Abstract
Background
Escherichia coli O157 (EcO157) infection has been recognized as an important global public health concern. But information on the prevalence of EcO157 in cattle at the global and at the wider geographical levels is limited, if not absent. This is the first meta-analysis to investigate the point prevalence of EcO157 in cattle at the global level and to explore the factors contributing to variation in prevalence estimates.
Methods
Seven electronic databases- CAB Abstracts, PubMed, Biosis Citation Index, Medline, Web of Knowledge, Scirus and Scopus were searched for relevant publications from 1980 to 2012. A random effect meta-analysis model was used to produce the pooled estimates. The potential sources of between study heterogeneity were identified using meta-regression.
Principal findings
A total of 140 studies consisting 220,427 cattle were included in the meta-analysis. The prevalence estimate of EcO157 in cattle at the global level was 5.68% (95% CI, 5.16–6.20). The random effects pooled prevalence estimates in Africa, Northern America, Oceania, Europe, Asia and Latin America-Caribbean were 31.20% (95% CI, 12.35–50.04), 7.35% (95% CI, 6.44–8.26), 6.85% (95% CI, 2.41–11.29), 5.15% (95% CI, 4.21–6.09), 4.69% (95% CI, 3.05–6.33) and 1.65% (95% CI, 0.77–2.53), respectively. Between studies heterogeneity was evidenced in most regions. World region (p<0.001), type of cattle (p<0.001) and to some extent, specimens (p = 0.074) as well as method of pre-enrichment (p = 0.110), were identified as factors for variation in the prevalence estimates of EcO157 in cattle.
Conclusion
The prevalence of the organism seems to be higher in the African and Northern American regions. The important factors that might have influence in the estimates of EcO157 are type of cattle and kind of screening specimen. Their roles need to be determined and they should be properly handled in any survey to estimate the true prevalence of EcO157.
Introduction
Enterohemorrhagic Escherichia coli O157 (EHEC O157), also known as verocytotoxin producing or shiga toxin producing EcO157, is a very important and world-wide reported food-borne pathogen. It causes hemorrhagic colitis with some severe sequelae including hemolytic uremic syndrome which is caused by the effect of shiga toxin produced by the organism that acts on kidney, intestine and other parenchymatous organs [1]. Cattle are the natural reservoir of it [1] contributing as a major source for human infections [2]–[6]. The public health concern of EcO157 came to light at first after its first outbreak reported in the USA in 1982 [7]. Although the organism does not produce any clinical illness in their natural reservoir, it can produce a broad spectrum of clinical abnormalities in humans including mild diarrhea, hemorrhagic colitis (HC), hemolytic uremic syndrome (HUS), bloody diarrhea and thrombotic thrombocytopenic purpura (TTP) [8], [9]. Harboring of EcO157 in cattle is a significant concern for public health because of their transmitting capability to humans through contaminated foods and water with feces from cattle [10]–[13]. A wide range of prevalence estimates ranging from 0.1% to 62% of EcO157 in cattle was reported worldwide [12], [14]–[19].
The inconsistent prevalence estimates of EcO157 reported in cattle in various geographical locations might be, to some extent, due to variable methodological modus operandi to identify the organism, such as sampling strategy, type of samples, enrichment procedures, immunomagnetic separation and cultural media of choice. Therefore, the factors that contribute to the variability in the detection of the organism and thus in the prevalence estimate need to be identified by analyzing the available published reports.
The aims of the study were to illustrate the prevalence estimates of EcO157 in cattle both at the global and at different geographical levels, and to generate empirical evidence on the sources/factors contributing to between study heterogeneity.
Methods
Search strategy
A systematic search strategy was used to identify all published studies reporting prevalence of EcO157 in cattle. Seven electronic databases-CAB Abstracts, PubMed, Biosis Citation Index, Medline, Web of Knowledge, Scirus and Scopus were searched for relevant studies published from 1980 to 2012. Two authors (MZI and KI) were assigned to search the databases. The search terms were adapted from Seargent et al. [20] and grouped into three categories: outcome, population and descriptive. Modified search terms are presented in Table 1.
Table 1. Algorithm for electronic database search to find published reports on prevalence of E. coli O157 in cattle.
| Search term | Boolean keywords |
| Descriptive term | Prevalence OR Incidence OR frequency OR occurrence OR Detection OR Identification OR Isolation OR characterization OR Investigation |
| Population term | Escherichia coli O157 OR E coli O157 OR O157 OR shiga toxin producing Escherichia coli O157 OR STEC O157 OR VTEC O157 OR verocytotoxin producing Escherichia coli O157 OR EHEC O157 OR Enterohaemorrhagic Escherichia coli O157 OR Enterohemorrhagic Escherichia coli O157 OR VTEC OR STEC OR EHEC OR Enterohemorragic OR Enterohaemorrhagic OR Enterohemorrhagic OR Enterohaemorragic |
| Outcome term | Cattle OR Bovine OR ruminant OR dairy OR Beef OR cow OR veal OR Calf OR calves OR heifer OR steer OR feedlot OR Bull OR Bullock OR yearling |
The Boolean operator “AND” was used to combine the categories and “OR” was used to join the terms within each category respectively. Search terms and keywords were altered as per specification of individual databases. No language restrictions were applied. The reference lists of retrieved articles were searched manually to identify all potential studies so that no articles have been missed by the electronic searches. Database search was undertaken on the 12th and 13th of February, 2013.
