Abstract
The accessory beta subunit (Cavβ) of calcium channels first appear in the same genome as Cav1 L-type calcium channels in single-celled coanoflagellates. The complexity of this relationship expanded in vertebrates to include four different possible Cavβ subunits (β1, β2, β3, β4) which associate with four Cav1 channel isoforms (Cav1.1 to Cav1.4) and three Cav2 channel isoforms (Cav2.1 to Cav2.3). Here we assess the fundamentally-shared features of the Cavβ subunit in an invertebrate model (pond snail Lymnaea stagnalis) that bears only three homologous genes: (LCav1, LCav2, and LCavβ). Invertebrate Cavβ subunits (in flatworms, snails, squid and honeybees) slow the inactivation kinetics of Cav2 channels, and they do so with variable N-termini and lacking the canonical palmitoylation residues of the vertebrate β2a subunit. Alternative splicing of exon 7 of the HOOK domain is a primary determinant of a slow inactivation kinetics imparted by the invertebrate LCavβ subunit. LCavβ will also slow the inactivation kinetics of LCav3 T-type channels, but this is likely not physiologically relevant in vivo. Variable N-termini have little influence on the voltage-dependent inactivation kinetics of differing invertebrate Cavβ subunits, but the expression pattern of N-terminal splice isoforms appears to be highly tissue specific. Molluscan LCavβ subunits have an N-terminal “A” isoform (coded by exons: 1a and 1b) that structurally resembles the muscle specific variant of vertebrate β1a subunit, and has a broad mRNA expression profile in brain, heart, muscle and glands. A more variable “B” N-terminus (exon 2) in the exon position of mammalian β3 and has a more brain-centric mRNA expression pattern. Lastly, we suggest that the facilitation of closed-state inactivation (e.g. observed in Cav2.2 and Cavβ3 subunit combinations) is a specialization in vertebrates, because neither snail subunit (LCav2 nor LCavβ) appears to be compatible with this observed property.
Introduction
Unique ancillary beta subunits are identifiable in the proteosomal complex with different voltage-gated ion channels including K [1]–[3], Na+ [4]–[6] and Ca2+ [7]–[9] channels. These accessory subunits are known to promote the membrane expression and trafficking of ion channel complexes, as well as to modify the biophysical features of voltage-gated ion channels [7]–[9]. Sodium channels (Nav1.x) and calcium channels (Cav1.x, Cav2.x, Cav3.x) bear a common structural template of four repeat domains of six transmembrane helices each, but they have distinct ancillary subunits which are known to regulate them [7]–[9]. Sodium channel beta subunits (Navβ) evolved separately in different animal groups such as insects, snails and vertebrates. TipE/Teh are insect Navβ subunits and have a likeness to (Slo/BK) Kvβ subunits with EGF-like domains [4], while gastropod snails Navβ subunits is a CUB domain containing protein family [10], while vertebrate Navβ subunits are CaM-like with a V-set Ig extracellular loop [5]. Calcium channel beta subunits (Cavβ) have common homologs in animal groups rooted in genomes of single cell organisms like coanoflagellates, which also possess an L-type calcium channel (Cav1) homolog [11]. From a likely primordial template of one Cav1 and Cavβ subunit in coanoflagellates [12], emerged the complexity in numbers in vertebrates, where there are four different Cavβ subunits, (β1, β2, β3, β4), which are expected to regulate seven pore-forming α1 subunits from the two high voltage-activated classes of calcium channels, Cav1.1–Cav1.4 (L-type) and Cav2.1–Cav2.3 (non-L-type) [13]. There are also low voltage-activated calcium channels, Cav3.1–Cav3.3 (T-type), but there is not strong evidence for their regulation by Cavβ subunits [7].
The pond snail Lymnaea stagnalis, possess a simple set of calcium channels: a single L-type channel gene (LCav1) [14], [15], a single, synaptic, non-L-type channel (LCav2) [16], [17], a T-type channel (LCav3) [18],[19] and a single beta subunit, LCavβ [20]. We use this simple model to address what are the core conserved features for β subunit regulation of calcium channels, and to determine what features are adaptive and unique to vertebrates.
We examine the common alternative splicing patterns in the N-terminus and HOOK domains between snail LCavβ and vertebrates Cavβ subunits. mRNA expression suggests a tissue specificity to the expression of N-terminal isoforms, with LCavβA (eg. structurally resembling vertebrate β1 isoform in sequence) possessing a more generalized expression, while the LCavβB (in exon position of the shorter vertebrate β3 isoforms) has a more discrete, brain specific expression pattern. Molluscs possess a common site of alternative splicing in the HOOK domain, which generates a variably sized exon 7 by use of alternative acceptor site, and is in the same location as mutually-exclusive spliced isoforms (exon 7a,7b, or 7c) [8], [9] in vertebrate Cavβ subunits. Cavβ subunits in invertebrates (eg. schistosomes [21], snails [20], squid [22] and honeybees [23]) commonly slow the inactivation kinetics of Cav2 channels in a manner like vertebrate β2a subunit [24]–[26], but without the canonical N-terminal palmitoylation site that primarily influences its slow inactivation. We show that the HOOK domain (- isoform) more than the N-terminus (A isoform) of snail LCavβ contributes to the slowing of inactivation kinetics of Cav2 channels. The snail Cavβ subunits can also slow the inactivation of snail LCav3 T-type channels, but this may be an observable phenomenon in vitro, because there is little evidence for Cavβ subunits associating with T-type channels in native cells [7]. And lastly, we examine that the capacity that Cavβ subunits to promote an increase in the inactivation rate of α subunits while the channels are largely in the closed state [27], [28]. This property known as “closed-state inactivation” is not observable for the snail LCavβ or synaptic LCav2 channel, which suggests that this property is more a specialization for specific vertebrate subunit combinations such as Cavβ3 and Cav2.2 [27], [28].
Results and Discussion
1. Conserved SH3-GK core of Cavβ subunits
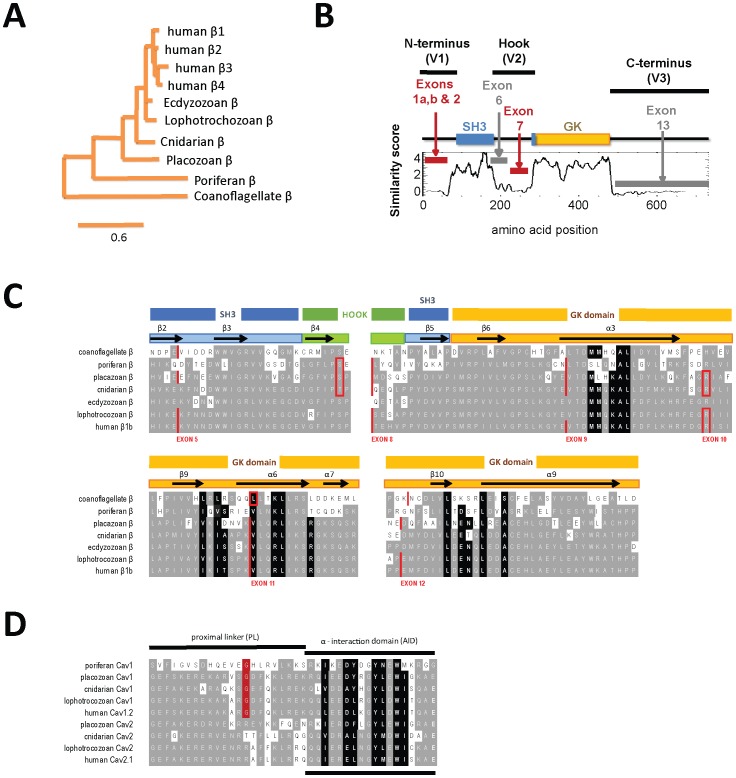
β subunits of calcium channels (Cavβ) contain four major representatives in vertebrates (β1 to β4), represented by a single gene in invertebrates ( Figure 1A ). Cavβ subunits are unique members within the MAGUK (membrane associated guanylate kinase) family of proteins [11], [29], [30], which share a structural core consisting of an SH3 domain and a guanylate kinase (GK) domain, separate by a variable HOOK region ( Figure. 1B, 1C ). MAGUKs, are noted for their role as scaffolding and cytoskeletal-organizing proteins, such as post-synaptic density protein (PSD-95), where the SH3 and GK domains form potential intramolecular or intermolecular interactions, with the same or differing MAGUKs, with neither the SH3 nor GK domains have active sites for standard polyproline interactions or nucleotide kinase activity respectively [11], [29], [30]. β subunits of calcium channels lack PDZ domains of standard MAGUKs, but does have a HOOK domain which splits the SH3 domain [31]–[33] ( Figure 1B, 1C ).
Figure 1. Conservation of calcium channel beta (Cavβ) subunits from single-celled organisms to humans.
(A) Gene tree derived from aligned sequences illustrating the relationship of the four vertebrate Cavβ subunits (β1, β2, β3, β4) with the single Cavβ representatives in non-vertebrates, including single-celled organisms. (B) Running average of similarity (window = 50 amino acids) of aligned invertebrate and human Cavβ subunits sequences (in Figure S1). Cavβ subunits have highly conserved SH3 and guanylate kinase (GK) domains, with variability in the N-terminus (V1), HOOK domain (V2) and C-terminus (V3). Alternative splicing shared in Cavβ subunits in Exons 1a/1b and exon 2 (N-terminus), and exon 7 (HOOK domain). (C) Multiple alignment of Cavβ subunits from single cell organisms to humans illustrating highly conserved SH3 and GK domains, conserved secondary structures (α helices and Cavβ sheets), and calcium channel (AID) binding residues (blackened residues) reported in crystal structures of Cavβ subunits [22]–[24]. Exon boundaries are indicated in red. (D) Alignment of cytoplasmic region post trans-membrane segment 6 in Domain I of Cav1 and Cav2 calcium channels which forms an expected α helix. Regions include a proximal linker (PL) and the Alpha1-Interaction Domain (AID) associated with binding Cavβ subunits (blackened residues).
The alpha helix at the end of hydrophobic segment 6 in Domain I of Cav1 and Cav2 α1 subunits, is largely expected to extend to an alpha1-interaction domain (AID) of conserved amino acids (blackened amino acids, Figure 1D ), which in a crystal structure embeds into a deep hydrophilic groove of the Cavβ subunit formed by the guanylate kinase domain [31]–[33] (blackened amino acids, Figure 1C ). The SH3 and GK domains of Cavβ subunits ( Figure 1B ), and most notably the key-in-keyhole interaction residues, between the AID sequences of calcium channel α1 subunits and a conserved binding pocket formed by the Cavβ subunit GK domain, respectively, are largely conserved down to the simplest known organisms that have calcium channels, the unicellular eukaryotes choanoflagellates ( Figure 1C, 1D ). The most basal unicellular organisms (protozoan) or multicellular animals (sponge, poriferans) have a single Cav1 L-type calcium channel homolog ( Figure 1A ). These Cav1 homologs, in the simplest organisms, are structural predecessors to the high voltage-activated, dihydropyridine-sensitive LCav1 homolog expressed in the pond snail, Lymnaea stagnalis [14], [15]. A second and third class of calcium channels likely derived from L-type calcium channels (in ancestral relatives of placozoans, cnidarians) include the non-L-type, Cav2 channel class, noted for their unique roles in mediating synaptic transmission [16], [17], and the low voltage-activated T-type, Cav3 channel class [18], [19]. All Cav1 and Cav2 channels from simple representatives (placozoan, cnidarians) have a hallmark AID sequence [34] for associating with Cavβ subunits, which enables a promiscuity and interchangeability of Cavβ subunits interacting with differing Cav1 and Cav2 α1 subunit classes.
2. Variable regions are associated with common alternative splicing
Variable regions outside of the conserved SH3 and GK domains of Cavβ subunits provide specificity and unique modulation of of different Cav1 and Cav2 channel types [8], [9], [13]. Cavβ subunits dramatically vary in the N-terminus (V1), HOOK region (V2) and C-terminus (V3) which is illustrated in a running similarity score ( Figure 1B ) of aligned sequences of invertebrate and mammalian Cavβ subunits (Figure S1). Alternative splicing of Cavβ subunits coincides with these variable regions, illustrated in the exon-intron structure of snail LCavβ and the four human Cavβ isoforms (shown in Figure 2A ).
