP estimates a greater FM then DXA. At the lower end of BMI, ADP underestimates FM compared to DXA, but, as BMI increases into the obese classification, ADP overestimates FM (Figure 3B). None of the possible covariates were significant predictors in the regression model (difference in BW, P=0.34, age, P=0.50, males, P=0.06 and race, P=0.64).
The results of the regression analysis were similar when examining the influence of BMI on fat content expressed as BF. The difference in the estimates of BF had a large, positive correlation with BMI (r=0.57, P<0.001). For every 5 kg/m2 increase in BMI, there was a 2.87 % increase in the difference in estimation of BF by ADP compared to DXA such that a higher BMI resulted in a higher BF estimated by ADP compared to DXA (R2=0.33; P<0.001). At the lower end of BMI, ADP underestimates BF compared to DXA, but, as BMI increases into the obese classification, ADP overestimates BF (Figure 3C). When examining potential covariates in the regression model, the difference in BW and male gender were significant predictors (P=0.003 and P=0.046, respectively). When these covariates were held constant in the regression model, BMI remained a significant predictor of the variance in the difference in BF as estimated by ADP compared to DXA (P<0.001).
Discussion
This study compared body composition estimates analyzed by ADP to another independent method of analysis based on the two compartment model approach, DXA. The study population included a heterogeneous population of adults with a range of ages and mixed races, as well as BMI classifications ranging from normal weight to extremely obese. The results confirmed that body composition parameters assessed by ADP and DXA were strongly correlated, but the precise estimates of body composition parameters significantly varied. The direction of differences between ADP and DXA was not uniform across the BMI spectrum, and the differences were influenced by BMI.
Overall, ADP overestimated FFM and underestimated fat content compared to DXA. This was in agreement with prior studies demonstrating ADP underestimated fat content compared to DXA analysis of adults with BMI values ranging from 19 to 36 kg/m2 15, 17, 22, 23. The current study extended these findings to a population with an even broader range of BMI values that included extremely obese adults with a maximum BMI of 52 kg/m2, but were limited to those that fit the requirements of the equipment (e.g. the weight limits of ADP and DXA and the field of view of the DXA scanner). Additional analyses were carried out to assess if differences in estimates of body composition parameters by ADP and DXA were influenced by BMI. Within the normal weight, overweight and extremely obese groups, significant differences were found in FFM, FM and BF estimated by these two laboratory techniques (Table 3). Previous research suggested that BMI might explain the outcome measurements of body composition analysis. Among participants with a BMI range of 17 to 42 kg/m2, the greatest difference between estimation of BF by ADP and DXA was among those participants with higher adiposity 15. More specifically, ADP tended to overestimate BF in those with higher adiposity, but underestimate BF in those with lower adiposity. This was shown in with comparisons between ADP and DXA in children 16, ADP and hydrostatic weighing in adults 17, and ADP and a three compartment model using body density by H218O dilution in adults with extreme obesity 24. This trend was not significant when comparing analyses by ADP to DXA in adults with a maximum BMI of 40 kg/m2 4, 20. However, the current study found that higher BMI values were associated with greater overestimation of fat content (expressed as either mass or percentage of total BW) and underestimation of FFM by ADP compared to DXA. At the lower end of BMI values, the opposite was true; ADP underestimated fat content (expressed as either mass or percentage of total BW) and overestimated FFM compared to DXA. A significant contributor to the variance in the differences in estimation of body composition outcome variables between ADP and DXA was BMI, and whether ADP under or overestimated a given outcome variable of body composition analysis in adults was dependent on which BMI classification group was of interest. In other words, the direction of the differences in the two methods’ estimations of body composition parameters varied across BMI classification groups. These results may contrast those of Levenhagen et al 17 and Nunez et al 16 due to the current study's larger sample size and the inclusion of extremely obese adults that extended the range of body fatness levels. In addition, differences between studies may be due to the estimations of body composition parameters estimated by different versions of the Lunar DXA software utilized by each study.
In addition to BMI, the male gender was also a significant confounding variable in the differences in FFM and BF (but not FM) measured by ADP and DXA. Differences between sexes may be more of a factor of body size and composition, but with males tendancy to present with greater BW and FFM and lower BF 17, extracting how sex influences the estimation of body composition parameters by different analyses is difficult. Such analysis was not a part of the current study's design, and may not be adequately powered to examine this relationship. However, the significant influence of body size (expressed as BMI in the current study) on differences in body composition parameters by ADP and DXA, suggests that this may have more of an effect on body composition analysis compared to a sex effect.