Inclusion and exclusion criteria
Two authors (MZI and KI) independently screened the titles and abstracts of search results to identify potential studies. Initial screening was performed according to some predefined inclusion and exclusion criteria based on research hypothesis. Full text articles were retrieved if they met the inclusion criteria.
Any article to be included in the meta-analysis had the following inclusion criteria: it had to be published between 1980 and 2012, reported with animal level prevalence data, any kind of cattle population from any place in the globe, type of specimens as intestinal content, feces, rectal swab and/or other enteric substances from cattle and the identity of bacterial isolates as EcO157 (H7 or not) by any of the recognized techniques: latex agglutination test, slide agglutination test, serotyping, and/or PCR for rfbO157 gene. Cross-sectional, case-control, longitudinal and cohort studies were eligible for inclusion. Studies were excluded if they had duplicate population group, insufficient prevalence data, farm level prevalence, pooled samples, and failing to meet the inclusion criteria mentioned above. Unpublished studies, conference abstracts, experimental and intervention studies were not included in this meta-analysis.
Data extraction
A pretested data extraction spreadsheet was developed and evaluated (File S1). Full text articles were screened independently by two authors (MZI and KI) and data were also extracted independently by them. Any disagreements between the two authors were resolved by consensus and/or cross-checking with a third author (SA). Data were extracted on first author, study location, year of publication, prevalence of EcO157, study date, study population, type of specimens, origin of sampled cattle, type of cattle, health status of animal, methods of pre-enrichment, isolation media and methods of confirmation. The characteristics of the studies included are mentioned in File S2.
Data analysis
All the data were analyzed using STATA 12.0 (StataCorp LP, College Station, Texas, USA). Between study variations in the prevalence of EcO157 in cattle were estimated using Chi-square test to evaluate whether the variation between studies exceeds the expected by chance and calculating I2 statistic which represents the proportion of total variation in effect estimates across the studies attributable to heterogeneity rather than by chance [21]. Only crude estimates of prevalence were used in this study. Prevalence was estimated by the number of cases divided by the total number of cattle in the sample, and expressed as a percent. The 95% confidence intervals (CI) were calculated using the standard formula for a proportion: p±1.96*sqrt[p*(100-p)/n]. In cases where the lower limit of the 95% CI was negative, we set the value to zero to avoid negative prevalence. The point estimates from separate studies were pooled using a random effect meta-analysis model. The meta-analysis was performed using STATA command ‘metan’ specifying random. The study estimates were combined by applying the DerSimonian-Laird random effects method [22]. The random effect model was selected because of high degree of heterogeneity (I2>75%) between the studies. Potential sources of between study heterogeneity were recognized from a group of possibly related variables by using a meta-regression model. Eight potential sources of heterogeneity were examined: region (world region), specimens (rectal swab/feces from rectum/feces from intestine/mixed type/voided feces), origin of sampled cattle (animal pen/others/slaughter house), type of cattle (dairy/beef cattle/others/feedlot), health status (healthy/not mentioned/diseased/mixed type), pre-enrichment (with inhibitors/others/without inhibitors/no pre-enrichment), immunomagnetic separation (yes/no/others) and isolation media (CT-SMAC, CHROMagar O157, MacConkey, SMAC, others). Initially a univariable meta-regression model was built to examine the association between a selected variable and prevalence of EcO157 in cattle separately. A random effect multivariable meta-regression model was built to assess the integrated association of prevalence with the variables. All the variables were tested separately in univariable analysis to identify their contribution to between study heterogeneity. Variables with p<0.2 in the univariable analysis were included into the multivariable model. The variable ‘region’ was at first entered into the multivariable meta-regression model. Manual forward selection, starting with variables that were more strongly associated with prevalence in univariable analyses, was applied and variables were retained in the multivariable model if p<0.2.The variables that were highly significant (p<0.05) in multivariable meta-regression model were considered as building blocks for interaction terms. The extent of publication bias was assessed using a funnel plot and the sources of funnel plot asymmetry were also tested to identify the small study effects.
Maintenance of study standard
The study was conducted following the guidelines for reporting meta-analysis of observational studies (MOOSE Statement) [23]. In addition, Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements [24] and PRISMA 2009 checklist (Checklist S1) were followed to maintain the study standard. There is no published protocol for this meta-analysis. We did not assess the quality of individual study, because this meta-analysis was based on observational findings of prevalence studies and was not proper for quantitative synthesis, but we assessed publication bias that may affect the cumulative evidence.
