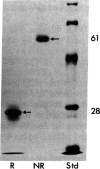
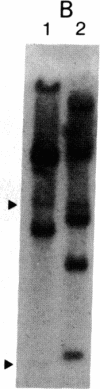
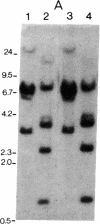
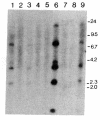
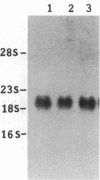
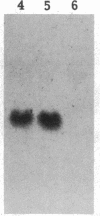
Abstract
The major forms of beta-hexosaminidase (2-acetamido-2-deoxy-beta-D-glucoside acetamidodeoxyglucohydrolase, EC 3.2.1.30) occur as multimers of alpha and beta chains--hexosaminidase A (alpha beta a beta b) and hexosaminidase B 2(beta a beta b). To facilitate the investigation of beta-chain biosynthesis and the nature of mutation in Sandhoff disease, a human hexosaminidase beta-chain cDNA clone was isolated. Hexosaminidase B (10 mg) was treated with CNBr, five peptide fragments were isolated by reverse-phase HPLC, and their amino acid sequences were determined. One of these contained a string of six amino acids from which an oligonucleotide probe was defined. The simian virus 40-transformed human fibroblast cDNA library of Okayama and Berg was screened by colony hybridization with the radiolabeled probe. Thirteen probe-binding clones were selected out of 50,000 clones screened. Four of these designated pHex were shown to be identical at their 3' ends by restriction enzyme mapping, differing only in their 5' extensions (1.4-1.7 kilobases). The nucleotide sequence of a 174-base-pair segment contained the deduced amino acid sequence of two of the five CNBr peptides, indicating that the pHex clones encode the beta subunit of hexosaminidase. In addition, pHex cDNA was found homologous to multiple bands in digests of genomic human DNA totaling 43 kilobases (kb), all of which were mapped to chromosome 5 in somatic cell hybrids, as expected of the HEXB gene. The pHex cDNA also hybridized to a 2.2-kilobase RNA that apparently codes for the pre-beta-polypeptide of hexosaminidase. This RNA species was absent in the fibroblasts of one of three patients with Sandhoff disease examined. We anticipate that these clones will be of value to diagnosis and carrier detection of Sandhoff disease in affected families.