There are several potential reasons for the disagreement between body composition measurements by ADP and DXA. Inherent errors of methodological assumptions were expected to affect the outcome measurements of body composition analyses between ADP and DXA. An assumption associated with ADP is that density of FFM is a constant 25. A violation to this assumption can occur with differences in hydration status and fluid distribution. Among extremely obese subjects, there is a significant variation in fluid distribution (examined by the ratio of extracellular to intracellular water) compared to reference values 24 and controls with normal weight and overweight BMI classifications 26. Greater hydration of FFM, resulting in lower FFM density and an underestimation of FFM, would explain the lower FFM found by ADP compared to DXA in the extremely obese group (Table 3). In an attempt to limit hydration status as a source of variability, the current study asked participants to not exercise the day of the study and to fast for 3 to 4 hours prior to testing. The assumption of a constant density of FFM may also be due to racial differences; it has been suggested that blacks have a higher FFM density than other racial groups, but previous studies have found this variable to be similar between blacks and whites 27. The current study did not find race to be a significant contributor to the difference in body composition parameters estimated by ADP and DXA, but, with the majority of the sample being of one race (i.e. Caucasian), the study may not have been powered to detect the effects of race.
The ability of both technologies to accurately estimate body composition parameters is more fraught with error with the differential effects of bone mineral density, which is a potential issue for obese adults who often present with higher bone content. DXA has greater error in estimating soft tissue under- and overlying bone, such that those with a higher bone mineral content leads to overestimation of FM 13. With ADP, higher bone content would lead to an overestimation of FFM. Variations in bone content and their potential effects on body composition analyses by each technology did not seem to influence the outcome measurements in the current study's population since estimation of FM was lower in DXA compared to ADP, and FFM was lower in ADP compared to DXA in the obese BMI classification groups (Table 3).
Deviations from protocols were another potential source of error. With ADP, the isothermal air trapped near skin, hair and clothing, as well as in the lungs must be accounted for 5. The current study limited this source of error by enforcing the recommendations of BOD POD to use swim caps and close fitting garments. To account for the isothermal conditions in the lungs 5, the lung volume can be measured or predicted with the equipment and software provided with the BOD POD. In the current study, lung volume was predicted instead of measured. Although this introduces a source of error, Collins and McCarthy 28 found no difference between predicted and measured lung volumes with BOD POD, nor did the use of either value of lung volume have a significant influence on the outcome measurements.
In conclusion, estimates of body composition parameters by ADP were strongly associated with DXA across a heterogeneous population with a wide range of BMI values. However, FFM, FM and BF estimated by ADP were systematically different from DXA and affected by BMI. The extent of the discrepancy between ADP and DXA was no greater in extremely obese then other BMI classifications, but the direction of the differences was not uniform. In comparison to DXA, ADP estimated a higher absolute and proportional fat content in adults with higher BMI, but estimated lower fat content in those with lower BMI. ADP's estimation of FFM was lower at higher BMI values, but higher at lower BMI values. These results do not support interchanging these two methodologies when a comparison of body composition is being conducted in only one BMI classification or across classifications.
What is already known about this subject
Body composition parameters assessed by air displacement plethysmography (ADP) and dual x-ray absorptiometry (DXA) are strongly correlated
ADP underestimates fat content expressed as fat mass (kg) or percentage of total body weight (%) compared to DXA in adults with body mass index (BMI) values ranging from normal weight to obese
Need for reliable and accurate assessment of body composition in the prevalent and growing population of extremely obese adults
What this study adds
Comparison of body composition analyses by ADP and DXA in adults with heterogeneous subject characteristics that include those classified as extremely obese (class 2 and 3 obese) by their BMI
Comparison of how these analyses differ across the range of BMI classifications and within BMI classifications
Information about how to interpret body composition analysis by ADP when including adults in all BMI classifications
Acknowledgements
Funding Sources. Obesity and Nutrition Research Center Grant (P30 DK46204) and R01 DK072507.
We would like to thank the participants and all staff at the Endocrinology and Metabolism Research Center for the integral part they played in making this research possible, with special thanks to George Grove, David Kelley and Carol Kelley.