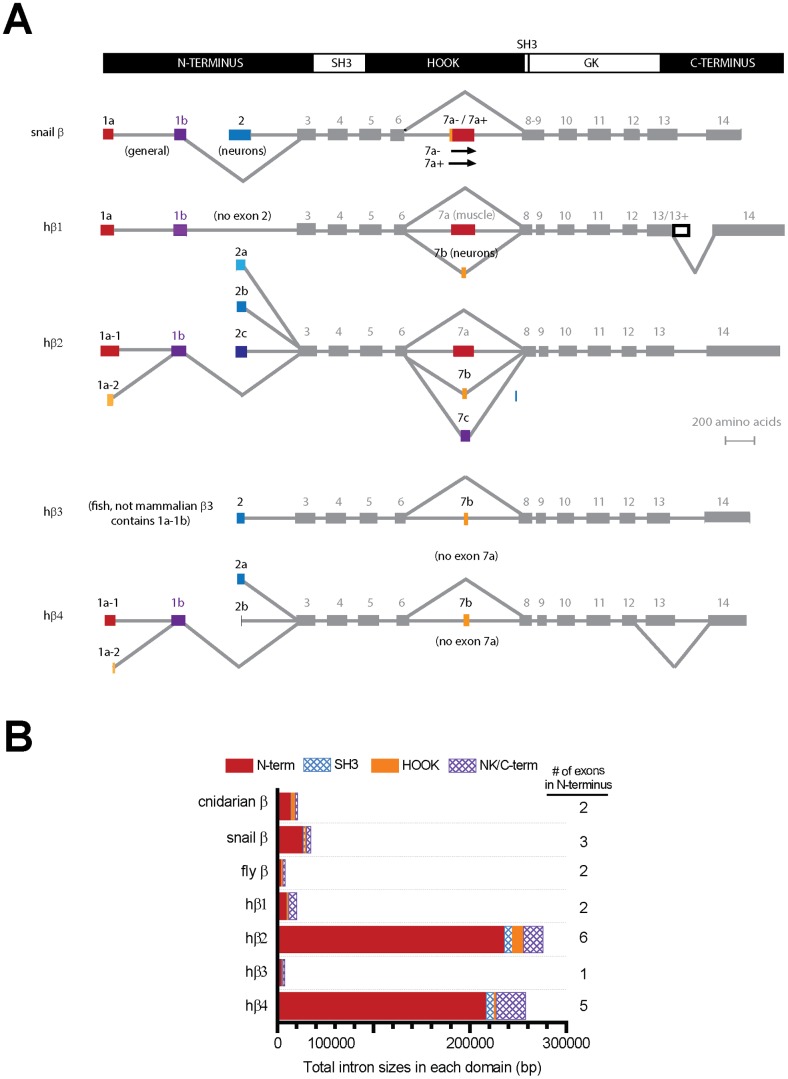
Figure 2. Conserved exon-intron organization, alternative splicing, and N-terminal intron sizes in the genomic sequence spanning calcium channel beta (Cavβ) subunits.
(A) Alignment of the 15 exons of Cavβ subunits comparing snail and human Cavβ subunit splicing. Cavβ subunits have mutually exclusive splicing of N-terminal exon 1a/1b or exon 2 isoforms. Exon 7 in the HOOK domain is subject to mutually-exclusive exon splicing (exon 7a or exon 7b or exon 7c) in vertebrates or splicing in mollusks generated by alternative acceptor sites (exon 7a+, exon 7a−). Molluscan and vertebrate have truncated forms of Cavβ subunits lacking the GK domain and C-terminus as a result of skipping of exon 7. (B) Most of the intron sizes of Cavβ subunits span the N-terminal exons, and the size of the total intron sequence in the N-terminus increases with the number of exons in the N-termini.
The major pattern of alternative splicing in the four vertebrate Cavβ subunits is represented in the molluscan LCavβ subunit (as shown in Figure 2A ), suggesting that the gene splicing patterns evolved in the common ancestor of mollusks and vertebrates, before the duplication that lead to the generation of the our vertebrate isoforms.
3. Conservation of N-terminal mutually-exclusive splicing in Cavβ subunits (exons 1a/1b and exon 2) and their consistent large intron sizes
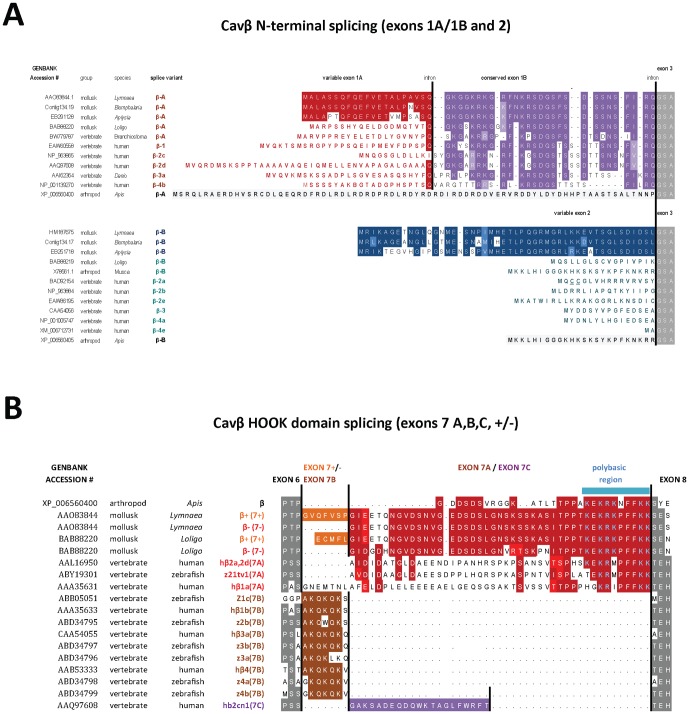
The two most upstream Cavβ subunit exons form one N-terminal splice variant, dubbed variant CavβA ( Figure 3A ), consisting of exon 1a with highly variable sequence, jointed to exon 2b which contains a common conserved amino acid clusters (KxSDSG…FIRQ) shared between molluscan CavβA and vertebrate spliced isoforms β1, β2c, β2d, β4b. The mutually exclusive N-terminal splice variant CavβB is formed by exon 2 ( Figure 3B ) and is downstream of exon 1a and exon 1b in the genomic structure of Cavβ subunits ( Figure 2A ). CavβB generates short N-terminal vertebrate isoforms β2b, β2e, β3, β4a including the canonical β2a which has doublet cysteines that are palmitoylated and confer slow inactivation to vertebrate Cav1 and Cav2 calcium channels [24]–[26], and to molluscan LCav2 channels [20]. Honeybee, as a representative insect and arthropod, has two N-terminal spliced isoforms [23], but does not possess a conserved exon 1B with the (KxSDGS…FIRQ) cluster found in molluscan [22] or vertebrate [8], [9] CavβB isoforms ( Figure 3 ).
Figure 3. Multiple sequence alignments illustrate conserved splicing in (A) the N-terminus and (B) HOOK domain of invertebrates and vertebrate Cavβ subunits.
Alternate N-terminal isoforms composed of exons 1a–1b (CavβA) or exon 2 (CavβB), and HOOK domain splicing includes optional short addendum to exon 7 (Cavβ−/Cavβ+) in invertebrates or mutually exclusive splicing, exon 7a, 7b, 7c. Note that Cavβ from snails, squid, schistosomes and bees and vertebrate Cavβ2a have slow inactivation kinetics and (B) possess HOOK domains with a long form of exon 7 (A form) with a common polybasic region at its 3′ end.
A general feature of N-terminal exons 1a, 1b and 2 is that the intervening non-coding sequences (introns) that span the region are so large that they compose the majority of the genomic region spanning most Cavβ subunits even in a simple cnidarian species, Nematostella ( Figure 2B ). The intron sizes are largest in vertebrates isoforms (such as fish and mammalian subunits [35], [36]) which also have the largest number of exon variants that code for exons 1a, and exon 2 in the N-terminus, which are 6 and 4 exons respectively, in vertebrate Cavβ2 and Cavβ4 subunits ( Figure 2A, 2B ). The genomic region spanning the N-termini for hCavβ4 and hCavβ2.is 217,000 bp and 236,000 bp. Gargantuan introns such as in the N-termini of hCavβ4 and hCavβ2 are highly unique, and represent ∼0.1% of all introns in the human genome [37]. The evolutionarily conserved, larger sized introns are expected to contain DNA elements which regulate the timing and tissue specificity of the expression of Cavβ subunit isoforms.
4. mRNA expression confirms a tissue specific expression pattern for N-terminal A and B isoforms
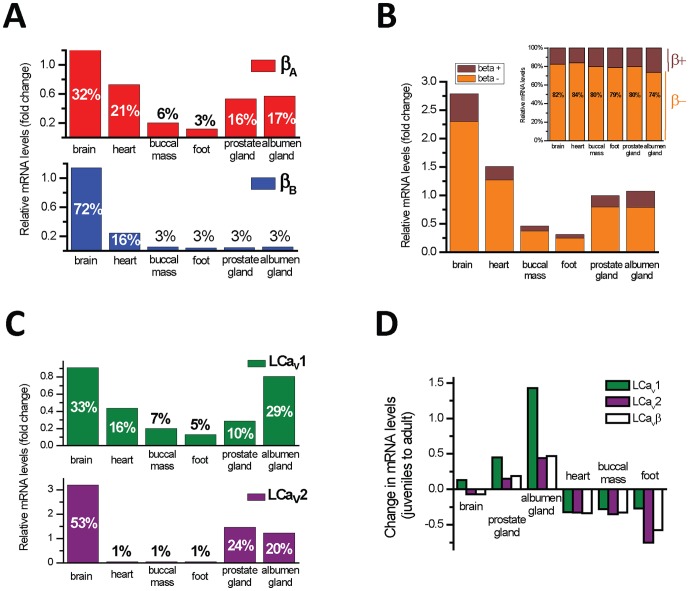
mRNA expression patterns suggest that the N-terminus of LCavβ, specifically, exons 1a/1b (dubbed “LCavβA”) and exon 2 (LCavβB”) are highly regulated in their tissue expression ( Figure 4A ), while the HOOK domain splice variants are not ( Figure 4B ). LCavβA is a widely expressed isoform in most tissues ( Figure 4A , top), and resembles the expression profile of the HOOK domain splice isoforms which lack a tissue specific distribution ( Figure 4B ). In contrast, LCavβB is more discretely expressed than LCavβA, as a mostly brain specific isoform (72%), with residual expression levels in the heart (16%) ( Figure 4A , bottom). Comparing the relative mRNA levels of the Cavβ ( Figure 4A ) with the corresponding mRNA expression of LCav1 and LCav2 channels in different snail tissues ( Figure 4C ) resembles similarities in expression patterns to mammalian gene spliced isoforms [8], [9]. LCavβA ( Figure 4A ) is the primary Cavβ subunit isoform expressed in the equivalent tissue resembling skeletal muscle in snails (buccal mass and foot), where LCav1 channel expression dominates ( Figure 4C ). In contrast, LCavβB and LCav2 channel are almost undetectable in the equivalent tissue of skeletal muscle in snails ( Figure 4A , Figure 4C ). The combination of mostly LCav1 and LCavβA in snail muscle is consistent with the exclusive pairing of Cav1.1 and Cavβ1a in vertebrate skeletal muscle [38]–[40], where vertebrate Cavβ1 bears only N-terminal exons 1a/1b like snail LCavβA ( Figure 2A ). It is also noteworthy that mammalian β3 lacks N-terminal exons 1a/1b and is composed of exon 2 only ( Figure 2A ) like the snail LCavβB isoform. Mammalian Cavβ3 is mostly a brain specific subunit that pairs more often with synaptic Cav2.2 channels [41], , and in this regard, resembles the splice variant of snail LCavβB which is also is more brain specific variant ( Figure 4A ) and composed of exon 2. These are examples of likely common pairings of calcium channel and beta subunit isoform between invertebrates and vertebrates, but outside of this, there appears to be a lot of promiscuity and overlap between Cav1 and Cav2 channels and their association with the differing Cavβ subunit isoforms. It is noteworthy that “A” isoforms are found in examples of all four vertebrate Cavβ subunits, and only Cavβ3 is lacking a “B” isoform in vertebrates ( Figure 4C ).
