Abstract
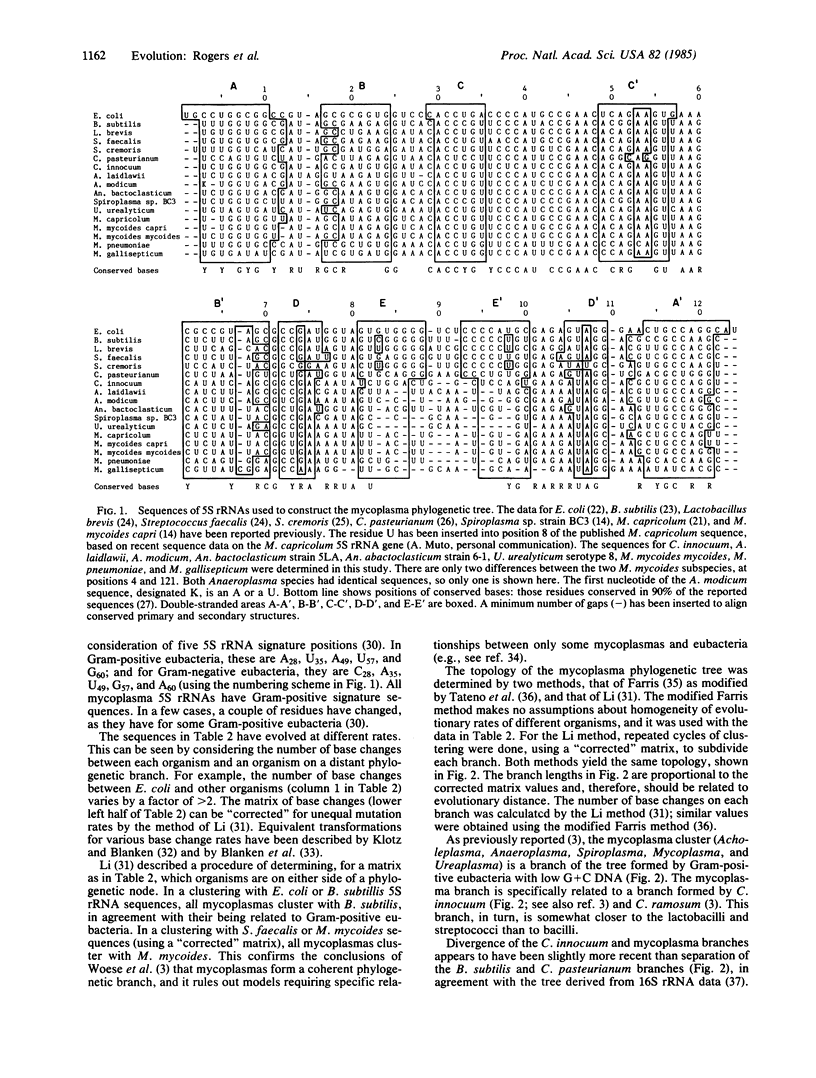
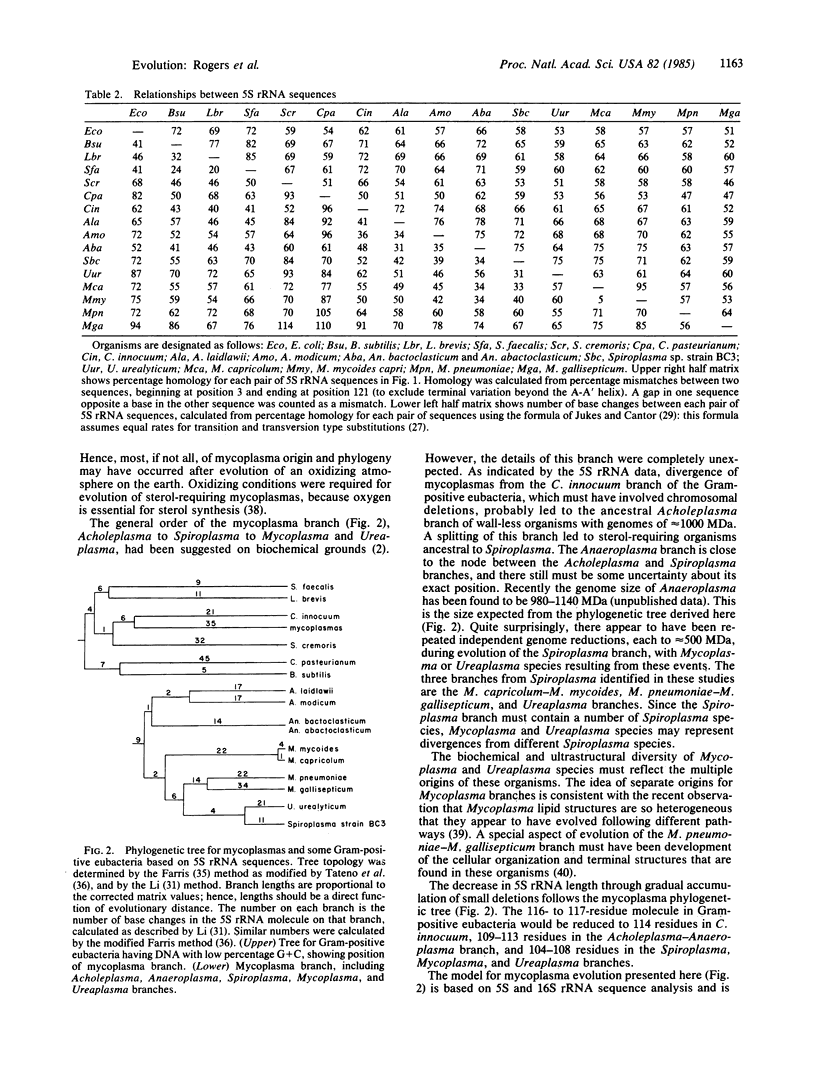
The 5S rRNA sequences of eubacteria and mycoplasmas have been analyzed and a phylogenetic tree constructed. We determined the sequences of 5S rRNA from Clostridium innocuum, Acholeplasma laidlawii, Acholeplasma modicum, Anaeroplasma bactoclasticum, Anaeroplasma abactoclasticum, Ureaplasma urealyticum, Mycoplasma mycoides mycoides, Mycoplasma pneumoniae, and Mycoplasma gallisepticum. Analysis of these and published sequences shows that mycoplasmas form a coherent phylogenetic group that, with C. innocuum, arose as a branch of the low G+C Gram-positive tree, near the lactobacilli and streptococci. The initial event in mycoplasma phylogeny was formation of the Acholeplasma branch; hence, loss of cell wall probably occurred at the time of genome reduction to approximately to 1000 MDa. A subsequent branch produced the Spiroplasma. This branch appears to have been the origin of sterol-requiring mycoplasmas. During development of the Spiroplasma branch there were several independent genome reductions, each to approximately 500 MDa, resulting in Mycoplasma and Ureaplasma species. Mycoplasmas, particularly species with the smallest genomes, have high mutation rates, suggesting that they are in a state of rapid evolution.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanken R. L., Klotz L. C., Hinnebusch A. G. Computer comparison of new and existing criteria for constructing evolutionary trees from sequence data. J Mol Evol. 1982;19(1):9–19. doi: 10.1007/BF02100219. [DOI] [PubMed] [Google Scholar]
- Bloch K. E. Speculations on the evolution of sterol structure and function. CRC Crit Rev Biochem. 1979 Nov;7(1):1–5. doi: 10.3109/10409237909102566. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F., Barrell B. G. The sequence of 5 s ribosomal ribonucleic acid. J Mol Biol. 1968 Jun 28;34(3):379–412. doi: 10.1016/0022-2836(68)90168-x. [DOI] [PubMed] [Google Scholar]
- Delihas N., Andersen J., Singhal R. P. Structure, function and evolution of 5-S ribosomal RNAs. Prog Nucleic Acid Res Mol Biol. 1984;31:161–190. doi: 10.1016/s0079-6603(08)60377-3. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H. Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res. 1980 Jul 25;8(14):3133–3142. doi: 10.1093/nar/8.14.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Das J., Maniloff J. Lack of repair of ultraviolet light damage in Mycoplasma gallisepticum. J Mol Biol. 1977 Oct 25;116(2):337–344. doi: 10.1016/0022-2836(77)90221-2. [DOI] [PubMed] [Google Scholar]
