Abstract
Corneal pain is mediated by primary afferent fibers projecting to the dorsal horn of the medulla, specifically the trigeminal nucleus caudalis. In contrast to reflex responses, the conscious perception of pain requires transmission of neural activity to higher brain centers. Ascending pain transmission is mediated primarily by pathways to either the thalamus or parabrachial nuclei. We previously showed that some corneal afferent fibers preferentially contact parabrachial-projecting neurons in the rostral pole of the trigeminal nucleus caudalis, but the role of these projection neurons in transmitting noxious information from the cornea has not been established. In the present study, we show that noxious stimulation of the corneal surface activates neurons in the rostral pole of the nucleus caudalis, including parabrachially projecting neurons that receive direct input from corneal afferent fibers. We used immunocytochemical detection of c-Fos protein as an index of neuronal activation after noxious ocular stimulation. Animals had previously received injections of a retrograde tracer into either thalamic or parabrachial nuclei to identify projection neurons in the trigeminal dorsal horn. Noxious stimulation of the cornea induced c-Fos in neurons sending projections to parabrachial nuclei, but not thalamic nuclei. We also confirmed that corneal afferent fibers identified with cholera toxin B preferentially target trigeminal dorsal horn neurons projecting to the parabrachial nucleus. The parabrachial region sends ascending projections to brain regions involved in emotional and homeostatic responses. Activation of the ascending parabrachial system may explain the extraordinary salience of stimulation of corneal nociceptors.
Keywords: parabrachial, thalamus, noxious, cornea, cholera toxin B
1. Introduction
The surface of the cornea is the most densely innervated structure in the body (Belmonte et al. 2004) and many forms of eye pain may originate from activation of corneal sensory fibers (Rosenthal and Borsook 2012). In spite of the prevalence of ocular pain, the central pathways involved in corneal pain transmission are poorly understood. The complexity of this system is due in part to the parallel pathways through the brainstem that mediate eye blink. The present studies used both anatomical and functional assessments to identify a central pathway involved in processing of ocular pain from the corneal surface.
The trigeminal dorsal horn, specifically the trigeminal nucleus caudalis (Vc) receives nociceptive primary afferent fibers from the cornea (Marfurt and Del Toro 1987;Hegarty et al. 2010;Meng and Bereiter 1996) and also contains neurons that project to both thalamic and parabrachial nuclei (Bereiter et al. 2000;Aicher et al. 2013;Mitchell et al. 2004;Guy et al. 2005;Cechetto et al. 1985;Saper 1995). There is conflicting evidence with regard to the role of thalamic-projecting neurons in corneal nociceptive processing. Electrophysiological studies suggest that thalamic-projecting neurons in trigeminal dorsal horn encode corneal nociceptive responses (Meng and Bereiter 1996;Meng et al. 2000). Yet in a previous study, we found that corneal primary afferent fibers preferentially target dorsal horn neurons that project to parabrachial nuclei over neurons that project to thalamic nuclei (Aicher et al. 2013). Our anatomical findings were consistent with other studies that failed to detect trigemino-thalamic neurons in the caudal ventrolateral region of Vc that receives corneal primary afferent fibers (Fukushima and Kerr 1979). The anatomical findings cannot exclude the possibility that polysynaptic pathways within trigeminal dorsal horn are involved in nociceptive transmission. Therefore, we sought to functionally assess the role of parabrachial- versus thalamic-projecting neurons in Vc with regard to encoding noxious corneal stimulation.
In the present study we used multi-modal noxious stimulation of the cornea together with an assessment of c-Fos induction. In the same animals, we determined whether the neurons responding to noxious stimulation of the cornea receive direct input from corneal afferent fibers, if they are projection neurons, and if they project to parabrachial or thalamic nuclei. This approach allows us to determine if projection neurons are responsive to noxious corneal stimulation based on c-Fos content and secondarily to determine if activated neurons are second-order (e.g. receive direct input from corneal afferent fibers) or if they are higher-order (e.g. respond to noxious stimuli but do not receive direct inputs and thus may receive indirect input). All assessments were conducted in regions of the ventrolateral Vc that receive the most abundant corneal afferent fibers (Aicher et al. 2013;Hegarty et al. 2010), consisting of two regions: an area at the caudal pole of Vc at the transition between Vc and cervical spinal cord level C1 (Vc/C1 level), which we will refer to as “caudal Vc” in this paper and area at the transition between Vc and the interpolaris region of the trigeminal nucleus (Vi/Vc level) which we will refer to as “rostral Vc”). This approach allows us to clarify both the anatomical and functional pathways within the trigeminal dorsal horn involved in nociceptive responses from the corneal surface.
2. Results
2.1. Retrograde Tracer Injection Sites and Labeling in Vc
Injections of FluoroGold into either the ipsilateral parabrachial nuclei (Figure 1A) or the contralateral thalamus (Figure 1B) produced retrograde labeling in Vc. Injection sites (Figure 1) were not confined to any specific parabrachial or thalamic subnuclei, in an effort to identify as many projection neurons to each target as possible. As with most tract tracers, it is possible that some uptake by fibers of passage is possible. However, if the tracer were taken up by trigemino-thalamic axons following parabrachial injections, the retrogradely labeled cells would be expected to be found on the contralateral side of the trigeminal dorsal horn which was not examined in the parabrachial cases for this study. These findings are consistent with our previous studies (Aicher et al. 2013;Mitchell et al. 2004) as well as results from other laboratories (Fukushima and Kerr 1979). We confined our analysis to the ventrolateral caudal and rostral transition levels of Vc that we and others have previously found to contain the greatest density of primary afferent fibers from the cornea (Aicher et al. 2013;Hegarty et al. 2010;Marfurt and Del Toro 1987).
Figure 1.