Footnotes
Author contributions. BHG conceived experiment. SJA and KCH carried out experiments. KCH, JCT, DG and BHG analyzed data. KCH and BHG interpreted data and generated figures. KCH, DG and BHG were involved in writing the paper, and all authors had final approval of the submitted and published versions.
Competing interests: the authors have no competing interests.
Contributor Information
Kazanna C. Hames, Department of Health and Physical Activity, University of Pittsburgh, Pittsburgh, PA Division of Endocrinology and Metabolism, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Steven J. Anthony, Division of Endocrinology and Metabolism, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA
John C. Thornton, The New York Obesity Nutrition Research Center, St. Luke's-Roosevelt Hospital Center, College of Physicians and Surgeons, Columbia University, New York, NY, USA
Dympna Gallagher, The New York Obesity Nutrition Research Center, St. Luke's-Roosevelt Hospital Center, College of Physicians and Surgeons, Columbia University, New York, NY, USA
Bret H. Goodpaster, Division of Endocrinology and Metabolism, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA
References
- 1.Freedman DS, Khan LK, Serdula MK, Galuska DA, Dietz WH. Trends and correlates of class 3 obesity in the United States from 1990 through 2000. JAMA. 2002;288(14):1758–61. doi: 10.1001/jama.288.14.1758. [DOI] [PubMed] [Google Scholar]
- 2.Ogden Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Frankenfield DC, Rowe WA, Cooney RN, Smith JS, Becker D. Limits of body mass index to detect obesity and predict body composition. Nutrition. 2001;17(1):26–30. doi: 10.1016/s0899-9007(00)00471-8. [DOI] [PubMed] [Google Scholar]
- 4.Prentice AM, Jebb SA. Beyond body mass index. Obesity Reviews. 2001;2(3):141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 5.Fields DA, Goran MI, McCrory MA. Body-composition assessment via air-displacement plethysmography in adults and children: a review. Am J Clin Nutr. 2002;75(3):453–67. doi: 10.1093/ajcn/75.3.453. [DOI] [PubMed] [Google Scholar]
- 6.Carrasco F, Ruz M, Rojas P, Csendes A, Rebolledo A, Codoceo J, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19(1):41–6. doi: 10.1007/s11695-008-9638-0. [DOI] [PubMed] [Google Scholar]
- 7.Peppo FD, Giorgio GD, Germani M, Ceriati E, Marchetti P, Galli C, et al. BioEnterics intragastric balloon for treatment of morbid obesity in Prader-Willi syndrome: specific risks and benefits. Obes Surg. 2008;18(11):1443–9. doi: 10.1007/s11695-008-9509-8. [DOI] [PubMed] [Google Scholar]
- 8.Garrapa G, Canibus P, Gatti C, Santangelo M, Frezza F, Feliciotti F, et al. Changes in body composition and insulin sensitivity in severely obese subjects after laparoscopic adjustable silicone gastric banding (LASGB). Med Sci Monit. 2005;11(11):CR522–8. doi: 10.12659/msm.430365. [DOI] [PubMed] [Google Scholar]
- 9.Giusti V, Gasteyger C, Suter M, Heraief E, Gaillard R, Burchardt P. Gastric banding induces negative bone remodelling in the absence of secondary hyperparathroidism: potential role of serum C Teloeptides for follow-up. Int J Obes (Lond) 2005;29(12):1429–35. doi: 10.1038/sj.ijo.0803040. [DOI] [PubMed] [Google Scholar]
- 10.Goodpaster B, DeLany J, Otto A, Kuller L, Vockley J, South-Paul J, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA. 2010;304(16):1795–802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boneva-Asiova Z, Boyanov M. Body Composition analysis by leg-to-leg bioelectrical impedance and dual-energy X-ray absorptiometry in non-obese and obese individuals. Diabetes Obes Metab. 2008;10(11):1012–8. doi: 10.1111/j.1463-1326.2008.00851.x. [DOI] [PubMed] [Google Scholar]