Results
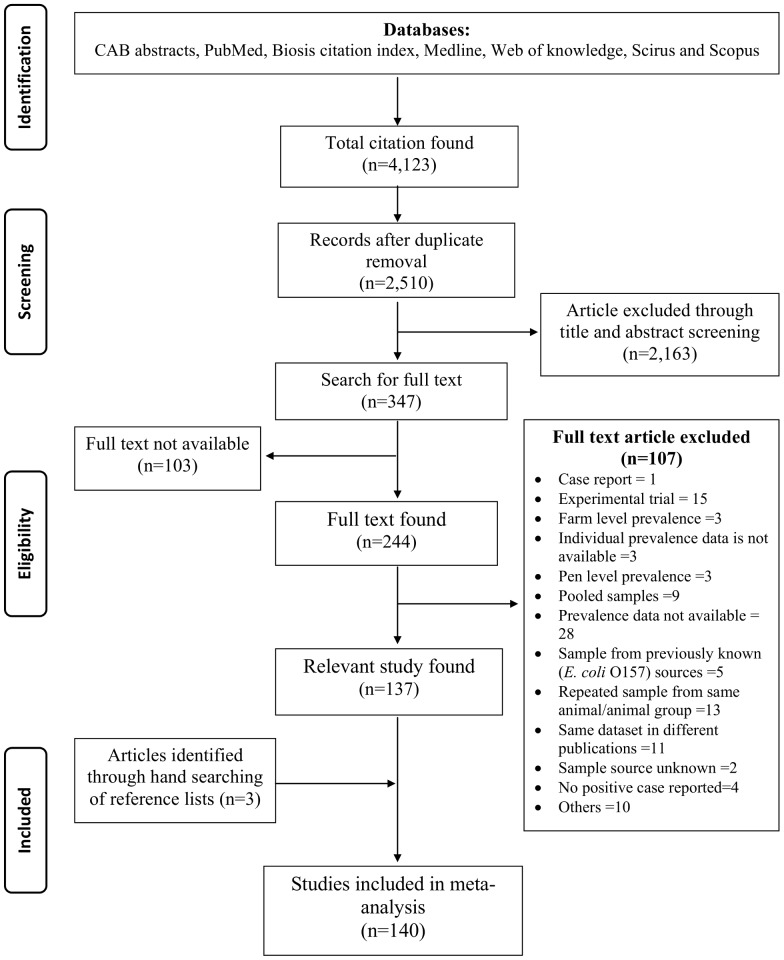
In the initial search, 2,510 potentially relevant studies were identified. After primary screening of titles and abstracts, 347 articles were selected for full text search. Among them 244 full text articles were retrieved to check the eligibility, of which 137 were included (Figure 1). Additional three eligible studies were identified by hand searches of reference lists of the selected articles. Therefore, finally, a total of 140 articles were included in the meta-analysis (File S3). Lists of excluded full text articles along with the reasons for their exclusion are provided in “File S4”. Description of characteristics of each study reporting the prevalence of EcO157 in cattle is shown in “File S2”.
Figure 1. Flow diagram of study selection for inclusion in the meta-analysis.
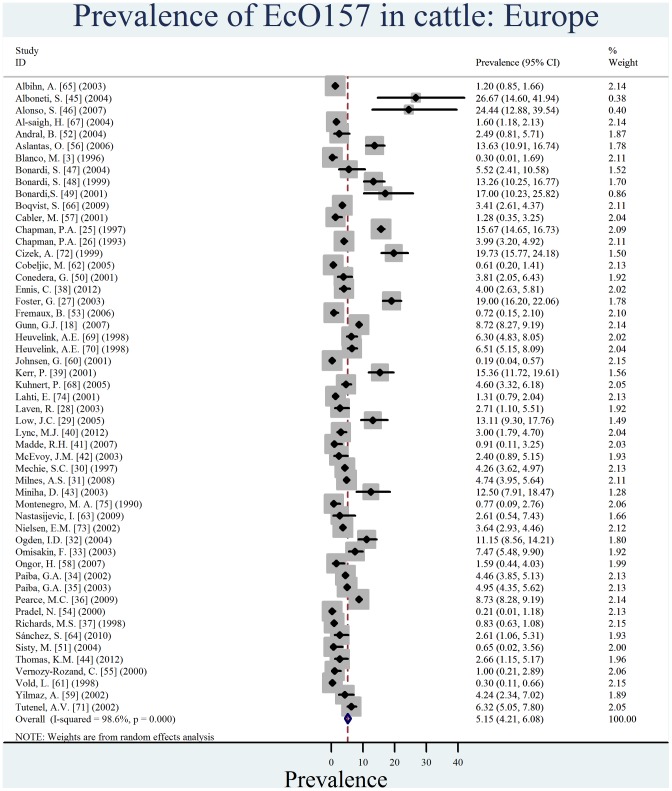
All the studies included represent data from 38 countries across the globe. The highest number (53) of studies (n = 88,643) was reported from Europe covering 16 countries. In Europe, 14 studies were from the United Kingdom [18], [25]–[37], seven from each of Ireland [38]–[44] and Italy [45]–[51], four from each of France [52]–[55] and Turkey [56]–[59], two from each of Norway [60], [61], Serbia [62], [63], Spain [3], [64], Sweden [65], [66], Switzerland [67], [68] and the Netherlands [69], [70], and one from each of Belgium [71], Czeck Republic [72], Denmark [73], Finland [74] and Germany [75].