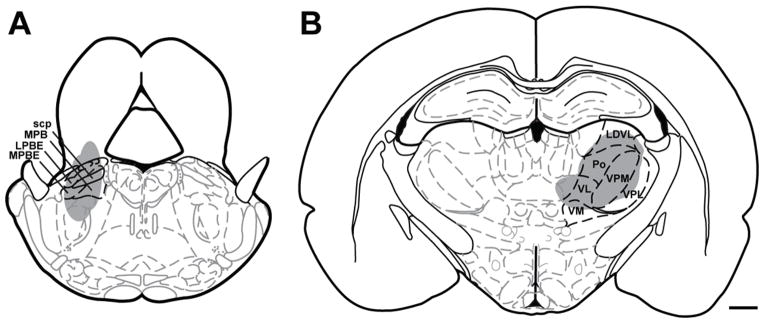
Schematic representation of FluoroGold injection sites into left parabrachial nuclei (A) or right thalamic nuclei (B) that yielded retrograde labeling in the left trigeminal dorsal horn (Paxinos and Watson atlas, with permission). Abbreviations: scp = superior cerebellar peduncle, MPB = medial parabrachial nucleus, LPBE = lateral parabrachial nucleus, external, MPBE = medial parabrachial nucleus, external. LDVL = laterodorsal thalamic nuclei, ventrolateral, Po = posterior thalamic nuclear group, VPM = ventral postermedial thalamic nuclei, VPL = ventrolateral posterolateral thalamic nuclei, VL = ventrolateral thalamic nuclei, VM = ventromedial thalamic nuclei. Scale bar = 1 mm
Injections of the retrograde tracer FG into parabrachial or thalamic nuclei yielded labeled neurons in the ventrolateral region of Vc (Figure 2). FG immunoreactivity was visible as punctate labeling within neurons and occasional proximal dendrites (Figure 2B). The punctate appearance of FG is consistent with it being sequestered into discrete lysosomes and endosomes (Mitchell et al. 2004;Aicher et al. 2013). Different patterns of retrograde labeling within the ventrolateral area of Vc were observed depending on the target nuclei injected, parabrachial or thalamic (Figures 3, 4). The caudal Vc contained no thalamic-projecting neurons (Figures 3E, 4), but some thalamic-projecting neurons (n = 8 ± 1) were present in the rostral Vc (Figures 3F, 4). Parabrachial-projecting neurons identified with FG (Figures 3C, 3D) were abundant at both caudal (n = 15 ± 4) and rostral (n = 34 ± 8) levels of Vc (Figure 4). These results are consistent with prior work from our lab (Aicher et al. 2013).
Figure 2.
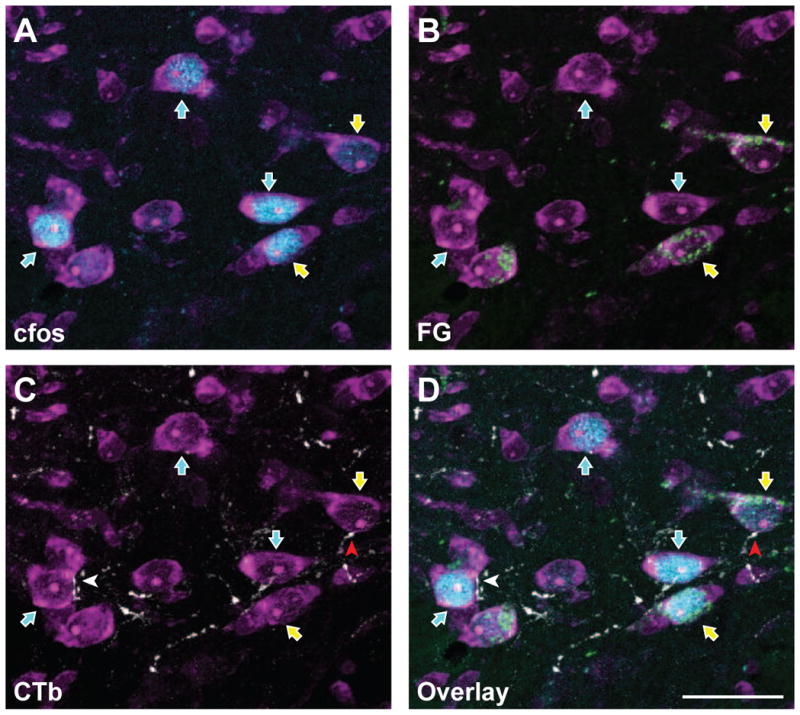
FluoroGold and c-Fos are co-localized in subsets of trigeminal dorsal horn neurons that also receive corneal afferent input. Micrographs of the same area showing the detection of individual markers are shown in panels A – C, while panel D shows all three channels in a single micrograph. A. Neurons were identified with NeuroTrace fluorescent Nissl stain (magenta). A subset of these neurons are immunoreactive for c-Fos (blue; blue arrows) following noxious stimulation of the cornea. B. Immunoreactivity for FluoroGold (green) shows a distinct subset of neurons containing both c-Fos and FluoroGold (yellow arrows). Thus, these FG-containing projection neurons are also activated by noxious corneal stimulation. C. CTb-containing corneal afferents (white) are seen in apposition to select subpopulations of trigeminal dorsal horn neurons. A neuron that receives a CTb apposition and also contains FG and c-Fos is indicated with a red arrowhead. D. Overlay of all four channels showing CTb appositions with cells containing both FG and c-Fos. Each panel is composed of a Z stack of 10 consecutive images for total depth of 3.6 μm. Scale bar = 25 μm
Figure 3.
