Abstract
Sequential palliative chemotherapy for metastatic breast cancer incorporating weekly gemcitabine administered as three-weeks-on, one-week-off schedule is widely adopted throughout the East Asia region. Hemolytic-uremic syndrome (HUS) associated with weekly gemcitabine for a breast cancer patient is extremely rare. We report here a case of 43-year-old woman with metastatic breast cancer who received weekly gemcitabine as a third-line palliative chemotherapy for her disease. She developed HUS after a cumulative dose of 11,000 mg/m2 gemcitabine, evidenced by microangiopathic hemolytic anemia (MAHA) with schistocytes seen in peripheral blood smear, decreased haptoglobin level (<0.29 mmol/L), thrombocytopenia, negative direct Coombs test, and acute kidney injury. Owing to the ease of administration of weekly gemcitabine, gemcitabine-induced thrombocytopenia, multifactorial anemia in metastatic breast cancer, and possibility of cancer progression, HUS could have gone unnoticed. Breast cancer oncologist should be cognizant of this rare HUS even during weekly gemcitabine treatment.
Keywords: breast neoplasms, gemcitabine, hemolytic-uremic syndrome
Introduction
Gemcitabine (2,2′-difluorodeoxycytidine), a nucleoside analog, given as single agent by weekly schedule for patients with advanced breast cancer has proven good anti-tumor activity and favorable toxicity profile known to the clinical oncology community as early as in 1995.1 Since then sequential palliative chemotherapy incorporating weekly gemcitabine is widely adopted throughout the East Asia region. Hemolytic-uremic syndrome (HUS) manifested as microangiopathic hemolytic anemia (MAHA) producing schistocytes, thrombocytopenia, and acute kidney injury commonly progresses to uremia, and even fatality has been reported as a rare complication of gemcitabine.2–4 To the best of our knowledge, there is not yet published literature linking weekly gemcitabine alone and HUS in advanced breast cancer patients. We thus report here such a case to arouse oncologists’ attention to this rare but potentially fatal form of gemcitabine-related HUS (Gr-HUS), particularly in patients with advanced breast cancer receiving weekly gemcitabine.
Case Report
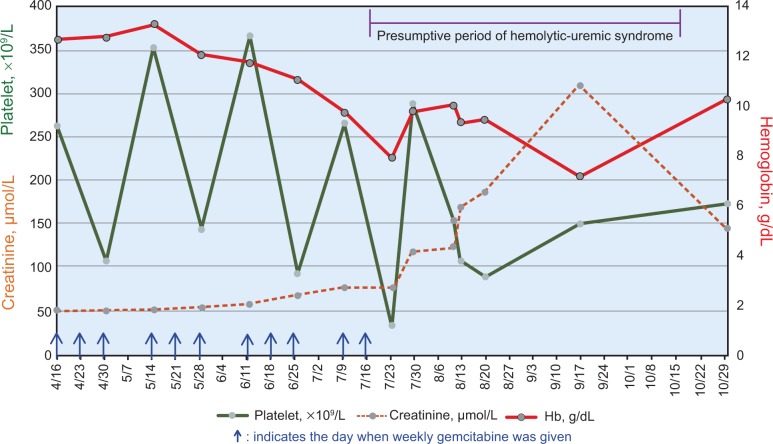
A 43-year-old Taiwan aboriginal resident presented to us with advanced left breast cancer with hepatic and osseous metastases. Left modified radical mastectomy on May 30, 2012 revealed grade 2 invasive ductal carcinoma staged as pT2 pN2a (4+/16) cM1 Stage IV. Estrogen receptor was positive in 95% of tumor cells and progesterone receptor in 40%. HER2 immunohistochemistry was 1+. From June 19, 2012 to April 2, 2013, five cycles of FEC (5- fluorouracil, epirubicin, and cyclophosphamide) followed by five cycles of three-weeks-on and one-week-off weekly paclitaxel were given sequentially. Since the beginning of chemotherapy, she also received monthly zoledronic acid for bone metastases. Owing to progressive disease, the patient was started on three-weeks-on, one-week-off weekly gemcitabine (Gemzar, Eli Lilly and Company) as third-line palliative chemotherapy. After a cumulative dose of 11,000 mg/m2 gemcitabine, the patient developed HUS evidenced by MAHA with schistocytes seen in peripheral blood smear, decreased haptoglobin level (<0.29 mmol/L), thrombocytopenia, negative direct and indirect Coombs test, and acute kidney injury (Fig. 1). Elevated lactate dehydrogenase (LDH) level is also an important indicator of HUS. However, in this case, it added no more information because bulky liver metastases had markedly elevated the LDH level. The patient had no diarrhea or non-steroidal anti-inflammatory drug-induced nephropathy. There was neither sepsis nor disseminated intravascular coagulation. She was hospitalized to the medical intensive care unit because of worsening chest pain suggesting unstable angina. After recognition of this condition, gemcitabine was discontinued, and blood component therapy and supportive oncology care were instituted. Ultimately the patient’s hemogram, cardiac function, and kidney function improved to the baseline state. The patient became able to receive further palliative biochemotherapy using bevacizumab and carboplatin plus weekly vinorelbine, and there was no recurrence of HUS after up to three cycles of new form of treatment.