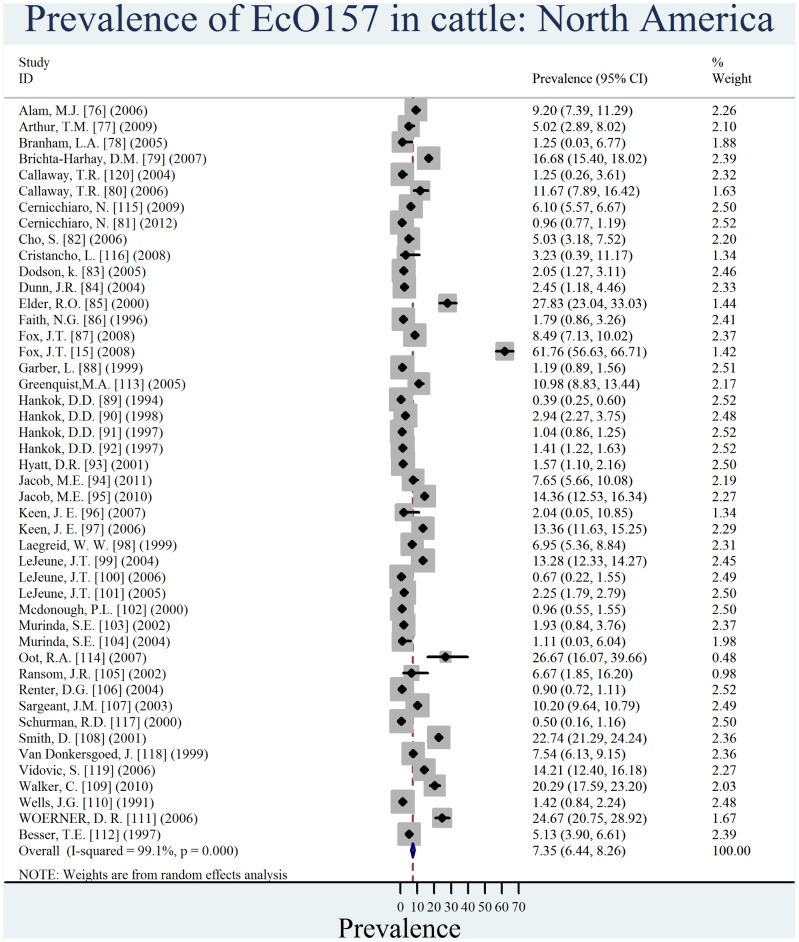
The second highest number (46) of studies (n = 110,641) was from Northern America.
Among the Northern American studies 40 were from the USA [15], [76]–[114], five from Canada [115]–[119] and one was from Mexico [120].
A total of 22 studies (n = 14,916) was identified in Asia, from 11 countries: eight were from Japan [121]–[128], three from India [129]–[131], two from each of South Korea [132], [133] and Thailand [134], [135], and one from each of Bangladesh [136], China [137], Hong Kong [138], Iran [139], Jordan [140], Taiwan [14], and Vietnam [141].
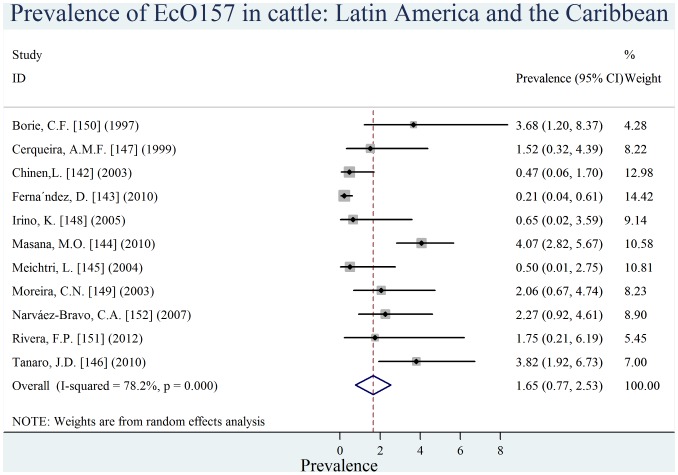
In total, 11 studies (n = 4,313) were reported from Latin America and Caribbean representing five countries. Among them, five were found from Argentina [142]–[146], three from Brazil [147]–[149], and one from each of Chile [150], Peru [151] and Venezuela [152].
Only four studies were identified from each of Africa (n = 626) and Oceania (n = 1,288) representing two and one countries, respectively. In Africa two studies were from each of Nigeria [153], [154] and South Africa [155], [156]. In Oceania, all the four studies were reported from Australia [157]–[160].
Prevalence of E. coli O157 in cattle
At the global level the estimated prevalence of EcO157 in cattle ranged from 0.13% (95% CI, 0.04–0.33) [14] to 61.77% (95% CI, 56.63–66.71) [15] with substantial heterogeneity (I 2 = 98.7% P<0.001). The random effect estimated pooled prevalence at the global level was 5.68% (95% CI, 5.16–6.20). Overall and stratified pooled prevalence estimates of EcO157 in cattle by world region are presented in Table 2.