Full text
PDF

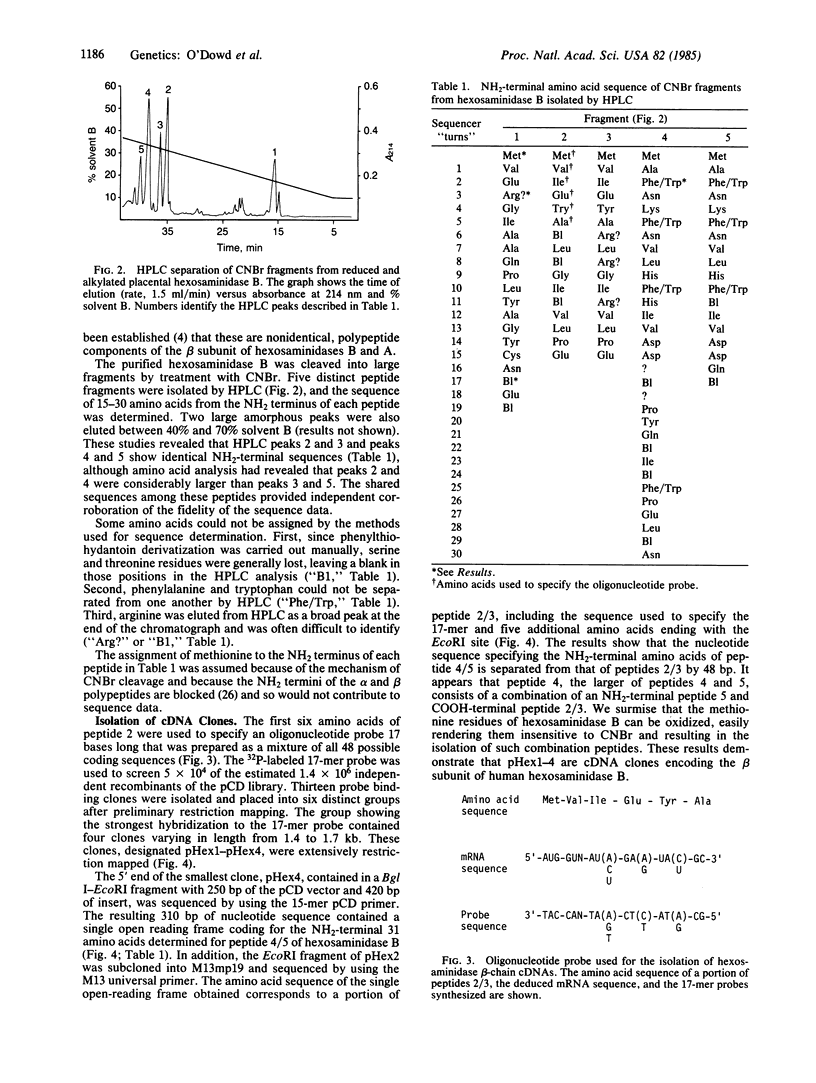
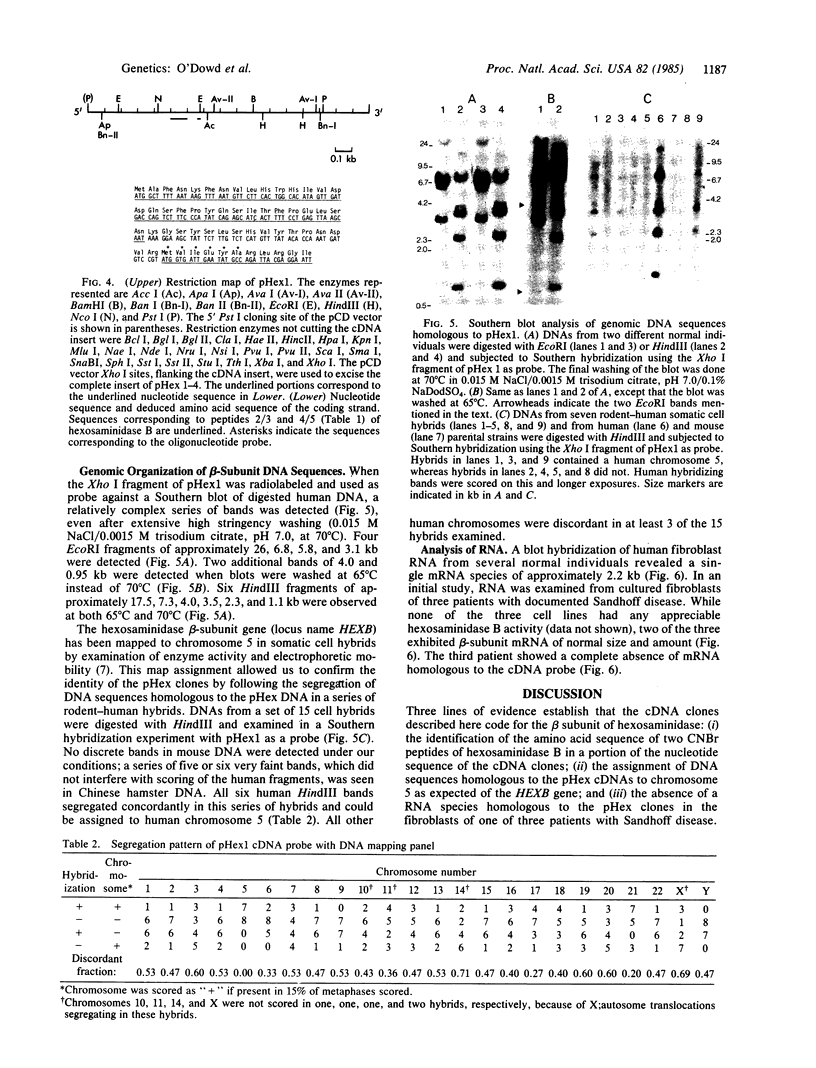

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Gilbert F., Kucherlapati R., Creagan R. P., Murnane M. J., Darlington G. J., Ruddle F. H. Tay-Sachs' and Sandhoff's diseases: the assignment of genes for hexosaminidase A and B to individual human chromosomes. Proc Natl Acad Sci U S A. 1975 Jan;72(1):263–267. doi: 10.1073/pnas.72.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Korneluk R. G., Quan F., Lewis W. H., Guise K. S., Willard H. F., Holmes M. T., Gravel R. A. Isolation of human fibroblast catalase cDNA clones. Sequence of clones derived from spliced and unspliced mRNA. J Biol Chem. 1984 Nov 25;259(22):13819–13823. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lalley P. A., Rattazzi M. C., Shows T. B. Human beta-D-N-acetylhexosaminidases A and B: expression and linkage relationships in somatic cell hybrids. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1569–1573. doi: 10.1073/pnas.71.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Mahuran D. J., Tsui F., Gravel R. A., Lowden J. A. Evidence for two dissimilar polypeptide chains in the beta 2 subunit of hexosaminidase. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1602–1605. doi: 10.1073/pnas.79.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahuran D., Lowden J. A. The subunit and polypeptide structure of hexosaminidases from human placenta. Can J Biochem. 1980 Apr;58(4):287–294. doi: 10.1139/o80-038. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattazzi M. C., Brown J. A., Davidson R. G., Shows T. B. Studies on complementation of beta hexosaminidase deficiency in human GM2 gangliosidosis. Am J Hum Genet. 1976 Mar;28(2):143–154. [PMC free article] [PubMed] [Google Scholar]