Figure 4. Quantitative RT-PCR results show that N-terminal splice isoforms (LCavβA and LCavβB), but not HOOK domain splice isoforms (LCavβ− and LCavβ+) of snail Cavβ subunits have tissue specific mRNA expression patterns.
mRNA levels are illustrated as fold change relative to HPRT mRNA levels. (A) More generalized pattern of splicing of LCavβA containing exons 1a/1b, than LCavβB containing exon 2. Exon 2 containing isoform is mostly expressed in the brain, and residual levels in the heart. (B) Exon 7 splicing generates seven extra amino acids (exon 7a− vs exon 7a+) and appears to have no tissue selectivity pattern of expression. (B, inset) Percent of LCavβ− vs LCavβ+, illustrating that 74%–84% of all transcripts lack the extra amino acids in exon 7. (C) LCav1 L-type channel has a more generalized mRNA expression pattern as LCavβA while more nervous system specific LCav2 has a more discrete expression pattern as LCavβB isoforms. (D) Rises and falls in the relative fold changes in mRNA levels from juvenile to adult animals are correlated between LCavβ, LCav1 and LCav2 channel subunits.
Changes in mRNA levels of Cavβ subunits from juvenile to adult snails, match the direction of change of the corresponding mRNA of calcium channels ( Figure 4D ). Heart and muscle mRNA levels fall from juvenile to adult animals for LCav1 and LCav2 channels and LCavβ subunits, while there is a rise in LCav1 and LCav2 channels and LCavβ subunits corresponding with the maturation of sexual organs from juvenile to adult animals, such as in prostate and albumen gland ( Figure 4D ).
5. Common HOOK domain splicing of exon 7 in molluscan and vertebrate Cavβ subunits
The HOOK domain is a second area of variability in Cavβ subunits and subject to alternative splicing. The HOOK domain is unstructured and not resolvable in the crystal structure of Cavβ subunits [31]–[33]. The HOOK domain splits the SH3 domain, separating the 5th Cavβ strand from the rest of the SH3 domain ( Figure 1B , Figure 1C and Figure S1). The variability in the HOOK domain is largely contained in exons 6 and 7, where exon 7 is subject to alternative splicing that is shared in molluscan and mammalian Cavβ subunits ( Figure 2A ).
Mutually-exclusive splicing of exon 7 in mammals leads to either a skipping of exon 7 altogether, or includes a choice of one of three exon 7 variants including, exon 7a (a long form: e.g. AIDID…PFFKK), exon 7b (a short form: AKQKQKQ) and more rarely exon 7c ( Figure 3B ) [8], [9]. Snails (and also squid [22]) can also exclude exon 7 altogether, via use of alternative acceptor sites ( Figure 2A ) or generating exon 7 containing seven or five extra amino acids (exon 7+), respectively, or versions lacking these extra amino acids (exon 7−) ( Figure 3B , Figure S2). Invertebrate exon 7 resembles the exon 7a (long form) of vertebrate Cavβ subunits, with a conserved polybasic region at its 3′ end (KRxPFFKK) ( Figure 3B ) [43]. The skipping of exon 7 is found in mammalian Cavβ subunits [44]–[46] and shared in molluscan Cavβ subunits and generates a frame shift and an immediately truncated Cavβ subunit that lacks the GK domain and C-terminus ( Figure 2A ). The frame shift occurs because exon 6 ends within a codon after the first nucleotide (phase 1), whereas the intron preceding exon 8 is located between codons (phase 0) (Figure S2). One role discovered for the truncated Cavβ subunit in vertebrates is that it transports into the nucleus and serve as a transcription factor [47]–[50]. Conservation of splicing that skips exon 7 suggests that truncated Cavβ subunits in invertebrates may also have similar roles outside of their association with Cav1 and Cav2 calcium channels.
6. HOOK domain spliced variants lack a tissue specific expression pattern
Snail LCavβ+ or LCavβ− containing or not containing the extension to exon 7, respectively, show no obvious tissue preference in their mRNA expression. Notably LCavβ− (lacking the extension to exon 7) is more common, being 74%–84% of the total mRNA transcript in all snail tissues ( Figure 4B ). The lack of tissue regulation for splicing in the HOOK domain contrasts with the more highly regulated N-terminal alternatively-spliced exons. There is also consistently smaller and more normal sizes of introns spanning exon 7, which contrasts with the very large to gargantuan introns spanning the N-terminal exons ( Figure 2B ).
7. Invertebrate Cavβ subunits slow the inactivation kinetics of Cav2 channels
We have co-expressed full-length isoforms of LCav1, LCav2 and LCav3 channels with mammalian α2δ1, and the four combinations of LCavβ isoforms in HEK-293T cells to assess the consequences of LCavβ splice isoforms on the expression of the calcium channels recorded using whole-cell patch clamp electrophysiology. Combinations that we assessed were LCavβ with exon1a/1b (A form) or exon 2 (B form), and LCavβ channels with or without the optional seven amino acids in exon 7 (i.e. + form or − form, respectively): (LCavβA+, LCavβB+, LCavβA−, LCavβB−) compared to the absence of co-expressed Cavβ subunit. We substituted native external calcium ions for barium ions to evaluate the consequences of calcium-independent effects on biophysical properties recorded in whole cell voltage clamp, avoiding the dramatic calcium dependent inactivation, characteristic of LCav1 channels, observed when external calcium in the charge carrier. Reported effects of Cavβ subunits have focused on the dramatic changes to the voltage-dependent properties [7]–[9], so we have limited our study to an examination of barium currents. All electrophysiology results +/− s.e.m. are tabulated in Table 1 .
Table 1. Summary of electrophysiology parameters for Figures 5–8.
| Figure 5 and 7. Voltage-sensitivities of LCav1, LCav2, LCav3 channels with and without LCavβ splice isoforms | ||||||||||||
| Activation V0.5 (mV) | p value | Activation Ka | n | p value | Inactivation V0.5 (mV) | n | p value | Inactivation Ki | n | p value | ||
| LCav1 & no β | 3.27±4.27 | 8 | 7.83±1.50 | 8 | −20.29±4.56 | 3 | 8.30±1.72 | 3 | ||||
| LCav1 & LCavβA+ | 2.07±1.05 | 7 | n.s. | 7.09±1.21 | 7 | n.s. | −16.47±4.27 | 3 | n.s. | 7.56±1.50 | 3 | n.s. |
| LCav1 & LCavβB+ | 2.47±1.03 | 24 | n.s. | 6.77±0.76 | 24 | n.s. | −21.33±2.18 | 5 | n.s. | 8.57±1.69 | 5 | n.s. |
| LCav1 & LCavβB− | 3.55±1.57 | 5 | n.s. | 6.96±1.74 | 5 | n.s. | −18.09±3.72 | 3 | n.s. | 8.25±1.45 | 3 | n.s. |
| LCav2 & no β | 12.11±2.20 | 5 | 6.43±1.91 | 5 | −31.28±6.76 | 4 | 10.90±1.21 | 4 | ||||
| LCav2 & LCavβA+ | 12.30±0.96 | 10 | n.s. | 6.74±0.27 | 10 | n.s. | −29.18±2.32 | 7 | n.s. | 11.02±1.63 | 7 | n.s. |
| LCav2 & LCavβB+ | 12.07±1.21 | 8 | n.s. | 6.49±1.74 | 8 | n.s. | −33.02±3.20 | 4 | n.s. | 9.15±2.01 | 4 | n.s. |
| LCav2 & LCavβB− | 9.95±1.07 | 5 | * | 6.13±0.30 | 5 | n.s. | −32.37±2.57 | 4 | n.s. | 9.26±1.21 | 4 | n.s. |
| LCav3 & no β | −67.65±1.20 | 9 | 4.68±0.20 | 9 | −84.12±0.38 | 3 | 3.16±0.15 | 3 | ||||
| LCav3 & LCavβA+ | −68.71±2.69 | 10 | n.s. | 4.64±0.61 | 10 | n.s. | −84.82±1.19 | 3 | n.s. | 3.28±0.24 | 3 | n.s. |
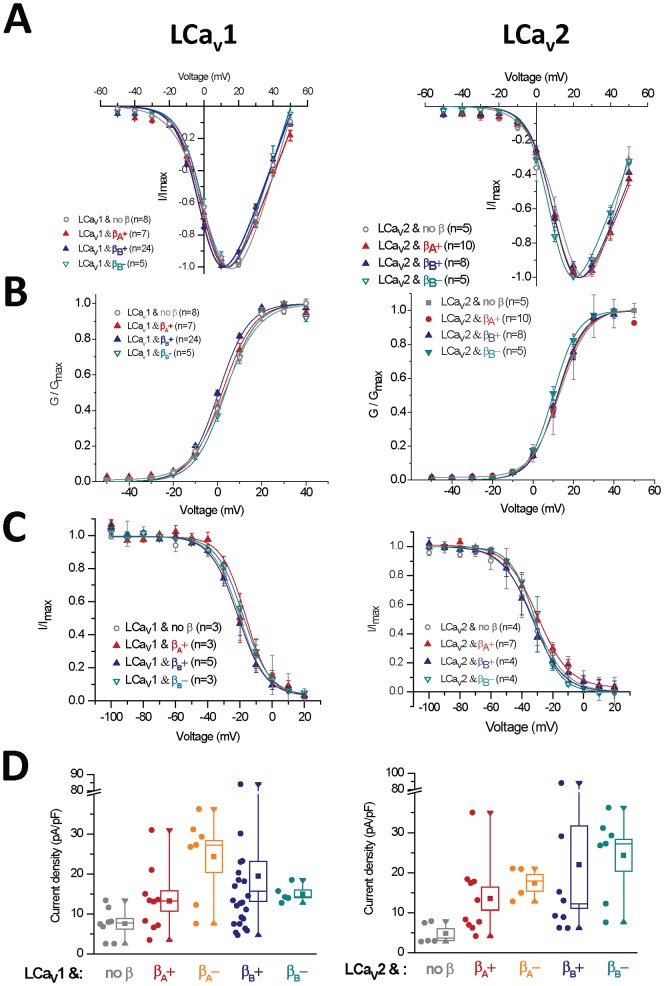
We found that none of the snail LCavβ isoforms influenced the current voltage-relationships such as the threshold voltage at which barium currents are beginning to be visible or the voltage of peak current for LCav1 or LCav2 channels ( Figure 5A ). This absence of a difference is reflected in the activation curves fitted with a Boltzmann equation ( Figure 5B ). Similarly there were also no effects of LCavβ subunits on steady-state inactivation ( Figure 5C ). This is different from the dramatic effects of mammalian Cavβ subunits on mammalian Cav1 and Cav2 channels [7]–[9]. Mammalian Cavβ subunits impart large shifts in the voltage-dependence of activation to more hyperpolarized voltages by approximately −10 mV to −20 mV [7], [8]. Mammalian Cavβ subunits, except for Cavβ2a also cause an approximately 10 mV hyperpolarizing shift in steady-state inactivation curves, and accelerate inactivation kinetics to varying degrees [7]–[9]. Cavβ2a has doublet cysteines that are palmitoylated in an exon 2 type N-terminus, which promote membrane association of Cavβ subunits [24]–[26]. Palmitoylation is considered to anchor and immobilize Cavβ2a, causing calcium channels to be more reluctant to inactivate (shifting inactivation curves in the depolarizing direction), and is responsible for much of the slowing of its inactivation kinetics [24]–[26]. Snail LCavβ resembles Cavβ2a in promoting the slowing of inactivation kinetics of LCav2 channels ( Figure 6B, 6C ), albeit to a lesser degree than the slowing of inactivation kinetics of LCav2 induced by Cavβ2a (see figure 3e in [20]). But notably, the slowing of inactivation kinetics is a consistent feature in other invertebrate Cavβ subunits (such as schistosome [21], squid [22] and honeybee [23] Cavβ subunits), and these subunits, including snail, lack N-terminal palymitoylation residues in the N-termini of their Cavβ subunits.