Table 2. Estimated pooled prevalence of E. coli O157 in cattle by world region.
| World region | No. study | No. Cattle sampled | No. Positive cattle | Pooled estimate (%) | 95% CI | Heterogeneity chi-squared (χ2) | I2 (%) | P-value |
| Global estimate | 140 | 220,427 | 12,683 | 5.68 | 5.16–6.20 | 98.7 | <0.001 | |
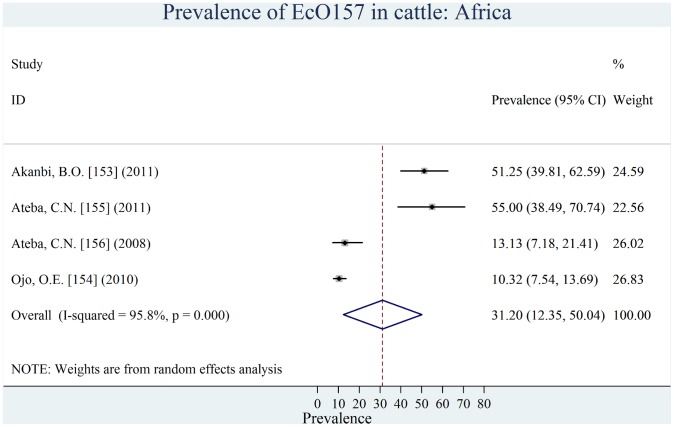
| Africa | 4 | 626 | 118 | 31.20 | 12.35–50.04 | 71.42 | 95.8 | <0.001 |
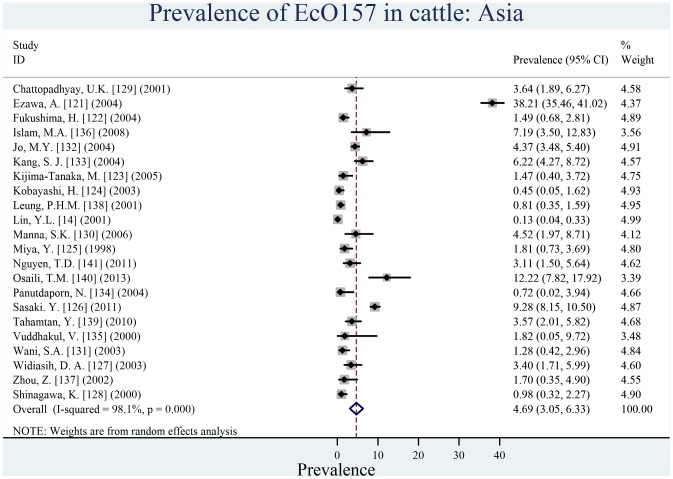
| Asia | 22 | 14,916 | 937 | 4.69 | 3.05–6.33 | 1108.3 | 98.1 | <0.001 |
| Europe | 53 | 88,643 | 5,425 | 5.15 | 4.21–6.09 | 3720.36 | 90.6 | <0.001 |
| Latin America & Caribbean | 11 | 4,313 | 73 | 1.65 | 0.77–2.53 | 45.97 | 78.2 | <0.001 |
| Northern America | 46 | 110,641 | 6,059 | 7.35 | 6.44–8.26 | 5187.73 | 99.1 | <0.001 |
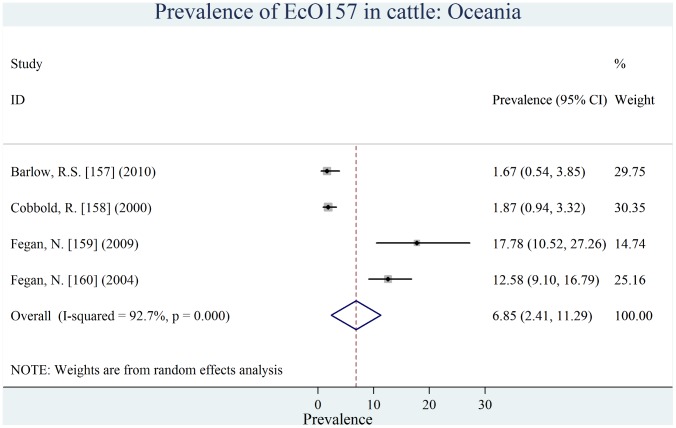
| Oceania | 4 | 1,288 | 71 | 6.85 | 2.41–11.29 | 41.15 | 92.7 | 0.002 |
Individual estimates of prevalence from contributing studies according to world region are outlined in Figures 2, 3, 4, 5, 6, 7. There was a wide regional variation in the prevalence of EcO157 in cattle, ranging from 1.65% (95% CI, 0.77–2.53) in Latin America and Caribbean to 31.20% (95% CI, 12.35–50.04) in Africa.
Figure 2. Forest plot of prevalence of E. coli O157 in cattle amongst studies conducted in Africa.
(In all forest plots, the gray square around the dot represents the contribution of each study (weight) to the meta-analysis and the center dot represents point estimate).
Figure 3. Forest plot of prevalence of E. coli O157 in cattle amongst studies conducted in Oceania.
Figure 4. Forest plot of prevalence of E. coli O157 in cattle amongst studies conducted in Asia.
Figure 5. Forest plot of prevalence of E. coli O157 in cattle amongst studies conducted in Latin America and Caribbean.
Figure 6. Forest plot of prevalence of E. coli O157 in cattle amongst studies conducted in Europe.
Figure 7. Forest plot of prevalence of E. coli O157 in cattle amongst studies conducted in North America.