- Sandhoff K., Conzelmann E., Nehrkorn H. Specificity of human liver hexosaminidases A and B against glycosphingolipids GM2 and GA2. Purification of the enzymes by affinity chromatography employing specific elution. Hoppe Seylers Z Physiol Chem. 1977 Jul;358(7):779–787. doi: 10.1515/bchm2.1977.358.2.779. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Hexosaminidase-A and hexosaminidase-B: studies in Tay-Sachs' and Sandhoff's disease. Nature. 1973 Feb 16;241(5390):463–463. doi: 10.1038/241463a0. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Yoshida A., Awasthi Y. C., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. II. Kinetic and structural properties. J Biol Chem. 1974 Apr 10;249(7):2049–2053. [PubMed] [Google Scholar]
- Tarr G. E., Crabb J. W. Reverse-phase high-performance liquid chromatography of hydrophobic proteins and fragments thereof. Anal Biochem. 1983 May;131(1):99–107. doi: 10.1016/0003-2697(83)90140-9. [DOI] [PubMed] [Google Scholar]
- Tsui F., Mahuran D. J., Lowden J. A., Mosmann T., Gravel R. A. Characterization of polypeptides serologically and structurally related to hexosaminidase in cultured fibroblasts. J Clin Invest. 1983 Apr;71(4):965–973. doi: 10.1172/JCI110851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard H. F., Holmes M. T. A sensitive and dependable assay for distinguishing hamster and human X-linked steroid sulfatase activity in somatic cell hybrids. Hum Genet. 1984;66(2-3):272–275. doi: 10.1007/BF00286615. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Smith K. D., Sutherland J. Isolation and characterization of a major tandem repeat family from the human X chromosome. Nucleic Acids Res. 1983 Apr 11;11(7):2017–2033. doi: 10.1093/nar/11.7.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S. Juvenile Sandhoff Disease: complementation tests with Sandhoff and Tay-Sachs disease using polyethylene glycol-induced cell fusion. Hum Genet. 1978 Apr 24;41(3):325–329. doi: 10.1007/BF00284766. [DOI] [PubMed] [Google Scholar]