- Haberer K., Klotz G., Maniloff J., Kleinschmidt A. K. Structural and biological properties of mycoplasmavirus MVL3: an unusual virus-procaryote interaction. J Virol. 1979 Oct;32(1):268–275. doi: 10.1128/jvi.32.1.268-275.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Hori H. Evolution of 5sRNA. J Mol Evol. 1975 Dec 31;7(1):75–86. doi: 10.1007/BF01732181. [DOI] [PubMed] [Google Scholar]
- Hori H., Osawa S. Evolutionary change in 5S RNA secondary structure and a phylogenic tree of 54 5S RNA species. Proc Natl Acad Sci U S A. 1979 Jan;76(1):381–385. doi: 10.1073/pnas.76.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., Sawada M., Osawa S., Murao K., Ishikura H. The nucleotide sequence of 5S rRNA from Mycoplasma capricolum. Nucleic Acids Res. 1981 Oct 24;9(20):5407–5410. doi: 10.1093/nar/9.20.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Francis B. S. Taxonomy of the Clostridia: ribosomal ribonucleic acid homologies among the species. J Gen Microbiol. 1975 Jun;88(2):229–244. doi: 10.1099/00221287-88-2-229. [DOI] [PubMed] [Google Scholar]
- Kimura M. Estimation of evolutionary distances between homologous nucleotide sequences. Proc Natl Acad Sci U S A. 1981 Jan;78(1):454–458. doi: 10.1073/pnas.78.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L. C., Blanken R. L. A practical method for calculating evolutionary trees from sequence data. J Theor Biol. 1981 Jul 21;91(2):261–272. doi: 10.1016/0022-5193(81)90233-2. [DOI] [PubMed] [Google Scholar]
- Küntzel H., Heidrich M., Piechulla B. Phylogenetic tree derived from bacterial, cytosol and organelle 5S rRNA sequences. Nucleic Acids Res. 1981 Mar 25;9(6):1451–1461. doi: 10.1093/nar/9.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küntzel H., Piechulla B., Hahn U. Consensus structure and evolution of 5S rRNA. Nucleic Acids Res. 1983 Feb 11;11(3):893–900. doi: 10.1093/nar/11.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langworthy T. A. Lipid tracers of mycoplasma phylogeny. Yale J Biol Med. 1983 Sep-Dec;56(5-6):385–390. [PMC free article] [PubMed] [Google Scholar]
- Li W. H. Simple method for constructing phylogenetic trees from distance matrices. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1085–1089. doi: 10.1073/pnas.78.2.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniloff J. Cytoskeletal elements in mycoplasmas and other prokaryotes. Biosystems. 1981;14(3-4):305–312. doi: 10.1016/0303-2647(81)90037-x. [DOI] [PubMed] [Google Scholar]
- Maniloff J. Evolution of wall-less prokaryotes. Annu Rev Microbiol. 1983;37:477–499. doi: 10.1146/annurev.mi.37.100183.002401. [DOI] [PubMed] [Google Scholar]