Figure 5. Snail beta subunit (LCavβ) splice isoforms containing either N-terminal exons 1a/1b or exon 2 (A or B) or insert in exon 7 (7a− vs 7a+), boost the membrane expression of snail LCav1 or LCav2 channels but has no effect on their voltage-sensitivities when external barium is the charge carrier.
(A) Current-voltage relationships (curve-fitted with Ohmic-Boltzmann equation) (B) Activation and (C) Steady-state inactivation curves (curve-fitted with Boltzmann equation). (D) Current densities (pA/pF) shown as box plot (box = s.e.m., whisker range = minimum/maximum values).
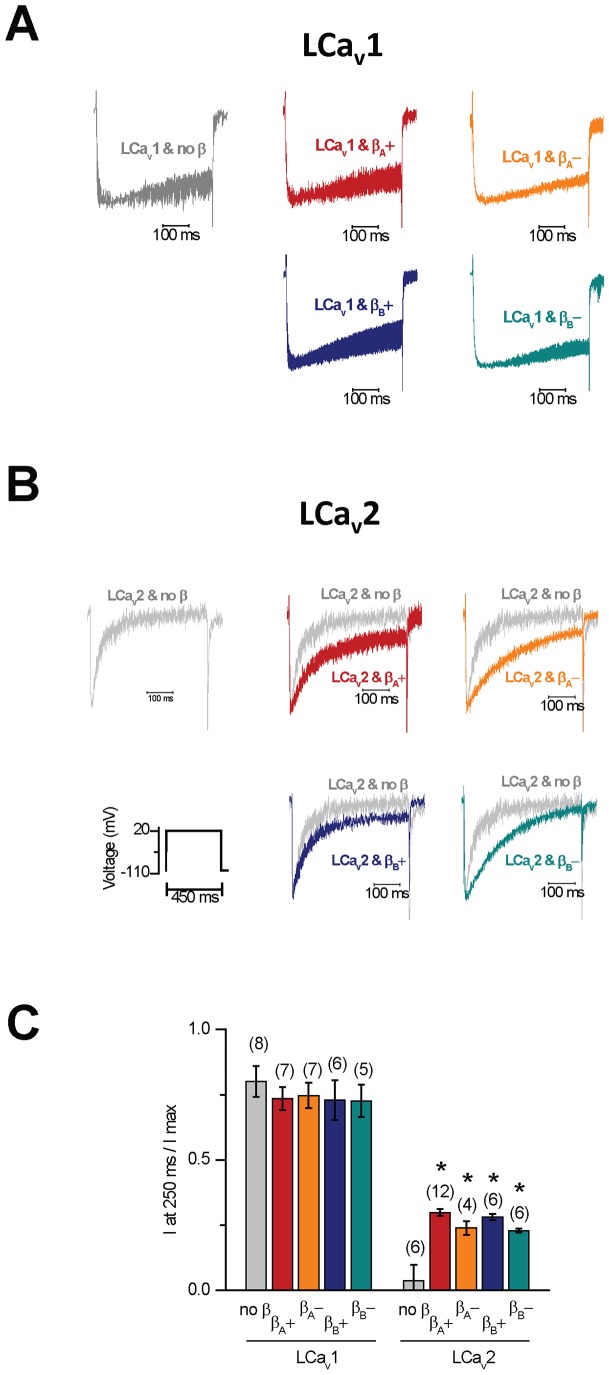
Figure 6. Snail beta subunit (LCavβ) splice isoforms containing either N-terminal exons 1a/1b or exon 2 (A or B) or insert in exon 7 (7a− vs 7a+), slow the inactivation kinetics of LCav2 channels.
Four representative and normalized peak barium current traces shown as mean, s.e.m. for (A) LCav1 and (B) LCav2 channels co-expressed with LCavβ splice isoforms or no Cavβ. (C) Rate of inactivation decay reflected in the fraction of maximal peak current at 250 ms time point. All LCavβ splice isoforms like vertebrate Cavβ2a slow inactivation kinetics of LCav2 channels. The slowing of inactivation kinetics is maximized with the exon 7a− configuration.
8. The HOOK domain more than the N-terminus contributes to the slowing of the inactivation kinetics imparted by invertebrate Cavβ subunits
We observe that splicing in the HOOK domain of snail LCavβ− lacking extra residues in exon 7, has a more dramatic effect on the slowing of the inactivation kinetics of LCav2 channels compared to snail LCavβ+ possessing extra residues in exon 7 ( Figure 6B, 6C ). In contrast, the differing N-termini of LCavβ do not influence the inactivation kinetics of LCav2 ( Figure 6B, 6C ). We observed no measureable electrophysiological differences between the expression of LCavβA (exon 1a/1b) and LCavβB (exon 2) isoforms, suggesting that the N-terminal splicing is more associated with tissue localization than to altering biophysical properties of snail Cav1 and Cav2 channels. Size of the N-terminus may also be a relevant parameter for LCavβA and LCavβB, since these variants have similarly sized N-termini (50 vs. 47 amino acids respectively). Differing lengths of N-termini of mammalian Cavβ1b [51] and Cavβ2 [52] channels are known to correlate with altering rates of inactivation in mammalian Cav2 channels.
Comparing different invertebrate Cavβ subunits (schistosome [21], snail [20], squid [22] and honeybee [23]), the N-termini are highly variable in sequence, yet all impart slow inactivation kinetics of Cav2 channels in the absence of palmitoylation residues found in vertebrate Cavβ2a Likely more relevant than the N-terminus for imparting slow inactivation kinetics is the HOOK domain which in invertebrate Cavβ subunits resembles Cavβ2a (longer spliced isoform, Exon 7A) with a conserved polybasic residue stretch at the 3′ end [43], [53]. The polybasic residue stretch is lacking in vertebrate β1b, β3 and β4 subunits, containing the shorter spliced isoform (Exon 7B instead of Exon 7A). These other channel types (Cavβ1b, Cavβ3 and Cavβ4) bear faster inactivation kinetics than Cavβ2a [8], [9]. The conserved polybasic residue stretch in Exon 7A contributes to the slow inactivation kinetic phenotype, even in the absence of palmitoylation residues in the N-terminus found in vertebrate Cavβ2a channels [43], [53]. The simplest evolutionary hypothesis is that vertebrate Cavβ2a retained the extended Exon 7A with the polybasic region in the HOOK domain shared in a common ancestral Cavβ subunit (resembling the invertebrate Cavβ subunit) to complement its very slow inactivation phenotype governed largely by its unique N-terminus of palmitoylation residues. Every invertebrate Cavβ subunit (eg. cnidarian, nematode, schistosome, mollusk, insect) resembles Exon 7A with the polybasic region in the HOOK domain, which may suggest that the shorter HOOK domain of Exon 7B was a vertebrate adaptation to promote a faster inactivation phenotype for Cavβ1b, Cavβ3 and Cavβ4 subunits.
9. Invertebrate Cavβ subunits do not alter the inactivation kinetics of LCav1 L-type channels
Snail LCav1 channels possess little voltage-dependent inactivation without Cavβ subunit expression, so it is not surprising that we did not observe additional slowing of inactivation kinetics imparted by LCavβ on LCav1 channels when barium is the external charge carrier ( Figure 6A, 6C ). Snail LCav1 channels have a prominent calcium dependent inactivation [14], [54] as mammalian Cav1.2 channels [55], which has been attributabed to a conserved C-terminal IQ motif and a N-terminal NSCATE motif for binding calmodulin in the presence of external calcium [14]. The much faster voltage-dependent inactivation of vertebrate Cav2 channels has been attributed to the more helical, and rigid proximal linker (PL) between the inactivation gate of the transmembrane segment 6 (S6) helix of domain I and the AID sequence for Cavβ subunit binding [56]–[58]. Both invertebrate and vertebrate Cav1 channels have a conserved glycine in the proximal linker ( Figure 1D ) which is expected to lower the alpha-helicity and rigidity of the helix, and weaken the observed influences of the Cavβ subunit on the inactivation kinetics of Cav1 channels [57]. The conservation of mammalian calcium channel features in the most primitive multicellular animals is consistent with the notion that a common ancestor before the appearance of nervous systems (i.e. resembling an extant placozoan), possessed a Cav2 channel homolog with a strongly voltage-dependent regulation by Cavβ subunits, and a Cav1 channel homolog with a calcium-dependent regulation of inactivation kinetics by calmodulin.
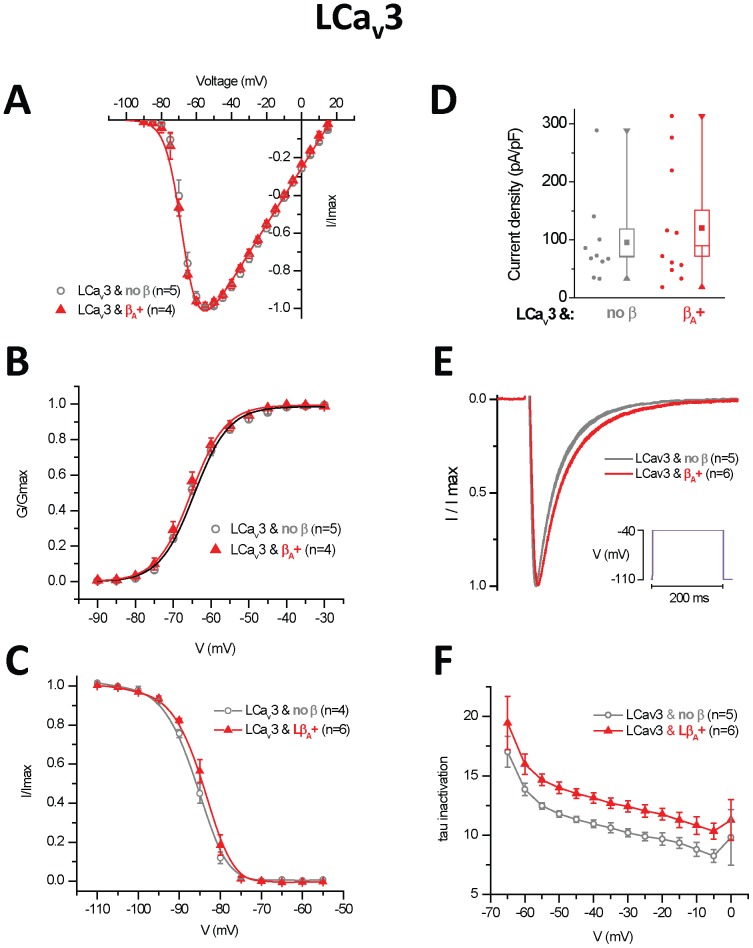
10. Invertebrate Cavβ subunits slow the inactivation kinetics of Cav3 channels in vitro
There have been reports that Cav3 T-Type channels can be modulated by the co-expression of Cavβ subunits in vitro [59]–[61]. We report that the snail Cavβ can promote the slowing of inactivation kinetics of LCav3 channels in a manner similar to LCav2 channels ( Figure 7E,F ), and this modulation occurs without any effect on other biophysical properties ( Figure 7A,B,C ) or membrane expression levels ( Figure 7E ). This commonly observed phenomenon could be an artifact of in vitro studies, since there isn't strong evidence that Cav3 channels are modulated with Cavβ subunits in vivo. Reported β subunit interactions are weak with T-type channels [59]–[61], and require the transfer of I–II linker sequences from Cav1 or Cav2 channels to acquire characteristic features of Cavβ subunit modulation [62]. Also, native Cav3-Cavβ protein complexes have never been identified, knockdown of Cavβ subunits in native cells do not alter T-type channel properties [63], [64], and snail LCav3 [18], [19] and mammalian [65] T-type channels are easily reconstituted and resemble the features of native currents in vitro, without having to co-express Cavβ subunits.
Figure 7. Snail beta subunit (LCavβA+) splice isoform slow the inactivation kinetics, but has no effect on any other biophysical property on snail T-type, LCav3 channels.