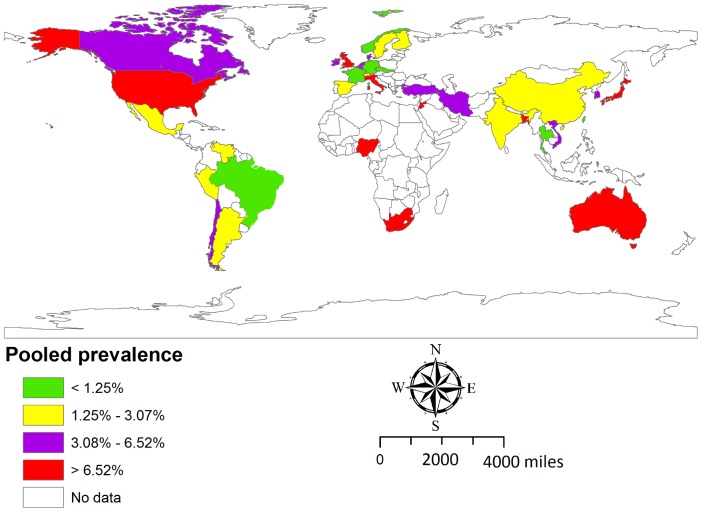
The prevalence of EcO157 in cattle was also varied in countries of different world region. The estimates of adjusted prevalence of EcO157 in cattle in different countries are shown in Figure 8, by quartiles of prevalence.
Figure 8. Estimated prevalence of E. coli O157 in cattle in different countries.
The prevalence is based on a meta-analysis of 140 studies comprising 220,427 cattle from different production system. Regional adjusted prevalence is denoted by different colors and it represents the quartile distribution of prevalence by country.
Sources of heterogeneity
In the prevalence of EcO157 in cattle four sources of heterogeneity were found in univariable meta-regression. They were world region (p<0.001), type of cattle (p<0.001), pre-enrichment (p = 0.027) and immunomagnetic separation (p = 0.024). The associations of the variables specimen (p = 0.066), health status (p = 0.080) and isolation media (p = 0.096) were borderline significant (Table 3). In the multivariable meta-regression model three variables world region (p<0.001), specimens (p = 0.074) and type of cattle (p<0.001) were found to be associated with the heterogeneity. An interaction term between ‘region’ and ‘type of cattle’ was added but it was not significant in the multivariable model.
Table 3. Meta-regression for prevalence of E. coli O157 in cattle.
| Variables | Covariates | No. study (N-140) | Prevalence* (95%CI) | Univariable | Multivariable | ||
| Coef. (95%CI) | P-value | Coef. (95%CI) | P-value | ||||
| World region | <0.001 | <0.001 | |||||
| Asia (Ref.) | 22 | 4.69 (3.05, 6.33) | |||||
| Africa | 4 | 31.20 (12.35, 50.04) | 23.17 (12.85, 33.49) | <0.001 | 22.37 (13.0, 31.75) | <0.001 | |
| Europe | 53 | 5.15 (4.21, 6.08) | 0.97 (−3.34, 5.28) | 0.658 | −1.01 (−5.03, 3.01) | 0.620 | |
| Latin America and Caribbean | 11 | 1.65 (0.77, 2.53) | −2.97 (−9.22, 3.28) | 0.350 | −3.67 (−9.64, 2.29) | 0.225 | |
| Northern America | 46 | 7.35 (6.44, 8.26) | 3.33 (−1.07, 7.73) | 0.137 | −0.24 (−4.57, 4.08) | 0.911 | |
| Oceania | 4 | 6.85 (2.41, 11.29) | 3.09 (−6.32, 12.50) | 0.517 | −0.99 (−9.56, 7.59) | 0.820 | |
| Specimens | 0.066 | 0.074 | |||||
| Rectal swab (Ref.) | 24 | 3.33 (2.24, 4.42) | |||||
| Feces from rectum | 55 | 6.67 (5.51, 7.82) | 4.24 (−0.16, 8.63) | 0.059 | −0.02 (−4.06, 4.02) | 0.992 | |
| Feces from intestine | 7 | 12.25 (4.48, 20.02) | 8.72 (0.77, 16.67) | 0.032 | 6.83 (−0.20, 13.87) | 0.057 | |
| Mixed type | 13 | 8.77 (7.12, 10.41) | 7.07 (0.87, 13.26) | 0.026 | 3.91 (−1.72, 9.53) | 0.171 | |
| voided feces | 41 | 5.39 (4.62, 6.18) | 2.07 (−2.53, 6.67) | 0.375 | −0.95 (−5.13, 3.23) | 0.653 | |
| Origin of sampled cattle | 0.321 | ||||||
| Animal pen (Ref.) | 70 | 5.12 (4.45, 5.79) | |||||
| Others | 22 | 5.25 (3.27, 7.23) | −0.90 (−5.37, 3.57) | 0.692 | |||
| Slaughter house | 48 | 7.10 (5.96, 8.24) | 2.21 (−1.25, 5.67) | 0.208 | |||
| Type of cattle | <0.001 | <0.001 | |||||
| Dairy (Ref.) | 18 | 1.75 (1.26, 2.24) | |||||
| Beef cattle | 14 | 6.84 (4.03, 9.65) | 4.96 (−0.77, 10.69) | 0.089 | 2.07 (−3.65, 7.79) | 0.476 | |
| Others | 96 | 4.85 (4.29, 5.41) | 4.04 (−0.08, 8.17) | 0.055 | 0.96 (−3.23, 5.16) | 0.650 | |
| Feedlot | 12 | 19.58 (15.57, 23.59) | 17.77 (11.65, 23.89) | <0.001 | 15.57 (9.54, 21.61) | <0.001 | |
| Health status | 0.080 | ||||||
| Healthy (Ref.) | 18 | 2.62 (1.40, 3.84) | |||||
| Not mentioned | 113 | 6.55 (5.94, 7.16) | 5.01 (0.44, 9.57) | 0.032 | |||
| Diseased | 3 | 1.34 (0.34, 2.35) | −0.99 (−12.16, 10.18) | 0.860 | |||