Figure 1.
Timeline, from initiation of weekly gemcitabine on April 16 to recovery from HUS after discontinuation of gemcitabine on July 30, showing the changes of hemoglobin level, platelet count, and creatinine level.
Discussion
Secondary atypical HUS caused by cancer chemotherapy is basically a diagnosis made on clinical grounds.5 Partial ADAMTS13 deficiency is a common finding in atypical HUS patients as reported recently by Feng et al from the MD Anderson Cancer Center, Houston.6 However, this test is not available for our patient in the region. Gemcitabine as one of the main antineoplastic drugs combined with other agents such as docetaxel, carboplatin, or pegylated liposomal doxorubicin has been reported to associate with HUS in previous case reports.7–9 To the best of our knowledge, this is the first case report of HUS caused by single agent weekly gemcitabine as the third-line palliative chemotherapy for metastatic breast cancer.
In this case report, the possibility of pure thrombotic microangiopathy from breast cancer per se as the etiology for HUS rather than from weekly gemcitabine chemotherapy has been refuted easily by the fact that the patient’s HUS improved after prompt discontinuation of gemcitabine. If the HUS was from the tumor thrombotic microangiopathy from cancer progression,10 discontinuation of gemcitabine would not help in this setting.
Literature review provides a glimpse of which risk factors predict the occurrence of gemcitabine-related HUS (Gr-HUS), namely, advanced-stage disease, heavily pretreated before gemcitabine, and prolonged gemcitabine treatment. While the detailed mechanism of Gr-HUS has yet to be studied, it is believed to be resulted from thrombotic microangiopathy.3,10 Although the incidence of Gr-HUS is rare, about 0.31–1.4%, it could go unnoticed if the oncologist might regard Gr-HUS as just clinical deterioration and keep dispensing gemcitabine for the purpose of palliation.2–4,10
Diagnosis of Gr-HUS may be difficult because thrombocytopenia is a common sequel of gemcitabine use and gemcitabine-induced nephropathy is also likely. We agree the recommendations of monitoring haptoglobin level and schistocytes when unexpected worsening of anemia and renal function occurs in patients receiving weekly gemcitabine. A written consent was obtained from the patient to reproduce information appearing in this work.
Footnotes
Author Contributions
Analyzed the data: VCK, SCW, CKL. Wrote the first draft of the manuscript: VCK. Contributed to the writing of the manuscript: VCK. Agree with manuscript results and conclusions: VCK, SCW, CKL. Jointly developed the structure and arguments for the paper: VCK, SCW, CKL. Made critical revisions and approved final version: VCK. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
REFERENCES
- 1.Carmichael J, Possinger K, Phillip P, et al. Advanced breast cancer: a phase II trial with gemcitabine. J Clin Oncol. 1995;13(11):2731–2736. doi: 10.1200/JCO.1995.13.11.2731. [DOI] [PubMed] [Google Scholar]
- 2.Muller S, Schutt P, Bojko P, et al. Hemolytic uremic syndrome following prolonged gemcitabine therapy: report of four cases from a single institution. Ann Hematol. 2005;84(2):110–114. doi: 10.1007/s00277-004-0938-8. [DOI] [PubMed] [Google Scholar]
- 3.Humphreys BD, Sharman JP, Henderson JM, et al. Gemcitabine-associated thrombotic microangiopathy. Cancer. 2004;100(12):2664–2670. doi: 10.1002/cncr.20290. [DOI] [PubMed] [Google Scholar]
- 4.Fung MC, Storniolo AM, Nguyen B, et al. A review of hemolytic uremic syndrome in patients treated with gemcitabine therapy. Cancer. 1999;85(9):2023–2032. doi: 10.1002/(sici)1097-0142(19990501)85:9<2023::aid-cncr21>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Loirat C, Fremeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis. 2011;6:60. doi: 10.1186/1750-1172-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng S, Eyler SJ, Zhang Y, et al. Partial ADAMTS13 deficiency in atypical hemolytic uremic syndrome. Blood. 2013;122(8):1487–1493. doi: 10.1182/blood-2013-03-492421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewin SN, Mutch DG, Whitcomb BP, Liapis H, Herzog TJ. Three cases of hemolytic uremic syndrome in ovarian cancer patients treated with combination gemcitabine and pegylated liposomal doxorubicin. Gynecol Oncol. 2005;97(1):228–233. doi: 10.1016/j.ygyno.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Citarrella P, Gebbia V, Teresi M, et al. Hemolytic uremic syndrome after chemotherapy with gemcitabine and taxotere: a case report. Anticancer Res. 2002;22(2b):1183–1185. [PubMed] [Google Scholar]
- 9.Gross M, Hiesse C, Kriaa F, Goldwasser F. Severe hemolytic uremic syndrome in an advanced ovarian cancer patient treated with carboplatin and gemcitabine. Anticancer Drugs. 1999;10(6):533–536. doi: 10.1097/00001813-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Regierer AC, Kuehnhardt D, Schulz CO, et al. Breast cancer-associated thrombotic microangiopathy. Breast Care (Basel) 2011;6(6):441–445. doi: 10.1159/000335201. [DOI] [PMC free article] [PubMed] [Google Scholar]