- 12.Gotfredsen A, Hendel HW, Andersen T. Influence of orlistat on bone turnover and body composition. Int J Obes Relat Metab Disord. 2001;25(8):1154–60. doi: 10.1038/sj.ijo.0801639. [DOI] [PubMed] [Google Scholar]
- 13.Jebb SA. Measurement of soft tissue composition by dual energy X-ray absorptiometry. Br J Nutr. 1997;77(2):151–63. doi: 10.1079/bjn19970021. [DOI] [PubMed] [Google Scholar]
- 14.Jebb SA, Goldberg GR, Jennings G, Elia M. Dual-energy X-ray absortiometry measurements of body composition: effects of depth and tissue thickness, including comparisons with direct analysis. Clin Sci. 1995;88(3):319–24. doi: 10.1042/cs0880319. [DOI] [PubMed] [Google Scholar]
- 15.Sardinha LB, Lohman TG, Teixeira PJ, Guedes DP, Going SB. Comparison of air displacement plethysmography with dual-energy X-ray absorptiometry and 3 field methods for estimating body composition in middle-aged men. Am J Clin Nutr. 1998;68(4):786–93. doi: 10.1093/ajcn/68.4.786. [DOI] [PubMed] [Google Scholar]
- 16.Nunez C, Kovera AJ, Pietrobelli A, Heshka S, Horlick M, Kehayias JJ, et al. Body composition in children and adults by air displacement plethysmography. Eur J Clin Nutr. 1999;53(5):382–7. doi: 10.1038/sj.ejcn.1600735. [DOI] [PubMed] [Google Scholar]
- 17.Levenhagen DK, Borel MJ, Welch DC, Piasecki JH, Piasecki DP, Chen KY, et al. A comparison of air displacement plethysmography with three other techniques to determine body fat in healthy adults. JPEN J Parenter Enteral Nutr. 1999;23(5):293–9. doi: 10.1177/0148607199023005293. [DOI] [PubMed] [Google Scholar]
- 18.Ginde SR, Geliebter A, Rubiano F, Silva AM, Wang J, Heshka S, et al. Air Displacement Plethysmography: Validation in Overweight and Obese Subjects. Obesity Research. 2005;13(7):1232–1237. doi: 10.1038/oby.2005.146. [DOI] [PubMed] [Google Scholar]
- 19.Das SK, Roberts SB, McCrory MA, Hsu LKG, Shikora SA, Kehayias JJ, et al. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003;78(1):22–30. doi: 10.1093/ajcn/78.1.22. [DOI] [PubMed] [Google Scholar]
- 20.Wagner DR, Heyward VH, Gibson AL. Validation of air displacement plethysmography for assessing body composition. Med Sci Sports Exerc. 2000;32(7):1339–44. doi: 10.1097/00005768-200007000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 22.Ball SD, Altena TS. Comparison of the Bod Pod and dual energy x-ray absorptiometry in men. Physiol Meas. 2004;25(3):671–8. doi: 10.1088/0967-3334/25/3/007. [DOI] [PubMed] [Google Scholar]
- 23.Weyers AM, Mazzetti SA, Love DM, Gomez AL, Kraemer WJ, Volek JS. Comparison of methods for assessing body composition changes during weight loss. Med Sci Sports Exerc. 2002;34(3):497–502. doi: 10.1097/00005768-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Das SK, Roberts SB, McCrory MA, Hsu LK, Shikora SA, Kehayias JJ, et al. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003;78(1):22–30. doi: 10.1093/ajcn/78.1.22. [DOI] [PubMed] [Google Scholar]
- 25.Lohman TG. Skinfolds and body density and their relation to body fatness: a review. Hum Biol. 1981;53(2):181–225. [PubMed] [Google Scholar]
- 26.Waki M, Kral JG, Mazariegos M, Wang J, Pierson RN, Jr., Heymsfield SB. Relative expansion of extracellular fluid in obese vs. nonobese women. Am J Physiol. 1991;261(2 Pt 1):E199–203. doi: 10.1152/ajpendo.1991.261.2.E199. [DOI] [PubMed] [Google Scholar]
- 27.Visser V, Gallagher D, Deurenberg P, Wang J, Pierson RN, Jr., Heymsfield SB. Density of fat-free body mass: relationship with race, age, and level of body fatness. Am J Physiol. 1997;272:E781–E787. doi: 10.1152/ajpendo.1997.272.5.E781. Endocrinol Metab 35. [DOI] [PubMed] [Google Scholar]
- 28.Collins AL, McCarthy HD. Evaluation of factors determining the precision of body composition measurements by air displacement plethysmography. European Journal of Clinical Nutrition. 2003;57:770–776. doi: 10.1038/sj.ejcn.1601609. [DOI] [PubMed] [Google Scholar]