| Mixed type | 6 | 2.55 (0.81, 4.29) | .054(−8.41, 8.52) | 0.990 | |||
| Pre-enrichment | 0.027 | 0.110 | |||||
| With inhibitors (Ref.) | 80 | 7.82 (6.83, 8.80) | |||||
| Others | 9 | 5.64 (3.62, 7.66) | −1.12 (−7.49, 5.25) | 0.729 | 4.79 (−2.50, 12.07) | 0.195 | |
| Without inhibitors | 35 | 3.92 (3.04, 4.80) | −3.64 (−7.28, 0.01) | 0.051 | −3.15 (−5.33, 0.04) | 0.053 | |
| No pre-enrichment | 15 | 1.42 (0.79, 2.06) | −6.91 (−11.92, −1.89) | 0.007 | −0.37 (−5.91, 5.17) | 0.894 | |
| IMS | 0.024 | 0.388 | |||||
| Yes (Ref.) | 90 | 7.67 (6.79, 8.54) | |||||
| Others | 6 | 1.41 (0.84, 1.97) | −5.09 (−12.60, 2.43) | 0.183 | −5.82 (−14.48, 2.83) | 0.185 | |
| No IMS | 44 | 2.41 (1.89, 2.92) | −4.33 (−7.64, −1.02) | 0.011 | −1.16 (−4.85, 2.53) | 0.535 | |
| Isolation media | 0.096 | 0.289 | |||||
| CT-SMAC (Ref.) | 91 | 6.93 (6.24, 7.62) | |||||
| CHROMagar O157 | 8 | 7.10 (2.76, 11.43) | −0.66 (−7.45, 6.13) | 0.848 | −4.03 (−9.91, 1.86) | 0.178 | |
| MacConkey | 6 | 1.34 (0.42, 2.27) | −6.36 (−13.94, 1.22) | 0.099 | −1.72 (−8.99, 5.56) | 0.641 | |
| SMAC | 13 | 2.22 (1.30, 3.15) | −5.17 (−10.51, 0.17) | 0.058 | −5.04 (−10.15, 0.07) | 0.053 | |
| Others | 22 | 3.93 (2.39, 5.47) | −4.06 (−8.34, 0.22) | 0.063 | −2.50 (−6.70, 1.71) | 0.242 | |
*Estimated prevalence was calculated separately, Coef. = Regression coefficient, Ref. = Reference category, IMS = Immunomagnetic separation.
Studies in which the type of cattle was feedlot animal had significantly (p<0.001) higher prevalence of EcO157 compared with the studies that surveyed on other types of animal. It was evidenced in multivariable model that studies conducted in Africa had significantly (p<0.001) higher prevalence compared with other world regions.
Discussion
This meta-analysis was based on a large number of cattle (220,427) derived from 140 studies representing 38 countries across the world, enabling us to assess reliable prevalence estimates of EcO157 at the global level.
To the best of our knowledge, this is the first meta-analysis of prevalence of EcO157 in cattle at the global level and the results indicate that the prevalence of EcO157 in cattle at the global level might be 5.68% (95% CI, 5.16–6.20), although the estimates varied ranging from 0.13% (95%CI, 0.04–0.33) [14] to 61.77% (95% CI, 56.63–66.71) [15]. The highest prevalence estimate (31.20%) was in African cattle and the estimates from each of the four studies from Africa [153]–[156] was comparably high, although each of two of them was based on the investigation of a sample size of only 120 cattle [153], [155].
On the other hand, a very low prevalence (1.65%) was from Latin America and Caribbean cattle [157]–[160]. A variable degree in prevalence was reported from Asia (4.69%), Europe (5.15%), Oceania (6.85%) and Northern America (7.35%). Compared with other countries in Asia, cattle in Jordan had the highest prevalence (12.22%, 95% CI, 7.82–17.92) [140] and the lowest (0.13%, 95% CI, 0.04–0.33) was estimated in Taiwan [14].
In Europe, the highest estimated prevalence was reported from Italy (10.45%, 95% CI, 5.30–15.61) [45]–[51] and the lowest from Norway (0.25%, 95% CI, 0.06–0.42) [60], [61].
Northern America was well represented in this study with 40 studies from the USA and the prevalence estimate was higher (7.60%) in this country compared with Canada and Mexico. The diverse prevalence estimates of EcO157 in cattle among the studies of different world regions might be due to reflections of geographical variations, or attributable to underlying risk factors.
