Abstract
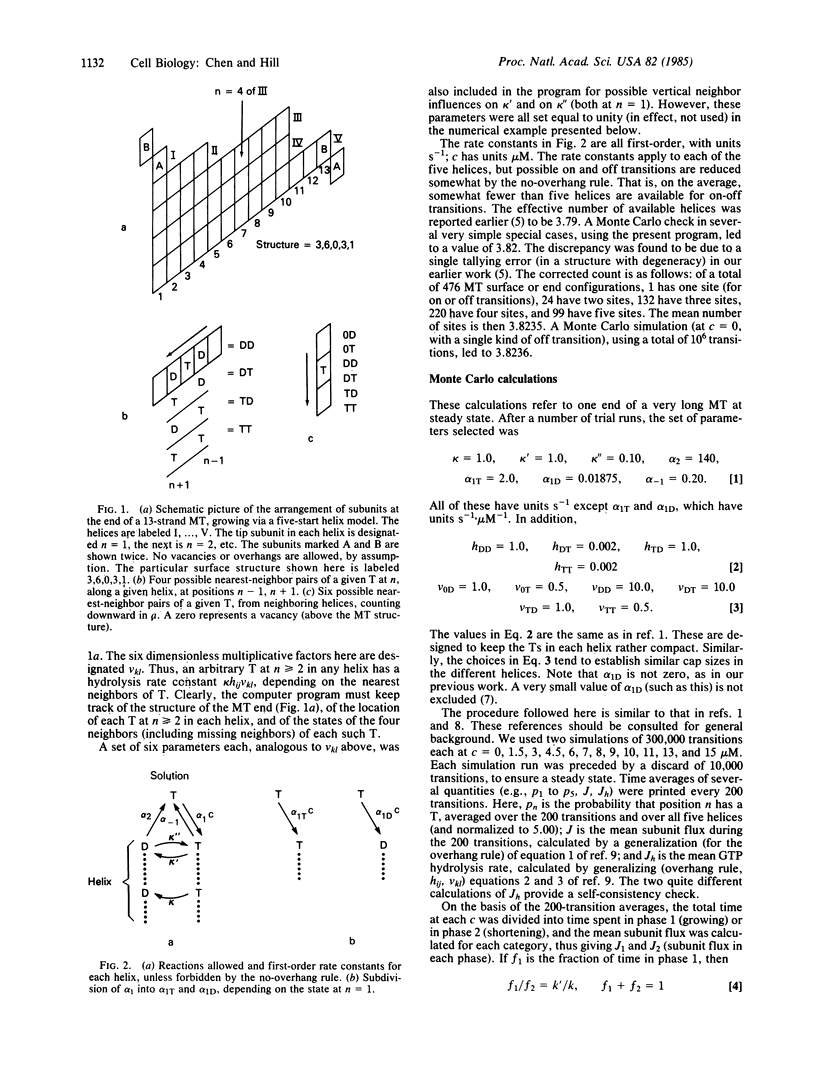
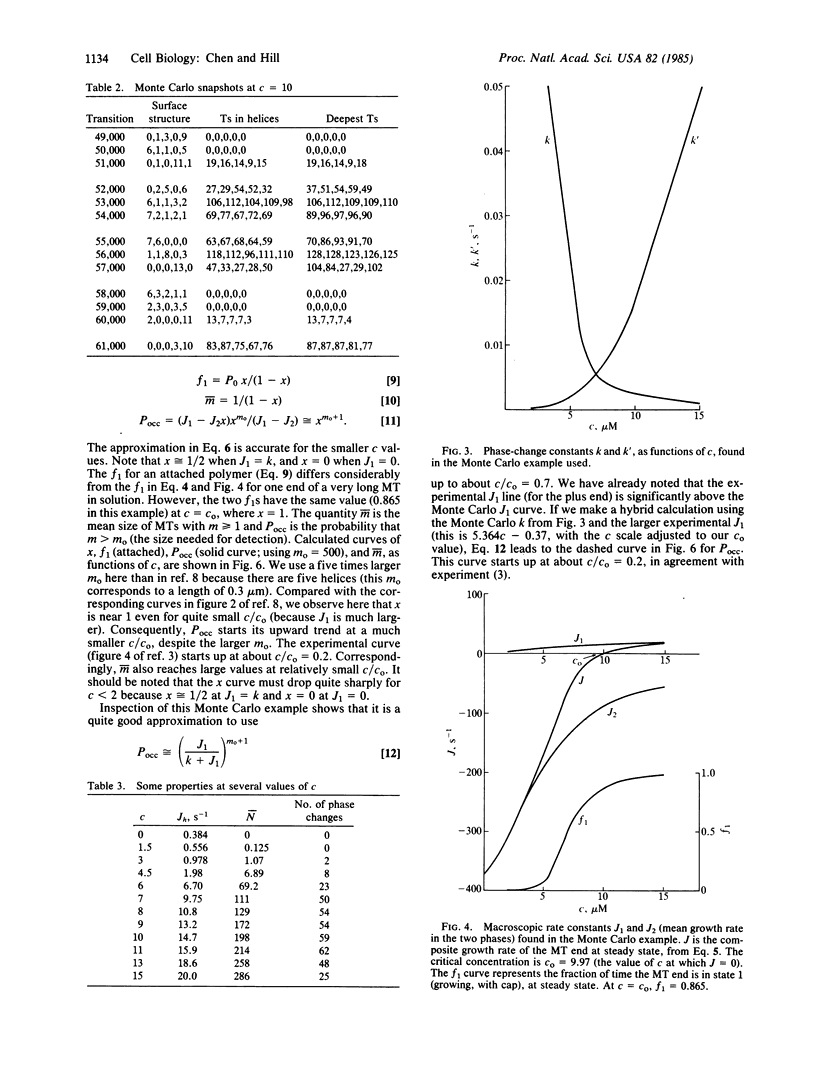
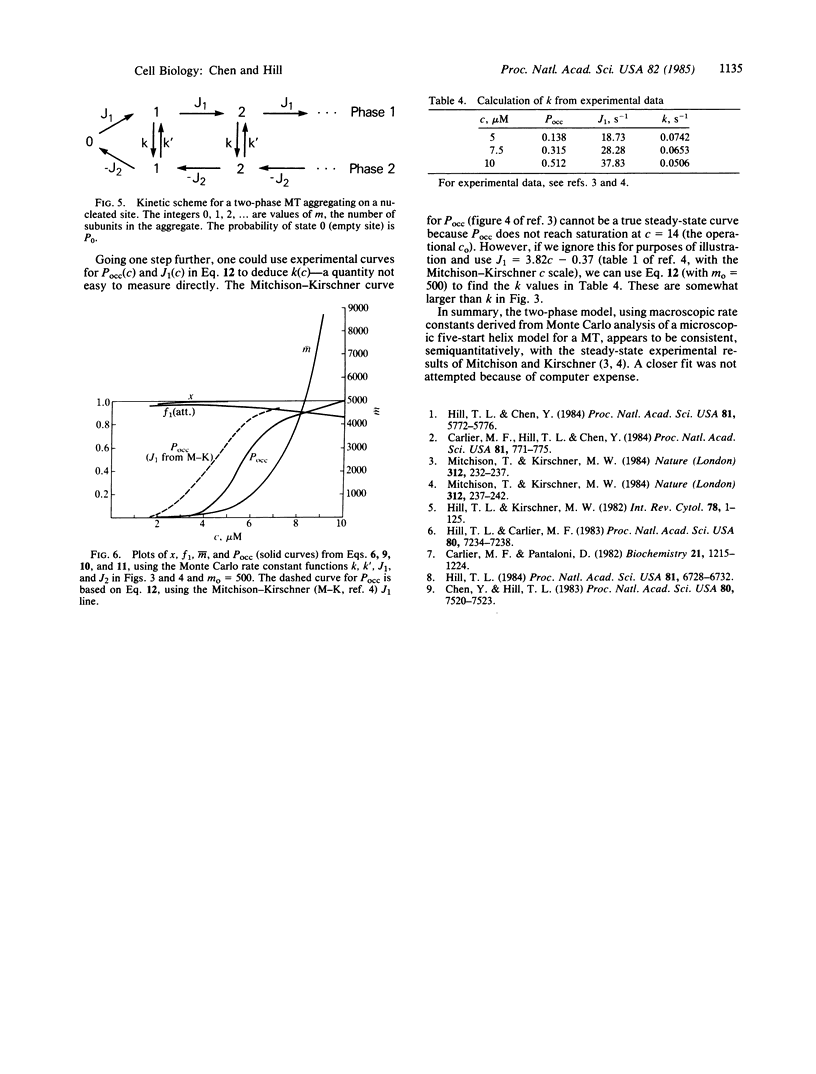
Earlier Monte Carlo studies on a single-helix model of the GTP cap at the end of a microtubule are extended here to a more realistic five-start helix model of the microtubule end. As in the earlier work, phase changes occur at the microtubule end: the end is either capped with GTP and growing slowly or uncapped and shortening rapidly, and these two regimes alternate (at a given tubulin concentration) at steady state. Macroscopic rate constants for the two-phase model are deduced from the Monte Carlo results. The macroscopic rate constants lead to properties that are in semiquantitative agreement with related experiments of Mitchison and Kirschner.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlier M. F., Hill T. L., Chen Y. Interference of GTP hydrolysis in the mechanism of microtubule assembly: an experimental study. Proc Natl Acad Sci U S A. 1984 Feb;81(3):771–775. doi: 10.1073/pnas.81.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D. Assembly of microtubule protein: role of guanosine di- and triphosphate nucleotides. Biochemistry. 1982 Mar 16;21(6):1215–1224. doi: 10.1021/bi00535a017. [DOI] [PubMed] [Google Scholar]
- Chen Y., Hill T. L. Use of Monte Carlo calculations in the study of microtubule subunit kinetics. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7520–7523. doi: 10.1073/pnas.80.24.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Carlier M. F. Steady-state theory of the interference of GTP hydrolysis in the mechanism of microtubule assembly. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7234–7238. doi: 10.1073/pnas.80.23.7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Chen Y. Phase changes at the end of a microtubule with a GTP cap. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5772–5776. doi: 10.1073/pnas.81.18.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Introductory analysis of the GTP-cap phase-change kinetics at the end of a microtubule. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6728–6732. doi: 10.1073/pnas.81.21.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Kirschner M. W. Bioenergetics and kinetics of microtubule and actin filament assembly-disassembly. Int Rev Cytol. 1982;78:1–125. [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature. 1984 Nov 15;312(5991):232–237. doi: 10.1038/312232a0. [DOI] [PubMed] [Google Scholar]