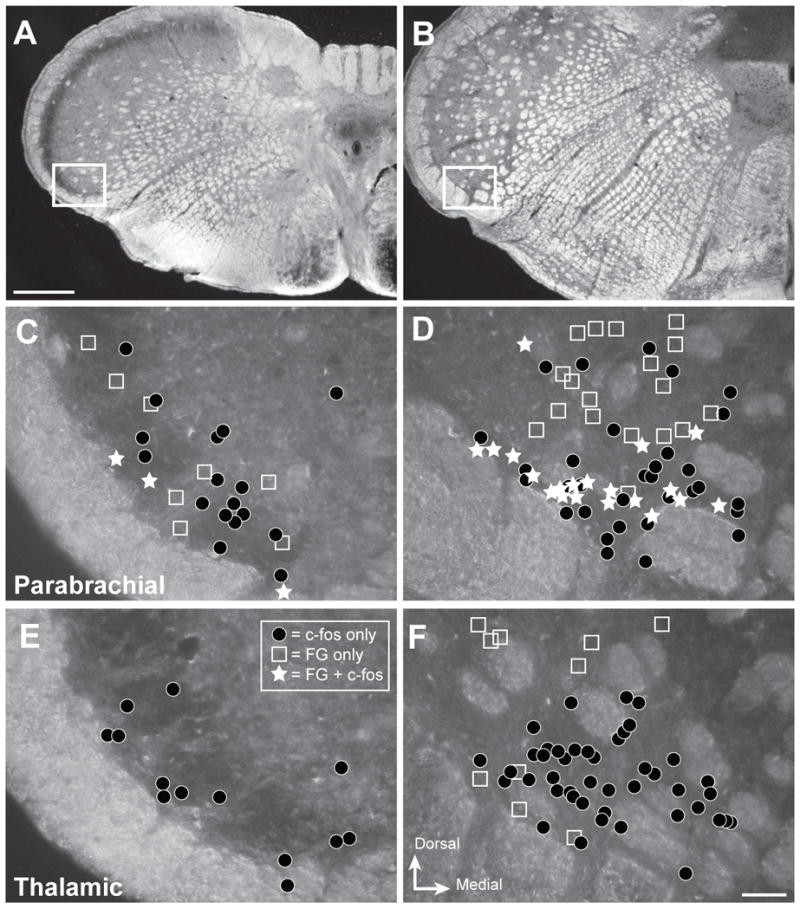
Projection neurons are interspersed with neurons activated by noxious ocular stimulation in trigeminal nucleus caudalis. Representative distribution of retrogradely labeled (FG) and c-Fos-immunoreactive (-ir) neurons within caudal and rostral regions of ventrolateral nucleus caudalis (Vc) that were analyzed in the present studies. A, B. Darkfield micrographs depicting the regions of interest for caudal Vc (A) and rostral Vc (B). White box shows the region examined in the analysis. C – F. Drawings of distribution of labeled neurons in representative cases. The area of interest contained neurons with FG only (open squares), c-Fos only (dark circles) or both (white stars) for injections into parabrachial (C, D) or thalamic (E, F) nuclei. Results show that c-Fos-ir neurons (black circles) are more abundant in rostral Vc (D, F) compared to caudal Vc in all cases. Injections into parabrachial nuclei produced more retrogradely labeled neurons (open squares; C, D) than injections into thalamic nuclei (E, F); and only parabrachial cases had retrogradely labeled neurons that also contained c-Fos (white stars; C, D) after noxious corneal stimulation. Scale bar = 500 μm for panels A and B, and 50 μm for panels C - F
Figure 4.
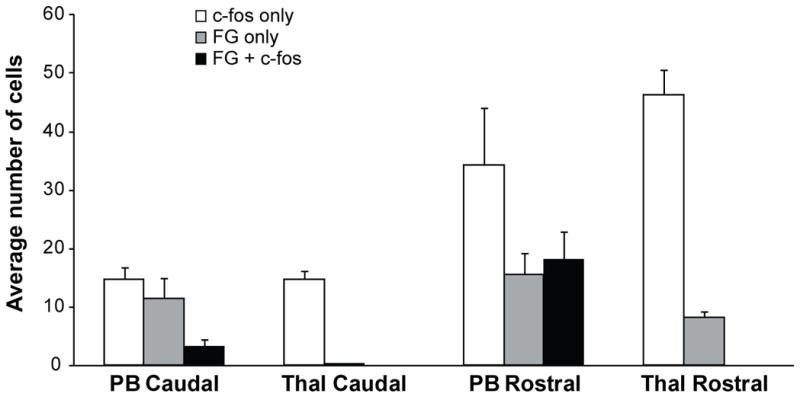
Quantification of cell counts within caudal and rostral levels of trigeminal nucleus caudalis (Vc) for cases that received retrograde tracer (FG) injections into either the parabrachial nuclei (PB) or thalamic nuclei (Thal). Each cell count classification is mutually exclusive. Cells containing c-Fos only (white bars) were detected in all cases in both caudal and rostral Vc, but were more abundant within rostral Vc. Cells containing FG only (gray bars) were detected in the caudal Vc for PB cases, but not for Thal cases. Cells containing FG only (gray bars) were present in rostral Vc for both PB and Thal cases, indicating that some projection neurons were not activated by the noxious stimuli applied to the cornea. Projection neurons that were activated by noxious stimulation of the cornea (FG + c-Fos; black bars) were only present in PB cases and were seen in both caudal and rostral Vc. In rostral Vc, more than half of the PB-projecting neurons identified were activated by noxious stimulation of the cornea (black versus gray bars).
2.2. Noxious Stimulation of the Cornea Activates c-Fos in the Trigeminal Nucleus Caudalis
A single one minute application of 10 μl of 0.1% capsaicin to the corneal surface followed by mechanical stimulation resulted in c-Fos-immunoreactive (-ir) cells within the ventrolateral aspect of the trigeminal nucleus caudalis (Vc) (Figure 2A). c-Fos immunoreactivity was observed in the nuclei of cells, confirmed by coincident NeuroTrace staining (Figure 2). Similar to our retrograde tracer analyses, we confined our counts of c-Fos neurons to the ventrolateral region of the caudal and rostral transition levels of Vc (Figures 3A, 3B). We counted cells within this region regardless of laminar distribution, but most neurons were found in the superficial laminae. In the caudal Vc (Figures 3A, 3C, 3E) an average of 17 ± 1 c-Fos-ir cells were observed per section per animal. The rostral Vc (Figures 3B, 3D, 3F) contained approximately three times more c-Fos-ir cells (50 ± 5 cells per section per animal) than the caudal Vc region. Group data are summarized in Figure 4. To ensure that there were no differences in the degree of c-Fos activation between cases with different retrograde tracer injections, we examined the number of c-Fos neurons based on the site of the retrograde tracer injection. Injections of the retrograde tracer FG into either parabrachial or thalamic nuclei did not affect the number of c-Fos-ir cells observed in Vc either caudally (t-test, p=0.237), or rostrally (t-test, p=0.591). These findings show that noxious stimulation of the cornea preferentially activates neurons in the rostral Vc compared to the caudal Vc (see Figure 3A, 3B for identification of regions of interest for analysis).