(A) Current-voltage relationships (B) Activation and (C) Steady-state inactivation curves. (D) Current densities (pA/pF) shown as box plot (box = s.e.m., whisker range = minimum/maximum values). (E) Normalized peak barium current traces illustrated as mean, s.e.m. for LCav3 channels co-expressed with LCavβ splice isoforms or no Cavβ. (F) Curve fitting of inactivation (Tau values) over the steps from −110 mV to the range of −70 mV to 0 mV).
11. A lack of closed state inactivation in the Cavβ subunit of invertebrates
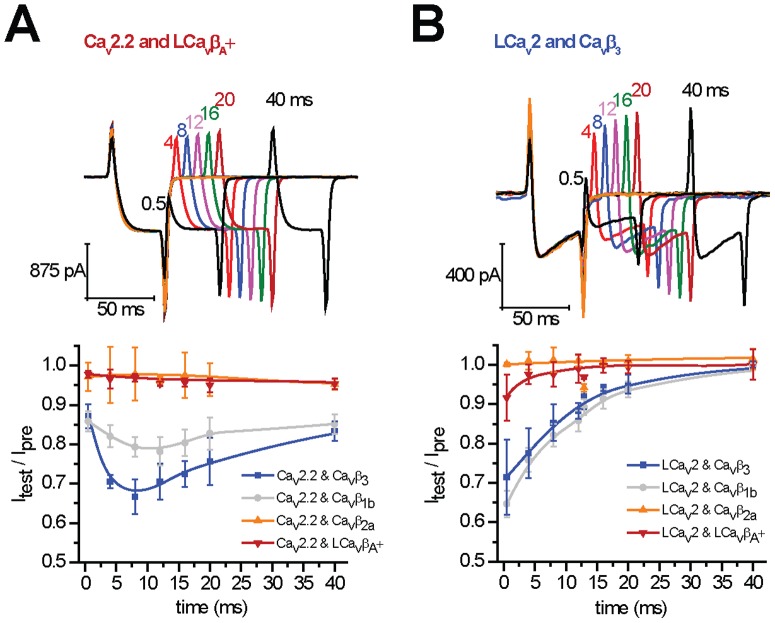
Another feature that has been attributed to Cavβ is the tendency of Cav2 channels to inactivate more than expected during a train of action potentials [27], [28]. This is a feature dubbed “closed-state inactivation”, where Cavβ subunits promote a greater inactivation during the repolarization period, when Cav2 channels are expected to be more unavailable, in non - open and inactivated states [27], [28]. Observation of this persistent inactivation can be obtained by using a two-pulse protocol, where a prepulse step depolarization is applied to generate maximal currents, and then the percent of recovery of the same peak currents assessed by a second voltage step after a time delay (e.g. 0.5 to 40 ms) [28]. With a short time delay between voltage pulses, such as 8 ms, there is a “dip” in the time of recovery observed for mammalian Cav2.2 channels co-expressed with Cavβ3, in particular where a greater than expected fraction of Cav2.2 channels are observed to be unavailable for opening compared to more brief time periods (0.5 ms) or longer time periods (40 ms) of inactivation recovery between the prepulse and the test pulse ( Figure 8A , bottom panel). We observe that the closed-state inactivation is a property that is most evident for Cavβ3 and less for Cavβ1b, and not observable for Cavβ2a ( Figure 8A , bottom panel), as has been reported previously [28]. Snail LCavβA+ is similar to Cavβ2a in lacking closed state inactivation ( Figure 8 , sample trace shown in upper panel). Not only was the snail Cavβ unable to bestow a closed state inactivation on mammalian Cav2.2 channels, but the snail LCav2 channel was not compatible with closed state inactivation either. None of the Cavβ subunits including LCavβA+ or mammalian Cavβ3 or Cavβ2a could generate the closed state inactivation channel behavior on LCav2 ( Figure 8B ).
Figure 8. Snail Cav2 channels nor snail LCavβ subunits do not promote the closed-state inactivation observed for mammalian Cav2.2 and Cavβ3 or Cavβ1b subunits.
The size of test barium currents relative to the prepulse current after time delays of 0.5, 4, 8, 12, 16, 20 and 40vβ3, Cavβ1b, Cavβ2a and snail LCavβA+ with mammalian Cav2.2 or snail LCav2 calcium channels. A closed-state inactivation exhibited where there is an increasing inactivation with increasing time delay, is found only with particular combination of subunits, which includes mammalian Cav2.2 and Cavβ3 or Cavβ1b [18], [19].
12. Calcium current density changes due to Cavβ subunit expression
Cavβ subunits are required for expression of Cav1 and Cav2 channels and little or no surface expression in vitro and in vivo is observed in their absence. Binding of Cavβ subunits to calcium channels in the Alpha1 Interaction Domain (AID) sequence of the linker is modeled to promote membrane trafficking and expression of calcium channels, by facilitating their export from the endoplasmic reticulum [66] and preventing ubiquitination and proteosomal degradation [67]–[69]. Previously we were unable to measure an increase in current density of expressed snail LCav2 channels with co-expressed snail LCavβ, when LCav2 channels contained an N-terminus of Cav2.2 [20]. We show here that expression of both LCav1 and LCav2 with its native N-terminus produced statistically significant increases in current density with the expression of all snail LCavβ subunit isoforms (boxplots in Figure 5D , and see statistics in Table 1 ).
13. Conclusions
Cavβ subunits found in single-celled coanoflagellates to humans, bear highly conserved functional core of SH3 and GK domains for associating and modulate high voltage-gated calcium channels. Variable regions in the N-terminus (exons 1a/1b and 2) and HOOK domain (exon 7) contain conserved alternative splicing that is shared amongst Cavβ subunits. Molluscan (squid [22], snail [20]) schistosome [21] and insect (bee) [23] Cavβ subunits all substantially slow inactivation kinetics of Cav2 channels despite differing spliced N-termini of exon 1a/1b or exon 2 isoforms. The slowing of inactivation kinetics of invertebrate Cavβ subunits occurs in the absence of the canonical palmitoylated residues found the N-terminus of vertebrate Cavβ2a subunit, but notably the slowing of inactivation kinetics correlates with the presence of a stretch of polybasic residues at the 3′ end of the HOOK domain common to vertebrate Cavβ1 and Cavβ2a subunits. While alternative splicing of the HOOK domain is primarily responsible for promoting the slowing of inactivation of invertebrate Cavβ subunits, alterative splicing of the N-terminus relates to a tissue specific expression pattern that is found in Cavβ subunits. An N-terminal “A isoform” composed of exon 1a/1b is the predominant form expressed in skeletal muscle (although A isoforms of Cavβ subunits are broadly expressed across tissues), while some “B isoforms” can have a highly brain-centric expression pattern (although this is not true of all “B isoforms” of Cavβ subunits). Specialization such as closed-state inactivation [18], [19] are features that evolved within vertebrates tailored to specific Cavβ isoforms and Cav2 calcium channel types. Further studies in invertebrates will further clarify the nature of the relationship between calcium channels and Cavβ, and it is a model where the numbers of possibilities are limited, given that there are only single genes coding for Cav1, Cav2, Cav3 and Cavβ in the snail genome.
Materials and Methods
Cloning of novel LCavβ subunit isoforms
The calcium channel beta subunit originally cloned and expressed from the pond snail Lymnaea stagnalis was LCavβA+ and previously described [20], and deposited as GenBank Accession # AF484087. Novel LCavβA−, LCavβB+ and LCavβB− splice isoforms have been deposited as HM187674.1, HM187675.1 and HM187676.1 respectively. Novel exons in the N-terminus and exon 7 were first identified by PCR amplification of λZAP cDNA libraries and freshly-isolated genomic DNA. The 5′ end of the LCavβB cDNA transcript was extended using 5′ RACE. Identified sequences were later confirmed by mRNA sequences from a published brain transcriptome of Lymnaea stagnalis [70], and unpublished genomic sequence spanning the LCavβ subunit made available by Daniel Jackson and Angus Davison (University of Nottingham). LCavβ subunit isoforms were cloned into mammalian expression vector pMT-2SX(R) using PCR inserts with flanking NotI and XhoI restriction sites spanning the start and stop codons of the LCavβ gene. DNA primers to create the 5′ end of the inserts using PCR were designed with a standard mammalian KOZAK sequence downstream of the Not1 site, GCGGCCGCCACCATGG, where the underlined ATG sequence is the expected start codon. Final expression constructs in pMT-2SX(R) vector were confirmed for their expected DNA sequences by dideoxy terminator sequencing in both orientations (TCAG DNA sequencing facility, The Hospital for Sick Children, Toronto, Ontario).
Sequence comparisons of calcium channel subunits
Multiple alignments and gene trees of sequences were generated in Phylogeny.fr [71]. The running average of similarity across Cavβ subunit sequences were generated with PLOTCON in EMBOSS [72]. Cavβ subunit sequences from differing exon 1a/1b, exon 2 and exon 7 splice isoforms in different organisms were identified by GenBank BLAST searches. The following are mostly full length Cavβ subunit sequences used for the generation of multiple alignments and gene trees shown in Figure 1 and S1 (Genus species, GenBank Accession #): Coanoflagellate (Monosiga brevicollis, XM_001748200.1), Poriferan (Amphimedon queenslandica, ACUQ01001402), Placozoan (Trichoplax adhaerens, ABGP01000238), Cnidarian (Nematostella vectensis, ABAV01020487), Lophotrochozoan (Lymnaea stagnalis, AF484087) and Ecdyzozyan (Drosophila melanogaster, U11074). The GenBank-derived Cav1 channel sequences for Figure 1D were: Poriferan (Amphimedon queenslandica, XM_003382988), Placozoan (Trichoplax adhaerens, XM_002108894), Cnidarian (Nematostella vectensis, XM_001639004), Lophotrochozoan (Lymnaea stagnalis, AF484079) and human Cav1.2 (BC146846). The GenBank-derived Cav2 channel sequences for Figure 1D were: Placozoan (Trichoplax adhaerens, XM_002109739), Cnidarian (Hydra magnipapillata, XM_004210303), Lophotrochozoan (Lymnaea stagnalis, AF484082) and human Cav2.1 (AB035727). Genomic and cDNA sequences for human Cavβ subunits were derived from NCBI Gene for CACNB1, CACNB2, CACNB3, CACNB4 genes. Genomic sequences for snail beta subunit were provided by Daniel Jackson and Angus Davison (University of Nottingham) and gaps in intron lengths were estimated from the genome sequence derived from the closely-related air-breathing, freshwater snail Biomphalaria glabrata.
Calcium channel subunit expression in HEK-293T cells
Snail LCav1 [15], [54] and LCav2 channels [16], [17] from pond snail Lymnaea stagnalis were previously cloned and characterized in HEK-293T cells, expressed in pIRES2-EGFP bicystronic vector. Mammalian Cav2.2 (α1B) channels were expressed in pMT2 expression vector, and co-transfected with pTRACER (EGFP). Positively-transfected channels were identified by green fluorescence with an Axovert 40 inverted CFL microscope using a GFP filter and mercury lamp excitation. Calcium channels were always co-transfected with mammalian α2δ1 subunit in pMT2 vector, with either no Cavβ subunits, mammalian Cavβ subunits (Cavβ1b or Cavβ2a or Cavβ3) or snail Cavβ subunit isoforms. Mammalian Cavβ and α2δ1 subunits were gifts from Gerald Zamponi (University of Calgary) and Terry Snutch (University of British Columbia).