- Marotta C. A., Varricchio F., Smith I., Weissman S. M. The primary structure of Bacillus subtilis and Bacillus stearothermophilus 5 S ribonucleic acids. J Biol Chem. 1976 May 25;251(10):3122–3127. [PubMed] [Google Scholar]
- Neimark H., Andersen J., Delihas N. Unusual structural features of the 5S ribosomal RNA from Streptococcus cremoris. Nucleic Acids Res. 1983 Nov 11;11(21):7569–7577. doi: 10.1093/nar/11.21.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neimark H., London J. Origins of the mycoplasmas: sterol-nonrequiring mycoplasmas evolved from streptococci. J Bacteriol. 1982 Jun;150(3):1259–1265. doi: 10.1128/jb.150.3.1259-1265.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribula C. D., Fox G. E., Woese C. R. Nucleotide sequence of Clostridium pasteurianum 5S rRNA. FEBS Lett. 1976 May 1;64(2):350–352. doi: 10.1016/0014-5793(76)80326-2. [DOI] [PubMed] [Google Scholar]
- Sankoff D., Cedergren R. J., McKay W. A strategy for sequence phylogeny research. Nucleic Acids Res. 1982 Jan 11;10(1):421–431. doi: 10.1093/nar/10.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Stahl D. A., Luehrsen K. R., Woese C. R., Pace N. R. An unusual 5S rRNA, from Sulfolobus acidocaldarius, and its implications for a general 5S rRNA structure. Nucleic Acids Res. 1981 Nov 25;9(22):6129–6137. doi: 10.1093/nar/9.22.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno Y., Nei M., Tajima F. Accuracy of estimated phylogenetic trees from molecular data. I. Distantly related species. J Mol Evol. 1982;18(6):387–404. doi: 10.1007/BF01840887. [DOI] [PubMed] [Google Scholar]
- Walker R. T., Chelton E. T., Kilpatrick M. W., Rogers M. J., Simmons J. The nucleotide sequence of the 5S rRNA from Spiroplasma species BC3 and Mycoplasma mycoides sp. capri PG3. Nucleic Acids Res. 1982 Oct 25;10(20):6363–6367. doi: 10.1093/nar/10.20.6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. Formylatable methionine transfer RNA from Mycoplasma: purification and comparison of partial nucleotide sequences with those of other prokaryotic initiator tRNAs. Nucleic Acids Res. 1975 Jan;2(1):61–78. doi: 10.1093/nar/2.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C., Morowitz H. J. Genome size and evolution. Chromosoma. 1973;40(2):121–126. doi: 10.1007/BF00321457. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Luehrsen K. R., Pribula C. D., Fox G. E. Sequence characterization of 5S ribosomal RNA from eight gram positive procaryotes. J Mol Evol. 1976 Aug 3;8(2):143–153. doi: 10.1007/BF01739100. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Maniloff J., Zablen L. B. Phylogenetic analysis of the mycoplasmas. Proc Natl Acad Sci U S A. 1980 Jan;77(1):494–498. doi: 10.1073/pnas.77.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]