2.4. c-Fos was Observed in Parabrachial-Projecting, But Not Thalamic-Projecting Neurons
Parabrachial-projecting neurons contained c-Fos immunoreactivity following noxious stimulation of the cornea (Figures 2, 3, 4). In caudal Vc, 23% of parabrachial-projecting neurons contained c-Fos immunoreactivity following stimulation of the cornea (Figures 3C, 4). Thalamic-projecting neurons were not detected in this caudal region and therefore, no c-Fos and FG dual-labeled neurons were detected (Figures 3E, 4). Within rostral Vc, 54% of parabrachial-projecting neurons contained immunoreactivity for c-Fos (Figures 3D, 4), but none of the thalamic-projecting neurons that were present contained c-Fos immunoreactivity (Figures 3F, 4). These results suggest differential transmission of noxious ocular stimuli from the periphery through parabrachial versus thalamic nuclei.
2.5. Corneal Afferent Fibers Differentially Target c-Fos Immunoreactive Parabrachial-Projecting Neurons
We examined whether parabrachial-projecting neurons that are activated by noxious corneal stimulation also received direct input from corneal afferent fibers. Following noxious corneal stimulation, parabrachial-projecting neurons that expressed c-Fos (FG + c-Fos) also received appositions from CTb-labeled corneal primary afferent fibers (Figure 1D). These appositions were observed frequently for neurons in rostral Vc (26%; 24/91), but only rarely in caudal Vc (6%; 1/17). None of the thalamic-projecting neurons in either caudal or rostral Vc contained c-Fos immunoreactivity; thus there were no instances of corneal afferent fibers contacting activated thalamic-projecting neurons.
3. Discussion
Consistent with our previous study (Aicher et al. 2013), we found that parabrachial-projecting neurons are more abundant than thalamic-projecting neurons in the caudal and rostral ventrolateral trigeminal dorsal horn regions that receive corneal primary afferent fibers. We also found that a majority of the parabrachial-projecting neurons in rostral Vc are activated by noxious stimulation of the cornea and many receive direct contact by corneal afferent fibers labeled with CTb. To our surprise, we were unable to activate thalamic-projecting neurons in caudal or rostral Vc even with different concurrent modalities of noxious corneal stimulation. If these thalamic-projecting neurons received indirect projections from corneal primary afferent fibers, or were activated by classes of afferent fibers not detected by CTb, we would have still expected them to express c-Fos after such robust noxious stimulation of the cornea. Together these findings support our hypothesis that corneal afferent fibers detected with CTb are nociceptors and that activity in these primary afferent fibers leads to excitation of second-order projection neurons. These second-order neurons project primarily to the parabrachial region, and thus this pathway is the primary nociceptive relay for ocular pain. Activation of an ascending parabrachial pathway has been reported following noxious stimulation of other specialized tissues innervated by the trigeminal nerve, including the meninges (Nozaki et al. 1992), mandibular incisor dentin and tooth pulp (Allen et al. 1996) and lower lip (Hermanson and Blomqvist 1997).
Previous anatomical studies from this laboratory have examined the distribution of, and corneal primary afferent fiber input to, parabrachial and thalamic projection neurons in Vc. We found that CTb-labeled corneal afferent fibers preferentially target parabrachial-projecting neurons (Aicher et al. 2013). Even though thalamic-projecting neurons were also present in the rostral trigeminal dorsal horn, very few of them received direct input from CTb-labeled corneal primary afferent fibers. This suggested the possibility that corneal afferent fiber activation of the trigemino-thalamic pathway might occur via a polysynaptic pathway, while corneal afferent fiber activation of the trigemino-parabrachial pathway occurs via a more direct monosynaptic pathway. In the present study, we used c-Fos to determine if noxious corneal stimulation would activate both populations of projection neurons. However, our results show that thalamic-projecting neurons did not express c-Fos following noxious stimulation of the cornea. It is possible that these neurons express a different immediate early gene, but other studies have found Vc neurons projecting to thalamus do express c-Fos after noxious TMJ stimulation (Chang et al. 2012). Our results are consistent with the finding that thalamic-projecting neurons do not receive monosynaptic input from corneal nociceptors, and therefore do not appear to be involved in transmitting corneal nociceptive information. We cannot rule out that thalamic-projecting neurons may respond exclusively to activation of cold fibers from the cornea (Belmonte and Gallar 2011;Robbins et al. 2012), as these were not examined in the present study.
We used cholera toxin subunit B (CTb) (Fishman 1982) to label corneal afferent fibers because it is the only tract tracer that we have found that will effectively label the central terminals of these afferent fibers (Hegarty et al. 2010). With CTb we found afferent fiber labeling to a similar extent and at similar locations in Vc as reported with other anterograde tracers (Marfurt and Del Toro 1987). It has been argued that CTb selectively labels myelinated afferent fibers (Todd et al. 2003) and since many corneal nociceptors in mice have conduction velocities in the c-fiber range (Belmonte et al. 2004) we may have not labeled all corneal afferent fibers. However, other studies suggest that corneal afferent fibers are myelinated and unmyelinated, and that all fibers demyelinate as the enter the cornea, potentially causing confusing results in terms of conduction velocities (MacIver and Tanelian 1993b;MacIver and Tanelian 1993a). Furthermore, some studies have demonstrated that CTb can label some unmyelinated fibers from a variety of tissues (Sugimoto et al. 1997;Corbett et al. 2005). We cannot exclude the possibility that CTb selectively labels myelinated fibers from the cornea which would primarily include mechanoreceptors and some polymodal nociceptors (Belmonte et al. 2004), but we cannot exclude the possibility that CTb also labeled unmyelinated afferent fibers. Temperature-sensitive and cold receptors in the cornea are thought to be primarily associated with unmyelinated c-fibers and thus may not be labeled by CTb, but these represent a small percentage of corneal nociceptors.