Quantitative RT-PCR of calcium channel subunits in snail tissues
mRNA expression was measured using quantitative Real-Time PCR (as previously published [14], [19], [73], [74]) using mRNA isolated from reproductively-active adult snails from Lymnaea stagnalis (shell lengths of 2.0 to 2.5 cm) illustrated in Figure 4 . Adult versus sexually immature juvenile mRNA expression was compared in Figure 4D , where juveniles have shell lengths of 1.0 to 1.5 cm. qPCR primers sets (Table S1) were designed to selectively amplify LCavβ, LCav1 and LCav2 subunits using a universal primer set, and additional primer sets designed to amplify specific exons, such as N-terminal exons: LCavβA and LCavβB and differing exon 7 isoforms: LCavβ− and LCavβ+. PCR primer specificity was confirmed by appropriate sized PCR products amplified from pooled template of cDNAs generated from freshly isolated mRNA and also cloned cDNAs. PCR primer efficiencies was then determined in relative standard curves using 1∶5 serial dilutions of pooled cDNA (1∶5, 1∶25, 1∶125; and 1∶625) as template for real time RT-PCR amplification. Triplicate reactions were carried out in 96-well PCR plates (Bio-Rad) for each dilution, with each well containing 0.5 µL of serially diluted cDNA, 5 µL of SsoFastTM EvaGreen Supermix (Bio-Rad), 0.5 µL of each 10 µM primer from a set, and 3 µL of water. PCR amplification, fluorescence reading, and melt curve analyses were carried out using a Bio-Rad C1000TM Thermal Cycler equipped with a CFX96TM Real-Time System and run by CFX Manager Software (Bio-Rad). All cycle threshold values used for analysis were determined relative to the average cycle threshold value of the control gene, HPRT1 (hypoxanthine phosphoribosyltransferase 1).
Mammalian HEK-293T cell lines
We have described our optimized methods for the expression of ion channels in mammalian HEK-293T cells and their recording using whole-cell patch clamp in an online JoVE video journal [75]. Briefly, HEK-293T are cultured in Dubecco's Modified Eagle's Medium (DMEM, Sigma, #D5796) supplemented with 10% Fetal Bovine Serum (FBS; Sigma, #F1051) that had been heat-inactivated at 56°C for 30 minutes and 2.5 mL of Penicillin-Streptomycin solution (5000 units of penicillin and 5 mg streptomycin/mL; Sigma, #P4458). The complete HEK-293T media (500 mL) was further supplemented with 5 mL of 100 mM sodium pyruvate (Sigma, #S8636). In all instances complete culture media was heated to 37°C in a water bath before use.
Subculturing of HEK-293T cells
To subculture cells, culturing media was removed from the flask and cells were washed twice with phosphate buffered saline (PBS; 6.70 mM KCl, 3.67 mM KH2PO4, 10.82 mM Na2HPO4-7H2O, 342 mM NaCl) that had been pre-warmed to 37°C. To detach cells, 1 mL of 0.25% Trypsin-EDTA (Sigma, #T4049) that had been heated to 37°C was added to the flask. The flask was then incubated at 37°C until the majority of cells had detached (to a maximum of five minutes). In the meantime, two new flasks were filled with 5.5 mL of fresh complete media (these will be used for the next passage). Also, flasks of cells for transfection were filled with 5 mL of fresh complete media. Once the cells were detached, 5 mL of fresh complete culture media was added and the cells were resuspended by pipetting up and down several times. The resuspended cells were then divided among the flasks so that each flask had a final volume of 6 mL, therefore the flasks to be used in the next passage were split 1∶12, while the flasks to be transfected were split 1∶6. These cells were then incubated under standard conditions. Cells were allowed to grow to 40–50% confluency prior to transfection.
Transient transfection of HEK-293T cells using calcium phosphate precipitation
Transfection of HEK 293T cells was done using a calcium phosphate transfection protocol that was carried out by diluting 4 µg of each plasmid to be transfected in 30 µL of 2.5M CaCl2 and sterile milli-Q water to 300 µL. This was then added dropwise to 300 µL of 2× HES buffer (280 mM NaCl, 50 mM Hepes, 1.5 mM Na2HPO4-7H2O, pH 7.0). The mixture was then mixed well and allowed to incubate at room temperature for 20 minutes to allow for the formation of calcium phosphate crystals. During the incubation, media was removed from the flask to be transfected and replaced with 5.4 mL of fresh complete medium. After incubation, the calcium phosphate solution was added dropwise into the flask of cells. The flask was then incubated under standard conditions for eight to 16 hours. After eight to16 hours, the cells were washed twice with PBS pre-heated to 37°C and 6 mL of fresh complete medium was added. These cells were then incubated at 28°C in a humidified 5% CO2 atmosphere for two days before plating onto glass coverslips.
Poly-L-Lysine coating of coverslips and plating cells onto coverslips
Prior to plating HEK cells, sterile, round glass coverslips (Fisher Scientific, #12-545-80) were coated in poly-L-lysine. The coverslips were spread out in a single layer at the bottom of a large (100 mm diameter) culture dish. A dilute poly-L-lysine solution was made by diluting 1.5 mL of 0.1% (w/v) poly-L-lysine (Sigma, #P8920) in 13.5 mL of milli-Q water. This solution was poured over the coverslips and they were allowed to incubate at room temperature for one hour. After one hour, the poly-L-lysine solution was removed and coverslips were washed twice in 15 mL of sterile water and then dried in a 56°C oven for two hours. After coating, coverslips were stored at 4°C for up to two weeks.
Two days after transfection, HEK cells were plated onto glass coverslips using the same method. Media was removed from the flask and the transfected cells were washed twice with PBS before the addition of 1 mL of trypsin, as in section 2.6.3. The cells were resuspended in 6 mL of complete media and added to 60 mm culture dishes containing the appropriate amount of complete media and four to six coverslips so as to give a split ratio of 1∶3 and 1∶4 for cells to be used in patch clamp experiments (HEK cells) and 1∶6 for cells to be used for antibody staining. These dishes were then incubated at standard conditions for three to four hours before they were moved to 28°C in a humidified 5% CO2 atmosphere.
Whole cell patch clamp recording
Cells were recorded by whole-cell patch clamp method three to seven days after plating onto coverslips using an AxoPatch 200B amplifier, combined with a Digidata 1440A Data Acquisition System and pCLAMP 10 Software (Molecular Devices). Whole cell patch clamp recordings were carried out with an Patch pipettes for recording with pipette resistances of 2–5 MΩ, and with typical access resistance maintained after breakthrough between 4 and 6 MΩ. Only recordings with minimal leak (<10% of peak) and small current sizes (<500 pA) in HEK-293T cells were used due to loss of voltage clamp above 500 pA. Series resistance was compensated to 70% (prediction and correction; 10-µs time lag). For all recordings, leak subtraction was preformed offline and data was filtered using a 500 Hz Gaussian filter using Clampfit 10.2 software (Molecular Devices) before further analysis.
Calcium channel currents were measured in a 20 mM Ba2+ external bath solution: 20 mM BaCl2, 40 mM tetramethylammonium chloride, 10 mM glucose, 64 mM CsCl, pH 7.2) and patch pipettes were filled with internal solution (108 mM cesium methane sulfonate, 4 mM MgCl2, 9 mM HEPES, 9 mM ethylene glycol tetraacetic acid, pH 7.2). During all recording sessions, cells were maintained at a holding potential of −100 mV. Prior to recording, a test step to peak current (10 mV for LCav1 or 30 mV for LCav2) was performed to indicate if the cell was producing a current and if the state of the patch was suitable for gathering data. All data derived from patch clamp recording was statistically analyzed using one-way analysis of variation (ANOVA) tests online at http://www.danielsoper.com/statcalc3.
Electrophysiology protocols for generating current-voltage relationships and steady-state inactivation
The current-voltage (IV) relationship was assessed by stepping cells from −100 mV to −50 mV for 450 ms and then increasing the voltage step by 10 mV in successive sweeps until reaching a potential of 50 mV. To analyze the effect of LCavβ subunits on the steady-state inactivation of the channels cells were stepped from −100 mV to peak voltage for 150 ms, then allowed to recover for 1 s before being held at −100 mV until all channels had reached inactivation (this conditioning step may take up to 15 seconds) then immediately stepping to peak again for 150 ms. In successive sweeps, the conditioning potential used to inactivate channels was increased by 10 mV each time until reaching 30 mV.
Analyses of activation and current-voltage curves
To create activation and IV plots, current (I in pA) at each voltage step was normalized to peak current (Imax in pA) for that cell. Each normalized IV relationship was plotted and the individual reversal potentials (Erev; in mV) were determined by calculating the y-intercept of the linear portion of the IV curve (+20 to +40 for LCav1; +30 to +50 for LCav2). The reversal potential represents the membrane potential at which the driving force for Ca2+ (or Ba2+) influx is equal to the driving force for Ca2+ (or Ba2+) efflux and there is no net movement of Ca2+ (or Ba2+) through the calcium channels. The conductance (rate of ions flowing through the channel) was then determined for each voltage step using the equation: G = (I/Imax)/(V−Erev), where G represents conductance (pS) of the channel and V represents the test voltage (mV). For each trace maximum conductance (Gmax) was then determined. The mean and standard error of the mean (s.e.m.) were calculated for (I/Imax), Erev and Gmax.
Activation plots were created using Origin 8 software (OriginLab, Northampton, MA) by importing IV data and then performing a Boltzmann transformation using the equation: Conductance (g) = I/(V−Erev). The Boltzmann-transformed data was then normalized and then a scatter of normalized activation versus voltage was created and curve fitted with the following Boltzmann equation: G/Gmax = 1/(1+e((V−V0.5)/Ka)), where V0.5 and Ka represent the half-activation voltage and slope factor of the activation curves, respectively. The half-activation potential is the voltage at which 50% of channels is expected to be open. The slope factor of the activation curve is a value that represents the delay of channels transitioning from the closed to active (open) position. The normalized activation data [(I/Imax), V0.5, Ka] were then averaged and the SEM was determined. The mean data was then plotted and a curve was simulated using the fitted parameters. IV plots were created by plotting voltage against mean (I/Imax) ± SEM and then fitted with an Ohmic-Boltzmann curve using the following equation: I/Imax = Gmax(V−Erev)/(1+eV−V0.5/Ka).
Analyses of steady-state inactivation
Steady-state inactivation values were determined by dividing the current after the inactivating (conditioning) pulse by the current prior to the inactivating pulse. Each data set was then imported into Origin and curve fit with the Boltzmann equation: (I/Imax) = 1/(1+e(V−V0.5)/Ki) where V0.5 and Ki represent the half-inactivation voltage and the slope factor of inactivation, respectively. The half-inactivation potential is the voltage at which 50% of the channels are available and the other 50% of channels have transitioned from the open to the inactivated state. The values of V0.5 and Ki (determined using Origin 8) and (I/Imax) were then averaged and the standard error of each means were calculated. The mean steady-state activation data was then plotted ± SEM and this data was used to simulate a Boltzmann curve derived from the fitted parameters. The relative inactivation of LCav1 and LCav2 currents were compared as the fraction of current remaining after 250 ms (R250).
Western blotting of LCavβ subunits
LCavβ protein was identified on Western blots with rabbit anti- LCavβ subunit antibody made against KLH-coupled synthetic peptide with LCavβ sequence: SLDEEKEALRRET, which is downstream of the N-terminus and common to all LCavβ isoforms [20]. Western blots were prepared from protein homogenates isolated from HEK cell lysates, five days post-transfection. Total protein was separated by SDS-PAGE, and the contents of the gels were transferred onto 0.45 µM nitrocellulose membrane (Mandel, # W-10401196) overnight at 90 mA at 30 V. Before applying antibody, the nitrocellulose membrane must be blocked to reduce nonspecific interactions. Blocking buffer [10 mM Tris, 100 mM NaCl, 0.1% Tween-20(v/v), 5%(w/v) skim milk powder] was added to the membrane in a shallow dish (enough to completely cover the nitrocellulose), placed on a shaker and allowed to incubate at room temperature for two hours.
After blocking, the membrane was washed five times with Tween-Tris-Buffered Saline [TTBS; 10 mM Tris, 100 mM NaCl, 0.1% Tween-20(v/v)] for five minutes at room temperature. Next, the primary antibody (rabbit anti-LCavβ) was diluted 1∶2000 in blocking buffer and poured over the membrane after the last wash with TTBS. The primary antibody was incubated with the membrane overnight at 4°C. The following morning, the antibody was removed and the membrane was washed five times in TTBS for five minutes at room temperature, and then once in blocking buffer for 15 minutes at room temperature. After the second block, the secondary antibody (Goat anti-rabbit IgG coupled to horseradish peroxidase; Invitrogen, #65-6120) was diluted 1∶5000 in blocking buffer and added to the dish containing the membrane. The secondary antibody was incubated with the membrane for 30 minutes at room temperature before it was decanted. The membrane was then washed five times in TTBS for five minutes at room temperature. The membrane was then ready for chemiluminescent staining to detect the presence of LCavβ subunits.
LCavβ subunits were detected using the chemiluminescent stain ECL which was made by combining 20 mL of freshly prepared solution one (2.5 mM luminol, 396 µM p-coumaric acid, 100 mM Tris, pH 8.5) and 20 mL of freshly prepared solution two (100 mM Tris, 12 uL of 30% H2O2, pH 8.5). Solutions one and two were combined, poured over the membrane and incubated for one minute at room temperature. The blots were then exposed to x-ray film (Kodak, #819 4540) for one minute in a darkroom before development using an automated developer. Films were then compared to nitrocellulose membranes and the ladder was marked onto the film by hand.
Potential caveats in studies of LCavβ subunits expressed in HEK-293T cells
We observe that snail LCavβ subunit isoforms promote membrane expression of calcium channels in HEK-293T cells, but the biophysical effects are relatively minor compared to the mammalian Cavβ subunits. A potential concern is a weak signal to background ratio perhaps due to a lack of saturation of heterologously-expressed Cavβ subunits in every cell recording that is also to contain calcium channels and a co-expressed α2δ subunit in a standard calcium-phosphate transfection protocol. Another issue is potentially contaminating, low levels of human calcium channels subunits reported in HEK-293T cells [76]. A fetal form of Cavβ3 subunit is detectable in Xenopus oocytes [77], [78], but little to no endogenous Cavβ subunits have been reported in HEK-293T cells [79]. We are confident that our data represents the snail Cavβ subunit isoforms effects on expressed snail calcium channels. Positively-expressing LCav1 and LCav2 HEK-293T cells corresponded largely to the green EGFP fluorescence of transfected cells, since EGFP and the calcium channels were derived from the same mRNA on bicistronic vector pIRES2-EGFP. LCavβA and LCavβB subunit expression can be identified by anti-rabbit LCavβ specific antibody in Western blots of transfected HEK cell lysates at appropriate protein sizes (Figure S3).
Expression of mammalian α2-δ subunits can cause dramatic shifts in voltage-sensitivities [7], [80], and perhaps the full contribution of molluscan Cavβ subunits will not be completely described without co-expression of native α2-δ subunits. Mining of molluscan genomes reveals three α2-δ subunits: a generalized one that resembles α2-δ1 and α2-δ2 subunit in structure, a second brain specific one that is a homolog to Drosophila straightjacket [81] and human α2-δ3 subunits, and a third more invertebrate specific α2-δ subunit.
Supporting Information
Multiple alignment of Cavβ subunits including those from a cnidarian (Nematostella), nematode (Caenorhabditis), ecdysozoan (Drosophila), lophotrochozoan (Lymnaea) and human gene isoforms (Cavβ1b, Cavβ2a, Cavβ3, Cavβ4b). Highly conserved SH3 and GK domains are illustrated, also conserved secondary structures (α helices and β sheets), and calcium channel (AID) binding residues (blue residues) reported in crystal structures of Cavβ subunits. Exon boundaries (red lines) are indicated. Red boxes surrounding a base indicates that the intron splits between an amino acid base.
(TIF)
Genomic sequences spanning exon 7 illustrating the alternate acceptor sites in Lymnaea snail calcium channel beta subunit, (LCavβ+ and LCavβ−) which generates a seven amino acid optional exon (7A+) or not exon (7A−). Note that skipping of exon 7 generates a change in reading frame and truncated LCavβ. The frame shift occurs because exon 6 ends within a codon after the first nucleotide (phase 1), whereas the intron preceding exon 8 is located between codons (phase 0).
(TIF)
Snail LCavβ - specific rabbit antibody localizes LCavβA+ and LCavβB+ proteins in transfected HEK cells by Western blotting. Snail LCav2 in pIRES2-EGFP vector were transfected alone (mock) or with coexpressed LCavβA+ and LCavβB+ plasmids in HEK cells. Expressed LCavβA+ and LCavβB+ proteins are identifiable on Western blots of transfected HEK cell lysates at appropriate size (62.8 kDa and 62.6 kDa), respectively.
(TIF)
Funding Statement
This work was funded through a Heart and Stroke Foundation of Canada Grant-In-Aid and NSERC Discovery Grant to J.D.S. and an NSERC Canada Graduate Scholarship and Ontario Graduate Scholarship award to A.S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hanlon MR, Wallace BA (2002) Structure and function of voltage-dependent ion channel regulatory beta subunits. Biochemistry 41: 2886–2894. [DOI] [PubMed] [Google Scholar]
- 2. Mangubat EZ, Tseng TT, Jakobsson E (2003) Phylogenetic analyses of potassium channel auxiliary subunits. J Mol Microbiol Biotechnol 5: 216–224. [DOI] [PubMed] [Google Scholar]
- 3. Pongs O, Schwarz JR (2010) Ancillary subunits associated with voltage-dependent K+ channels. Physiol Rev 90: 755–796. [DOI] [PubMed] [Google Scholar]
- 4. Li J, Waterhouse RM, Zdobnov EM (2011) A remarkably stable TipE gene cluster: evolution of insect Para sodium channel auxiliary subunits. BMC Evol Biol 11: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patino GA, Isom LL (2010) Electrophysiology and beyond: multiple roles of Na+ channel beta subunits in development and disease. Neurosci Lett 486: 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tseng TT, McMahon AM, Johnson VT, Mangubat EZ, Zahm RJ, et al. (2007) Sodium channel auxiliary subunits. J Mol Microbiol Biotechnol 12: 249–262. [DOI] [PubMed] [Google Scholar]
- 7. Dolphin AC (2012) Calcium channel auxiliary alpha2delta and beta subunits: trafficking and one step beyond. Nat Rev Neurosci 13: 542–555. [DOI] [PubMed] [Google Scholar]
- 8. Buraei Z, Yang J (2012) Structure and function of the beta subunit of voltage-gated Ca(2+) channels. Biochim Biophys Acta [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buraei Z, Yang J (2010) The beta subunit of voltage-gated Ca2+ channels. Physiol Rev 90: 1461–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fux J, Hseuh N, Spafford JD Accessory beta subunits for molluscan Nav1 sodium channels are members of a novel CUB domain containing protein family. [Google Scholar]
- 11. de MA, Suga H, Ruiz-Trillo I (2010) Evolution of the MAGUK protein gene family in premetazoan lineages. BMC Evol Biol 10: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. King N, Westbrook MJ, Young SL, Kuo A, Abedin M, et al. (2008) The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 451: 783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dolphin AC (2009) Calcium channel diversity: multiple roles of calcium channel subunits. Curr Opin Neuro biol 19: 237–244. [DOI] [PubMed] [Google Scholar]
- 14. Taiakina V, Boone AN, Fux J, Senatore A, Weber-Adrian D, et al. (2013) The calmodulin-binding short linear motif NSCaTE is conserved in L-type channel ancestors of vertebrate Cav1.2 and Cav2 channels. PLoS One [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Senatore A, Boone A, Lam S, Dawson TF, Zhorov B, et al. (2011) Mapping of dihydropyridine binding residues in a less sensitive invertebrate L-type calcium channel (LCa v 1). Channels (Austin) 5: 173–187. [DOI] [PubMed] [Google Scholar]
- 16. Spafford JD, Chen L, Feng ZP, Smit AB, Zamponi GW (2003) Expression and modulation of an invertebrate presynaptic calcium channel alpha1 subunit homolog. J Biol Chem 278: 21178–21187. [DOI] [PubMed] [Google Scholar]
- 17. Huang X, Senatore A, Dawson TF, Quan Q, Spafford JD (2010) G-proteins modulate invertebrate synaptic calcium channel (LCav2) differently from the classical voltage-dependent regulation of mammalian Cav2.1 and Cav2.2 channels. J Exp Biol 213: 2094–2103. [DOI] [PubMed] [Google Scholar]
- 18. Senatore A, Spafford JD (2010) Transient and big are key features of an invertebrate T-type channel (LCav3) from the central nervous system of Lymnaea stagnalis. J Biol Chem 285: 7447–7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Senatore A, Spafford JD (2012) Gene transcription and splicing of T-type channels are evolutionarily-conserved strategies for regulating channel expression and gating. PLoS One 7: e37409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spafford JD, van MJ, Larsen P, Smit AB, Syed NI, et al. (2004) Uncoupling of calcium channel alpha1 and beta subunits in developing neurons. J Biol Chem 279: 41157–41167. [DOI] [PubMed] [Google Scholar]
- 21. Salvador-Recatala V, Schneider T, Greenberg RM (2008) Atypical properties of a conventional calcium channel beta subunit from the platyhelminth Schistosoma mansoni. BMC Physiol 8: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kimura T, Kubo T (2003) Cloning and functional characterization of squid voltage-dependent Ca2+ channel beta subunits: involvement of N-terminal sequences in differential modulation of the current. Neurosci Res 46: 105–117. [DOI] [PubMed] [Google Scholar]
- 23. Cens T, Rousset M, Collet C, Raymond V, Demares F, et al. (2013) Characterization of the first honeybee Ca(2)(+) channel subunit reveals two novel species- and splicing-specific modes of regulation of channel inactivation. Pflugers Arch 465: 985–996. [DOI] [PubMed] [Google Scholar]
- 24. Chien AJ, Carr KM, Shirokov RE, Rios E, Hosey MM (1996) Identification of palmitoylation sites within the L-type calcium channel beta2a subunit and effects on channel function. J Biol Chem 271: 26465–26468. [DOI] [PubMed] [Google Scholar]
- 25. Qin N, Platano D, Olcese R, Costantin JL, Stefani E, et al. (1998) Unique regulatory properties of the type 2a Ca2+ channel beta subunit caused by palmitoylation. Proc Natl Acad Sci USA 95: 4690–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Restituito S, Cens T, Barrere C, Geib S, Galas S, et al. (2000) The [beta]2a subunit is a molecular groom for the Ca2+ channel inactivation gate. J Neurosci 20: 9046–9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yasuda T, Lewis RJ, Adams DJ (2004) Overexpressed Ca(v)beta3 inhibits N-type (Cav2.2) calcium channel currents through a hyperpolarizing shift of ultra-slow and closed-state inactivation. J Gen Physiol 123: 401–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patil PG, Brody DL, Yue DT (1998) Preferential closed-state inactivation of neuronal calcium channels. Neuron 20: 1027–1038. [DOI] [PubMed] [Google Scholar]
- 29. Hanlon MR, Berrow NS, Dolphin AC, Wallace BA (1999) Modelling of a voltage-dependent Ca2+ channel beta subunit as a basis for understanding its functional properties. FEBS Lett 445: 366–370. [DOI] [PubMed] [Google Scholar]
- 30. te Velthuis AJ, Admiraal JF, Bagowski CP (2007) Molecular evolution of the MAGUK family in metazoan genomes. BMC Evol Biol 7: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen YH, Li MH, Zhang Y, He LL, Yamada Y, et al. (2004) Structural basis of the alpha1-beta subunit interaction of voltage-gated Ca2+ channels. Nature 429: 675–680. [DOI] [PubMed] [Google Scholar]
- 32. Opatowsky Y, Chen CC, Campbell KP, Hirsch JA (2004) Structural analysis of the voltage-dependent calcium channel beta subunit functional core and its complex with the alpha 1 interaction domain. Neuron 42: 387–399. [DOI] [PubMed] [Google Scholar]
- 33. Van PF, Clark KA, Chatelain FC, Minor DL Jr (2004) Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature 429: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De WM, Witcher DR, Pragnell M, Liu H, Campbell KP (1995) Properties of the alpha 1-beta anchoring site in voltage-dependent Ca2+ channels. J Biol Chem 270: 12056–12064. [DOI] [PubMed] [Google Scholar]