In the rostral pole of the ventrolateral trigeminal dorsal horn, where most of our projection neurons were located, more than half of the parabrachial-projecting neurons were activated by noxious chemical and mechanical stimulation of the cornea. This supports the notion that these parabrachial-projecting neurons receive corneal nociceptor input and mediate the transmission of nociceptive information from the cornea. Corneal afferent fibers are polymodal (Acosta et al. 2001), so we expect some neurons to be activated by multiples types of stimuli. The manipulations used in the current study would not activate all nociceptors including those that transduce noxious cold (Belmonte et al. 2004), but these represent a small subset of corneal afferent fibers and some of these afferent fibers are activated by capsaicin. Our goal was to activate as many nociceptors as possible to avoid false negative conclusions regarding the activation of thalamic neurons. Trigeminal dorsal horn neurons are also activated by innocuous stimuli (Robbins et al. 2012) that evoke lacrimation, but this pathway would involve relays within the hindbrain to superior salivatory nucleus (Cuthbertson et al. 2003) not an ascending pathway. However, neurons in the region that were not ascending projection neurons and were activated by corneal stimulation may be components of the blink reflex (Henriquez and Evinger 2007).
We found almost a quarter of parabrachial-projecting ascending neurons that respond to noxious stimulation also received direct somatic input from CTb-labeled corneal afferent fibers. Since we are unlikely to label all afferent fibers with a single tracer injection and we confined our analyses to somatic, but not dendritic targets, it is possible that a larger proportion of activated parabrachial-projecting neurons receive direct corneal afferent fiber input. However, our findings support the notion that the parabrachial projection neurons are second-order neurons and the ocular pain pathway does not require an interneuron within the trigeminal dorsal horn. We cannot rule out the possible presence of some polysynaptic circuits in trigeminal dorsal horn, but our data are more consistent with a single synapse in dorsal horn between the corneal nociceptor and an ascending projection neuron.
Previous studies (Meng and Bereiter 1996) have reported that the caudal region of Vc contains more c-Fos activated neurons than the rostral region following noxious thermal stimulation of the cornea; we have found the opposite. One possible reason for this discrepancy is that we only examined the small ventrolateral region of Vc that receives the densest innervation from CTb-labeled corneal afferent fibers. We chose to not count more dorsal regions of Vc that receive primary afferent fibers from periorbital tissues that could confound our results (Panneton et al. 2010). Noxious stimulation of the cornea, particularly using a liquid may activate nociceptors in tissues other than the corneal surface, including the conjunctiva. Due to this possible confound, we only examined the area of Vc where we find the peak input from CTb-labeled corneal afferent fibers (Aicher et al. 2013). A second difference between our experiments and previous studies is that in our studies, the area of analysis for quantification is the same size, allowing for more direct quantification of results between caudal and rostral Vc. Using this approach we feel confident that we have made the most careful comparison possible between the rostral and caudal Vc with regard to quantitative comparisons of the degree of c-Fos induction. Our findings support the notion that both regions of Vc contain neurons that are activated by noxious corneal stimulation, but within the rostral region a greater portion of these cells are parabrachial-projecting neurons. These studies add to our findings that the neurotransmitter content of corneal afferent fibers projecting to rostral and caudal Vc are also distinct, with a greater CGRP content in the afferents projecting to the rostral Vc (Hegarty et al. 2010). Thus, the morphology of the corneal afferents projecting to rostral Vc is also consistent with their identification as nociceptors.
4. Experimental procedures
4.1. Animals
Rats were treated according to the guidelines of the National Institutes of Health publication “Guide for the Care and Use of Laboratory Animals”. All protocols were approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University.
Male Sprague-Dawley rats (n = 10; 224 – 330 g; Charles River Laboratories, Wilmington, MA) were used in this study. Rats were housed on a 12/12 light/dark cycle and were given access to food and water ad libitum.
4.2. Retrograde tracing of projection neurons
Rats were placed into a stereotaxic frame (David Kopf Instruments, Tujunga, CA) and anesthetized with vaporized isoflurane in oxygen (Isotex Tec3, Datex-Ohmeda, Madison, WI; 5% induction, 3% maintenance). FluoroGold (FG, 2% in saline; Fluorochrome, LLC, Denver, CO) was picospritzed into thalamic (100–135 nl) or parabrachial (65–100 nl) nuclei as previous described (Aicher et al. 2013). FG was selected as the retrograde tracer of choice because it produces only retrograde labeling, appears to be taken up by all neurons, and it can be detected using immunocytochemical methods (Aicher et al. 1995;Van Bockstaele et al. 1994). Incisions were closed with 3-0 monocryl suture (Ethicon, Cornelia, GA), antiseptic was applied and animals were monitored during recovery.
4.3. Anterograde tracing of corneal afferent fibers
Cholera toxin B subunit (CTb; 1% solution in 0.1 M phosphate buffer; List Biological Laboratories, Inc., Campbell, CA) was used as an anterograde tracer to identify central projections of corneal afferent fibers to the trigeminal dorsal horn (Hegarty et al. 2010;Todd et al. 2003;Aicher et al. 2013). Following FG injections, several drops of ophthalmic proparacaine hydrochloride, 5% solution (Bausch and Lomb, Tampa, FL) were placed in the left eye to reduce spontaneous eye movements and a 1% CTb solution (8 – 12 μl) was placed on the corneal surface inside a steel ring for 30 min as previously described (Hegarty et al. 2010). Rats received a subcutaneous injection of ketoprofen (2.5 mg/kg) to reduce discomfort and were monitored during recovery from anesthesia.
4.4. Chemical and mechanical corneal stimulation
Multiple modalities of noxious stimulation were applied briefly to the corneal surface in order to activate a substantial population of corneal nociceptors. Six to seven days following tract tracer injections, each rat was anesthetized with isoflurane in oxygen, and a 0.1% capsaicin solution (10 ul; in 1% Tween, 1% ethanol in saline, Sigma-Aldrich) was applied for 1 min to the left eye inside a metal retaining ring. Following copious rinsing with saline, the left eye was mechanically stimulated by rubbing a dry surgical eye spear across the cornea 20 times. Rats were removed from anesthesia following completion of corneal stimulation.
4.5. Tissue preparation and immunocytochemistry
Two hours after corneal stimulation, corresponding to the time of peak c-Fos activation (Gogas et al. 1996;Bullitt et al. 1992), rats were overdosed with pentobarbital sodium (150 mg/kg) and perfused transcardially through the ascending aorta with 3.8% acrolein in 2% paraformaldehyde as previously described (Hegarty et al. 2010). Brain regions of interest were placed in 2% paraformaldehyde for 30 minutes, rinsed in 0.1 M phosphate buffer, sectioned (40 μm) on a vibrating microtome (Leica, Malvern, PA) and processed for immunocytochemistry. Correct placement of FG injections was verified in tissue sections that contained thalamic or parabrachial nuclei (Figure 1) using an Olympus BX51 light and epifluorescent microscope interfaced with a DP71 digital camera.
4.6. Fluorescent Immunocytochemistry
For triple-labeling studies (Aicher et al. 2003;Bailey et al. 2006;Winkler et al. 2006), tissue sections from the brainstem were incubated in a 1% sodium borohydride solution for 30 minutes, rinsed (0.1 M phosphate buffer), and incubated for 30 minutes in a 0.5% bovine serum albumin (BSA, Sigma-Aldrich) solution in 0.1 M Tris-Saline (TS). Tissue sections were then placed in a primary antibody cocktail with 0.25% Triton X-100 (Sigma-Aldrich, St. Louis, MO) and 0.1% BSA in 0.1 M TS, for 40 hours at 4°C consisting of a polyclonal goat anti-c-Fos antibody (1:3,000; Santa Cruz Biotechnology, Santa Cruz, CA) and polyclonal rabbit anti-FG antibody (1:15,000; Fluorochrome LLC). Sections were rinsed and placed in a secondary antibody cocktail containing Alexa Fluor® 546 donkey anti-rabbit IgG (1:800, Life Technologies, Grand Island, NY) and Alexa Fluor® 647 donkey anti-goat IgG (1:800, Life Technologies) for 2 hours at room temperature. Next, tissue sections were rinsed and incubated with an additional primary antibody directed against CTb prepared in goat (1:25,000; List Biological Laboratories) for 40 hours at 4°C. After rinsing, tissue sections were incubated with another secondary antibody, Alexa Fluor® 488 donkey anti-goat IgG (1:800, Life Technologies) for 2 hours. After additional rinses, sections were incubated in NeuroTrace™ Fluorescent Nissl Stain 435/455 (Life Technologies) for 20 minutes at room temperature. After a last rinse, sections were mounted on gelatin-coated slides, coverslipped with Prolong Gold™ Antifade reagent (Life Technologies) and stored at −20°C. All rinses and incubations, including and following the first secondary antibody cocktail, were protected from ambient light.
The specificity of primary and secondary antibodies has been previously validated in our laboratory and others. The goat anti-CTb antibody was raised against purified cholera toxin beta subunit (Llewellyn-Smith et al. 1995;Hegarty et al. 2010;Aicher et al. 2013). The rabbit anti-FG antibody recognizes FluoroGold, also known as hydroxystilbamidine (Aicher et al. 1995;Van Bockstaele et al. 1994) and staining is abolished by preadsorption of the antigen (Lee et al. 2009). The pattern of immunocytochemical labeling matched the intrinsic fluorescence of FG emitted under ultraviolet light.
4.7. Inclusion criteria, imaging and analytic methodology
FG, c-Fos and CTb were examined at both the caudal and rostral transition zones of trigeminal nucleus caudalis (Vc), regions we previously demonstrated contain both corneal afferent fibers and ascending projection neurons (Aicher et al. 2013;Hegarty et al. 2010). In order to minimize false negative conclusions, we established inclusion criteria for both retrograde and anterograde labeling. FG cases required an appropriately located injection site (Figure 1) and the presence of labeled neurons within the brainstem (Figure 2). CTb cases required at least 25 varicosities at the caudal transition zone of Vc and 50 at the rostral transition (Hegarty et al. 2010). One animal that received a successful FG injection into the thalamus but did not meet the minimum criteria for CTb was excluded from the analysis of CTb appositions. All cases with successful retrograde labeling were analyzed for c-Fos content after noxious corneal stimulation.
4.8. Confocal imaging and analysis
Images were collected of the caudal and rostral Vc transition zones as Z stacks bounded by the vertical extent of immunoreactivity for c-Fos, CTb, and FG on a Zeiss LSM780 confocal microscope (Carl Zeiss MicroImaging, Thornwood, NY) (Hegarty et al. 2010;Aicher et al. 2013;Marfurt and Del Toro 1987). Despite sequential application of the two goat primary antibodies, a small amount of overlap was observed with c-Fos immunoreactivity as faint nuclear staining seen in the CTb channels. This was likely due to the secondary antibody used to detect CTb also binding to the primary c-Fos antibody. However, knowing a priori that co-localization of a cellular nuclear marker (c-Fos) and a marker of primary afferent fibers (CTb) was likely an artifact of the experimental conditions, c-Fos immunoreactivity and CTb immunoreactivity were captured as separate channels, according to their respective fluorophores, and any nuclear labeling seen in the CTb channel was subtracted from the images utilizing the capabilities of the Zeiss ZEN software. Confocal micrographs used for publication are projections of several optical sections that were adjusted for optimal brightness and contrast using Zeiss ZEN software.