- 35. Ebert AM, McAnelly CA, Handschy AV, Mueller RL, Horne WA, et al. (2008) Genomic organization, expression, and phylogenetic analysis of Ca2+ channel beta4 genes in 13 vertebrate species. Physiol Genomics 35: 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ebert AM, McAnelly CA, Srinivasan A, Mueller RL, Garrity DB, et al. (2008) The calcium channel beta2 (CACNB2) subunit repertoire in teleosts. BMC Mol Biol 9: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shepard S, McCreary M, Fedorov A (2009) The peculiarities of large intron splicing in animals. PLoS One 4: e7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Currie KP (2010) G protein modulation of CaV2 voltage-gated calcium channels. Channels (Austin) 4: 497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Obermair GJ, Tuluc P, Flucher BE (2008) Auxiliary Ca(2+) channel subunits: lessons learned from muscle. Curr Opin Pharmacol 8: 311–318. [DOI] [PubMed] [Google Scholar]
- 40. Schredelseker J, Di BV, Obermair GJ, Felder ET, Flucher BE, et al. (2005) The beta 1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc Natl Acad Sci USA 102: 17219–17224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murakami M, Nakagawasai O, Yanai K, Nunoki K, Tan-No K, et al. (2007) Modified behavioral characteristics following ablation of the voltage-dependent calcium channel beta3 subunit. Brain Res 1160: 102–112. [DOI] [PubMed] [Google Scholar]
- 42. Witcher DR, De WM, Sakamoto J, Franzini-Armstrong C, Pragnell M, et al. (1993) Subunit identification and reconstitution of the N-type Ca2+ channel complex purified from brain. Science 261: 486–489. [DOI] [PubMed] [Google Scholar]
- 43. Miranda-Laferte E, Schmidt S, Jara AC, Neely A, Hidalgo P (2012) A short polybasic segment between the two conserved domains of the beta2a-subunit modulates the rate of inactivation of R-type calcium channel. J Biol Chem 287: 32588–32597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cohen RM, Foell JD, Balijepalli RC, Shah V, Hell JW, et al. (2005) Unique modulation of L-type Ca2+ channels by short auxiliary beta1d subunit present in cardiac muscle. Am J Physiol Heart Circ Physiol 288: H2363–H2374. [DOI] [PubMed] [Google Scholar]
- 45. Foell JD, Balijepalli RC, Delisle BP, Yunker AM, Robia SL, et al. (2004) Molecular heterogeneity of calcium channel beta-subunits in canine and human heart: evidence for differential subcellular localization. Physiol Genomics 17: 183–200. [DOI] [PubMed] [Google Scholar]
- 46. Harry JB, Kobrinsky E, Abernethy DR, Soldatov NM (2004) New short splice variants of the human cardiac Cavbeta2 subunit: redefining the major functional motifs implemented in modulation of the Cav1.2 channel. J Biol Chem 279: 46367–46372. [DOI] [PubMed] [Google Scholar]
- 47. Hibino H, Pironkova R, Onwumere O, Rousset M, Charnet P, et al. (2003) Direct interaction with a nuclear protein and regulation of gene silencing by a variant of the Ca2+-channel beta 4 subunit. Proc Natl Acad Sci USA 100: 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Subramanyam P, Obermair GJ, Baumgartner S, Gebhart M, Striessnig J, et al. (2009) Activity and calcium regulate nuclear targeting of the calcium channel beta4b subunit in nerve and muscle cells. Channels (Austin) 3: 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu X, Lee YJ, Holm JB, Terry MD, Oswald RE, et al. (2011) The Ca2+ channel beta4c subunit interacts with heterochromatin protein 1 via a PXVXL binding motif. J Biol Chem 286: 9677–9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Y, Yamada Y, Fan M, Bangaru SD, Lin B, et al. (2010) The beta subunit of voltage-gated Ca2+ channels interacts with and regulates the activity of a novel isoform of Pax6. J Biol Chem 285: 2527–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jangsangthong W, Kuzmenkina E, Khan IF, Matthes J, Hullin R, et al. (2010) Inactivation of L-type calcium channels is determined by the length of the N terminus of mutant beta(1) subunits. Pflugers Arch 459: 399–411. [DOI] [PubMed] [Google Scholar]
- 52. Herzig S, Khan IF, Grundemann D, Matthes J, Ludwig A, et al. (2007) Mechanism of Ca(v)1.2 channel modulation by the amino terminus of cardiac beta2-subunits. FASEB J 21: 1527–1538. [DOI] [PubMed] [Google Scholar]
- 53. Richards MW, Leroy J, Pratt WS, Dolphin AC (2007) The HOOK-domain between the SH3 and the GK domains of Cavbeta subunits contains key determinants controlling calcium channel inactivation. Channels (Austin) 1: 92–101. [DOI] [PubMed] [Google Scholar]
- 54. Spafford JD, Dunn T, Smit AB, Syed NI, Zamponi GW (2006) In vitro characterization of L-type calcium channels and their contribution to firing behavior in invertebrate respiratory neurons. J Neurophysiol 95: 42–52. [DOI] [PubMed] [Google Scholar]
- 55. Christel C, Lee A (2012) Ca2+-dependent modulation of voltage-gated Ca2+ channels. Biochim Biophys Acta 1820: 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Almagor L, Chomsky-Hecht O, Ben-Mocha A, Hendin-Barak D, Dascal N, et al. (2012) The role of a voltage-dependent Ca2+ channel intracellular linker: a structure-function analysis. J Neurosci 32: 7602–7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vitko I, Shcheglovitov A, Baumgart JP, Arias-Olguin II, Murbartian J, et al. (2008) Orientation of the calcium channel beta relative to the alpha(1)2.2 subunit is critical for its regulation of channel activity. PLoS One 3: e3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang Y, Chen YH, Bangaru SD, He L, Abele K, et al. (2008) Origin of the voltage dependence of G-protein regulation of P/Q-type Ca2+ channels. J Neurosci 28: 14176–14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bae J, Suh EJ, Lee C (2010) Interaction of T-type calcium channel Ca(V)3.3 with the beta-subunit. Mol Cells 30: 185–191. [DOI] [PubMed] [Google Scholar]
- 60. Dubel SJ, Altier C, Chaumont S, Lory P, Bourinet E, et al. (2004) Plasma membrane expression of T-type calcium channel alpha(1) subunits is modulated by high voltage-activated auxiliary subunits. J Biol Chem 279: 29263–29269. [DOI] [PubMed] [Google Scholar]
- 61. Lacerda AE, Perez-Reyes E, Wei X, Castellano A, Brown AM (1994) T-type and N-type calcium channels of Xenopus oocytes: evidence for specific interactions with beta subunits. Biophys J 66: 1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Arias JM, Murbartian J, Vitko I, Lee JH, Perez-Reyes E (2005) Transfer of beta subunit regulation from high to low voltage-gated Ca2+ channels. FEBS Lett 579: 3907–3912. [DOI] [PubMed] [Google Scholar]
- 63. Lambert RC, Maulet Y, Mouton J, Beattie R, Volsen S, et al. (1997) T-type Ca2+ current properties are not modified by Ca2+ channel beta subunit depletion in nodosus ganglion neurons. J Neurosci 17: 6621–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Leuranguer V, Bourinet E, Lory P, Nargeot J (1998) Antisense depletion of beta-subunits fails to affect T-type calcium channels properties in a neuroblastoma cell line. Neuropharmacology 37: 701–708. [DOI] [PubMed] [Google Scholar]
- 65. Senatore A, Zhorov BS, Spafford JD (2012) Cav3 T-type calcium channels. WIREs Membr Transp Signal 1: 467–491. [Google Scholar]
- 66. Fang K, Colecraft HM (2011) Mechanism of auxiliary beta-subunit-mediated membrane targeting of L-type (Ca(V)1.2) channels. J Physiol 589: 4437–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Altier C, Garcia-Caballero A, Simms B, You H, Chen L, et al. (2011) The Cavbeta subunit prevents RFP2-mediated ubiquitination and proteasomal degradation of L-type channels. Nat Neurosci 14: 173–180. [DOI] [PubMed] [Google Scholar]
- 68. Rougier JS, Albesa M, Abriel H, Viard P (2011) Neuronal precursor cell-expressed developmentally down-regulated 4-1 (NEDD4-1) controls the sorting of newly synthesized Ca(V)1.2 calcium channels. J Biol Chem 286: 8829–8838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Waithe D, Ferron L, Page KM, Chaggar K, Dolphin AC (2011) Beta-subunits promote the expression of Ca(V)2.2 channels by reducing their proteasomal degradation. J Biol Chem 286: 9598–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sadamoto H, Takahashi H, Okada T, Kenmoku H, Toyota M, et al. (2012) De novo sequencing and transcriptome analysis of the central nervous system of mollusc Lymnaea stagnalis by deep RNA sequencing. PLoS One 7: e42546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, et al. (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36: W465–W469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rice P, Longden I, Bleasby A (2000) EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16: 276–277. [DOI] [PubMed] [Google Scholar]
- 73. Senatore A, Spafford JD (2013) A uniquely adaptable pore is consistent with NALCN being an ion sensor. Channels (Austin) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Senatore A, Guan W, Boone AN, Spafford JD (2014) T-type calcium channels become sodium channels using alternate extracellular turret residues outside the selectivity filter. Journal of Biological Chemistry [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Senatore A, Boone AN, Spafford JD (2011) Optimized transfection strategy for expression and electrophysiological recording of recombinant voltage-gated ion channels in HEK-293T cells. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Berjukow S, Doring F, Froschmayr M, Grabner M, Glossmann H, et al. (1996) Endogenous calcium channels in human embryonic kidney (HEK293) cells. Br J Pharmacol 118: 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Canti C, Davies A, Berrow NS, Butcher AJ, Page KM, et al. (2001) Evidence for two concentration-dependent processes for beta-subunit effects on alpha1B calcium channels. Biophys J 81: 1439–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tareilus E, Roux M, Qin N, Olcese R, Zhou J, et al. (1997) A Xenopus oocyte beta subunit: evidence for a role in the assembly/expression of voltage-gated calcium channels that is separate from its role as a regulatory subunit. Proc Natl Acad Sci USA 94: 1703–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yasuda T, Chen L, Barr W, McRory JE, Lewis RJ, et al. (2004) Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells. Eur J Neurosci 20: 1–13. [DOI] [PubMed] [Google Scholar]
- 80. Dolphin AC (2012) The alpha(2)delta subunits of voltage-gated calcium channels. Biochim Biophys Acta [DOI] [PubMed] [Google Scholar]
- 81. Ly CV, Yao CK, Verstreken P, Ohyama T, Bellen HJ (2008) straightjacket is required for the synaptic stabilization of cacophony, a voltage-gated calcium channel alpha1 subunit. J Cell Biol 181: 157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple alignment of Cavβ subunits including those from a cnidarian (Nematostella), nematode (Caenorhabditis), ecdysozoan (Drosophila), lophotrochozoan (Lymnaea) and human gene isoforms (Cavβ1b, Cavβ2a, Cavβ3, Cavβ4b). Highly conserved SH3 and GK domains are illustrated, also conserved secondary structures (α helices and β sheets), and calcium channel (AID) binding residues (blue residues) reported in crystal structures of Cavβ subunits. Exon boundaries (red lines) are indicated. Red boxes surrounding a base indicates that the intron splits between an amino acid base.
(TIF)
Genomic sequences spanning exon 7 illustrating the alternate acceptor sites in Lymnaea snail calcium channel beta subunit, (LCavβ+ and LCavβ−) which generates a seven amino acid optional exon (7A+) or not exon (7A−). Note that skipping of exon 7 generates a change in reading frame and truncated LCavβ. The frame shift occurs because exon 6 ends within a codon after the first nucleotide (phase 1), whereas the intron preceding exon 8 is located between codons (phase 0).
(TIF)
Snail LCavβ - specific rabbit antibody localizes LCavβA+ and LCavβB+ proteins in transfected HEK cells by Western blotting. Snail LCav2 in pIRES2-EGFP vector were transfected alone (mock) or with coexpressed LCavβA+ and LCavβB+ plasmids in HEK cells. Expressed LCavβA+ and LCavβB+ proteins are identifiable on Western blots of transfected HEK cell lysates at appropriate size (62.8 kDa and 62.6 kDa), respectively.
(TIF)