Analyses were limited to optical sections (within one caudal, and one rostral section per animal) within Z-stacks that contained immunoreactivity for all three antigens of interest: FG, c-Fos, and CTb. All c-Fos cells within the ventrolateral trigeminal dorsal horn were enumerated, as were all FG cells, then all numbered cells were evaluated for coincident c-Fos and FG immunoreactivity.
All CTb corneal afferent fiber varicosities that were directly adjacent to NeuroTrace-stained somata in two consecutive 0.4 μm thick optical sections (Bailey et al. 2006;Hegarty et al. 2010) were counted. NeuroTrace-stained somata that contained FG, c-Fos, or both antigens that received appositions from CTb varicosities were then tabulated. All analyses were verified by two independent observers.
4.9. Statistical analyses
T-tests compared number of c-Fos activated neurons at both rostral and caudal levels of Vc. Average numbers of neurons and CTb-labeled corneal afferent fibers apposed to somata are reported with standard error of the mean (SigmaStat 2.03, Systat Software, Inc., San Jose, CA).
Highlights.
Noxious stimulation of the corneal surface activates rostral Vc neurons.
Parabrachial-projecting neurons are activated by corneal capsaicin.
Corneal afferent fibers contact neurons activated by capsaicin.
Acknowledgments
This work was supported by grants from the NIH: NIH R01 DE012640, a shared instrumentation grant from NIH RR016858 (confocal) and NIH P30 NS061800 (Neuroscience Imaging Center at OHSU).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sue A. Aicher, Email: aichers@ohsu.edu.
Deborah M. Hegarty, Email: hegartyd@ohsu.edu.
Sam M. Hermes, Email: hermess@ohsu.edu.
References
- Acosta MC, Belmonte C, Gallar J. Sensory experiences in humans and single-unit activity in cats evoked by polymodal stimulation of the cornea. J Physiol. 2001;534:511–525. doi: 10.1111/j.1469-7793.2001.t01-1-00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher SA, Hermes SM, Hegarty DM. Corneal afferents differentially target thalamic- and parabrachial-projecting neurons in spinal trigeminal nucleus caudalis. Neuroscience. 2013;232:182–193. doi: 10.1016/j.neuroscience.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher SA, Mitchell JL, Swanson KC, Zadina JE. Endomorphin-2 axon terminals contact mu-opioid receptor-containing dendrites in trigeminal dorsal horn. Brain Res. 2003;977:190–198. doi: 10.1016/s0006-8993(03)02678-7. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Reis DJ, Nicolae R, Milner TA. Monosynaptic projections from the medullary gigantocellular reticular formation to sympathetic preganglionic neurons in the thoracic spinal cord. J Comp Neurol. 1995;363:563–580. doi: 10.1002/cne.903630405. [DOI] [PubMed] [Google Scholar]
- Allen GV, Barbrick B, Esser MJ. Trigeminal-parabrachial connections: Possible pathway for nociception-induced cardiovascular reflex responses. Brain Res. 1996;715:125–135. doi: 10.1016/0006-8993(95)01580-9. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci. 2006;26:11893–11902. doi: 10.1523/JNEUROSCI.2044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–525. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Gallar J. Cold thermoreceptors, unexpected players in tear production and ocular dryness sensations. Invest Ophthalmol Vis Sci. 2011;52:3888–3892. doi: 10.1167/iovs.09-5119. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Hirata H, Hu JW. Trigeminal subnucleus caudalis: Beyond homologies with the spinal dorsal horn. Pain. 2000;88:221–224. doi: 10.1016/S0304-3959(00)00434-6. [DOI] [PubMed] [Google Scholar]
- Bullitt E, Lee CL, Light AR, Willcockson H. The effect of stimulus duration on noxious-stimulus induced c-fos expression in the rodent spinal cord. Brain Res. 1992;580:172–179. doi: 10.1016/0006-8993(92)90941-2. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Standaert DG, Saper CB. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol. 1985;240:153–160. doi: 10.1002/cne.902400205. [DOI] [PubMed] [Google Scholar]
- Chang Z, Okamoto K, Bereiter DA. Differential ascending projections of temporomandibular joint-responsive brainstem neurons to periaqueductal gray and posterior thalamus of male and female rats. Neuroscience. 2012;203:230–243. doi: 10.1016/j.neuroscience.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett EK, Sinfield JK, McWilliam PN, Deuchars J, Batten TF. Differential expression of vesicular glutamate transporters by vagal afferent terminals in rat nucleus of the solitary tract: projections from the heart preferentially express vesicular glutamate transporter 1. Neuroscience. 2005;135:133–145. doi: 10.1016/j.neuroscience.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Cuthbertson S, LeDoux MS, Jones S, Jones J, Zhou Q, Gong S, Ryan P, Reiner A. Localization of preganglionic neurons that innervate choroidal neurons of pterygopalatine ganglion. Invest Ophthalmol Vis Sci. 2003;44:3713–3724. doi: 10.1167/iovs.02-1207. [DOI] [PubMed] [Google Scholar]
- Fishman PH. Role of membrane gangliosides in the binding and action of bacterial toxins. J Membr Biol. 1982;69:85–97. doi: 10.1007/BF01872268. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Kerr FW. Organization of trigeminothalamic tracts and other thalamic afferent systems of the brainstem in the rat: presence of gelatinosa neurons with thalamic connections. J Comp Neurol. 1979;183:169–184. doi: 10.1002/cne.901830112. [DOI] [PubMed] [Google Scholar]
- Gogas KR, Cho HJ, Botchkina GI, Levine JD, Basbaum AI. Inhibition of noxious stimulus-evoked pain behaviors and neuronal Fos-like immunoreactivity in the spinal cord of the rat by supraspinal morphine. Pain. 1996;65:9–15. doi: 10.1016/0304-3959(95)00141-7. [DOI] [PubMed] [Google Scholar]
- Guy N, Chalus M, Dallel R, Voisin DL. Both oral and caudal parts of the spinal trigeminal nucleus project to the somatosensory thalamus in the rat. Eur J Neurosci. 2005;21:741–754. doi: 10.1111/j.1460-9568.2005.03918.x. [DOI] [PubMed] [Google Scholar]
- Hegarty DM, Tonsfeldt K, Hermes SM, Helfand H, Aicher SA. Differential localization of vesicular glutamate transporters and peptides in corneal afferents to trigeminal nucleus caudalis. J Comp Neurol. 2010;518:3557–3569. doi: 10.1002/cne.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez VM, Evinger C. The three-neuron corneal reflex circuit and modulation of second-order corneal responsive neurons. Exp Brain Res. 2007;179:691–702. doi: 10.1007/s00221-006-0826-7. [DOI] [PubMed] [Google Scholar]
- Hermanson O, Blomqvist A. Subnuclear localization of FOS-like immunoreactivity in the parabrachial nucleus after orofacial nociceptive stimulation of the awake rat. J Comp Neurol. 1997;387:114–123. [PubMed] [Google Scholar]
- Lee SB, Beak SK, Park SH, Waterhouse BD, Lee HS. Collateral projection from the locus coeruleus to whisker-related sensory and motor brain regions of the rat. J Comp Neurol. 2009;514:387–402. doi: 10.1002/cne.22012. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Pilowsky P, Minson JB, Chalmers J. Synapses on axons of sympathetic preganglionic neurons in rat and rabbit thoracic spinal cord. J Comp Neurol. 1995;354:193–208. doi: 10.1002/cne.903540204. [DOI] [PubMed] [Google Scholar]
- MacIver MB, Tanelian DL. Free nerve ending terminal morphology is fiber type specific for Ad and C fibers innervating rabbit corneal epithelium. J Neurophysiol. 1993a;69:1779–1783. doi: 10.1152/jn.1993.69.5.1779. [DOI] [PubMed] [Google Scholar]
- MacIver MB, Tanelian DL. Structural and functional specialization of Ad and C fiber free nerve endings innervating rabbit corneal epithelium. J Neurosci. 1993b;13:4511–4524. doi: 10.1523/JNEUROSCI.13-10-04511.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfurt CF, Del Toro DR. Corneal sensory pathway in the rat: A horseradish peroxidase tracing study. J Comp Neurol. 1987;261:450–459. doi: 10.1002/cne.902610309. [DOI] [PubMed] [Google Scholar]
- Meng ID, Bereiter DA. Differential distribution of Fos-like immunoreactivity in the spinal trigeminal nucleus after noxious and innocuous thermal and chemical stimulation of rat cornea. Neuroscience. 1996;72:243–254. doi: 10.1016/0306-4522(95)00541-2. [DOI] [PubMed] [Google Scholar]
- Meng ID, Hu JW, Bereiter DA. Parabrachial area and nucleus raphe magnus inhibition of corneal units in rostral and caudal portions of trigeminal subnucleus caudalis in the rat. Pain. 2000;87:241–251. doi: 10.1016/S0304-3959(00)00289-X. [DOI] [PubMed] [Google Scholar]
- Mitchell JL, Silverman MB, Aicher SA. Rat trigeminal lamina I neurons that project to thalamic or parabrachial nuclei contain the mu-opioid receptor. Neuroscience. 2004;128:571–582. doi: 10.1016/j.neuroscience.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Boccalini P, Moskowitz MA. Expression of c-fos-like immunoreactivity in brainstem after meningeal irritation by blood in the subarachnoid space. Neuroscience. 1992;49:669–680. doi: 10.1016/0306-4522(92)90235-t. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Hsu H, Gan Q. Distinct central representations for sensory fibers innervating either the conjunctiva or cornea of the rat. Exp Eye Res. 2010;90:388–396. doi: 10.1016/j.exer.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A, Kurose M, Winterson BJ, Meng ID. Menthol activation of corneal cool cells induces TRPM8-mediated lacrimation but not nociceptive responses in rodents. Invest Ophthalmol Vis Sci. 2012;53:7034–7042. doi: 10.1167/iovs.12-10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf. 2012;10:2–14. doi: 10.1016/j.jtos.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Saper CB. The spinoparabrachial pathway: shedding new light on an old path. J Comp Neurol. 1995;353:477–479. doi: 10.1002/cne.903530402. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Fujiyoshi Y, He YF, Xiao C, Ichikawa H. Trigeminal primary projection to the rat brain stem sensory trigeminal nuclear complex and surrounding structures revealed by anterograde transport of cholera toxin B subunit-conjugated and Bandeiraea simplicifolia isolectin B4-conjugated horseradish peroxidase. Neurosci Res. 1997;28:361–371. doi: 10.1016/s0168-0102(97)00064-3. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2003;17:13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Wright AM, Cestari DM, Pickel VM. Immunolabeling of retrogradely transported Fluoro-Gold: Sensitivity and application to ultrastructural analysis of transmitter-specific mesolimbic circuitry. J Neurosci Methods. 1994;55:65–78. doi: 10.1016/0165-0270(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Winkler CW, Hermes SM, Chavkin CI, Drake CT, Morrison SF, Aicher SA. Kappa opioid receptor (KOR) and GAD67 immunoreactivity are found in OFF and NEUTRAL cells in the rostral ventromedial medulla. J Neurophysiol. 2006;96:3465–3473. doi: 10.1152/jn.00676.2006. [DOI] [PubMed] [Google Scholar]