Strong evidence was found leading to the variation in the prevalence of EcO157 in cattle. Meta-regression model enabled us to evaluate the impact of both methodological differences and some other factors on the prevalence estimates of EcO157 in cattle. About 46% between study heterogeneity was explained by the final multivariable model, indicating that other biological and/or methodological factors are responsible for the remaining between study variance. The residual heterogeneity was 98%. The Joint test for all covariates (P<0.001) also showed evidence for an association of covariates with the prevalence of EcO157. The interaction between variables ‘world region’ and ‘type of cattle’ was included as an interaction term in multivariable meta-regression model but this interaction term was found non-significant (p>0.20). The study revealed that type of cattle (dairy/beef/feedlot/others) plays a vital role in the variation of regional prevalence (Table 3). This finding is supported by Jeon et al. [161] who stated that the level of EcO157 carriage in cattle is influenced by many animal related factors. In this study the prevalence of EcO157 was estimated to be 19.58% (95% CI, 15.57–23.59) in feedlot cattle, substantially higher to the level (1.75%; 95% CI, 1.26–2.24) estimated in dairy cattle. The types of specimen collected from cattle were also responsible for the variability in the estimates. There were several types of specimen used to isolate the organism namely rectal swab, feces from intestine, feces from rectum, voided feces and mixed types. When feces were collected directly from the intestine then the estimates were higher compared to other types of specimen investigated. On the other hand, diversity in the methodological steps followed in the isolation of the organism, especially pre-enrichment and type of isolation media was found responsible for the global variation in the prevalence of EcO157 in cattle. In relation to the methods of pre-enrichment, the highest estimated prevalence (7.82%) was found in the studies in which specific inhibitors were used compared to other pre-enrichment methods. In addition, the amount of specimen matrix, particularly feces could be an important factor to influence the detection rate of EcO157 in cattle [79]. This factor might also have a role in the sources of between study heterogeneity, but we couldn't include it (amount of feces) in this meta-analysis because not feces but rectal swabs or swabs plus feces, in which the amounts of investigated matrix could not be estimated, were also the primary samples used in many studies. Similarly, a higher level of prevalence (7.67%) was reported where IMS techniques were employed. Season is a potential explanatory variable in the prevalence of EcO157 in cattle [32], [45], [65], [76], [77]. This was also not included in this study because of the limitation of data sources and different seasonal parameters used in different studies.
However, the outputs of meta-regression need to be explained with caution. Some covariates were reported in a few studies, for example, the covariate ‘disease’ was found only in three studies [37], [131], [141], shrinking the association detection power of meta-regression. Furthermore, some categories were explored as sources of heterogeneity between studies but were classified as ‘others’ or ‘unknown’, thereby failing to give sufficient information. Residual confounding is also an important issue to consider when dealing with observational studies.
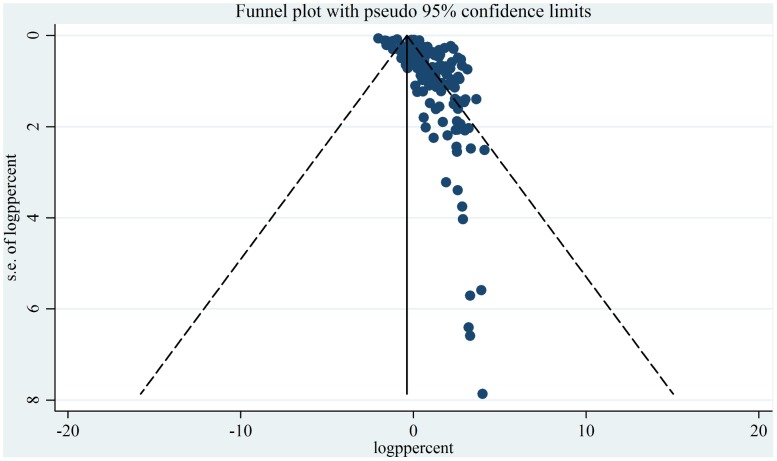
The extent of publication bias in the selected studies was measured and demonstrated by the funnel plot (Figure 9). It is clearly not symmetrical and some of the points fall outside of the funnel, indicating publication bias. The sources of the funnel plot asymmetry were tested by Egger test [162], the result of which confirmed the small study effects. The estimated bias co-efficient was 6.79 with a standard error of 0.084 providing a p-value of <0.001. Thus the test demonstrates strong evidence to the presence of small study effects. However, there are many different possible factors for funnel plot asymmetry, namely selection bias, true heterogeneity, data irregularities, artifact as well as by chance [162].
Figure 9. Funnel plot for examination of publication bias.
(ppercent, prevalence percent; se, standard error).
There are some limitations in this study. Firstly, there were a very few reports from some world regions, especially Africa and Oceania. Thus a true reflection of the prevalence from these regions could not be obtained. Furthermore, all the four studies from Oceania are in fact from Australia [157]–[160] and out of the 53 countries in Africa only two: Nigeria [153], [154] and South Africa [155], [156] are represented in this study with two studies from each of them.
Secondly, most of the studies drew samples from large commercial cattle production system and none of the studies reported any prevalence in backyard or smallholdings' cattle population which occupy a large proportion of the total cattle population in the developing world, especially in Asia and Africa.
Thirdly, unpublished studies, conference abstract, experimental and intervention studies were not included in this meta-analysis though it could bring some more publications. Besides, the authors of this study could not find 103 full text papers even after contacting the corresponding authors of all these studies. Additionally, some other potential factors like, age dependent factors and seasonal pattern in the prevalence are to be included in the study to explore their effects on the regional estimates. Due to unavailability of data these factors could not be evaluated in this study. Finally, a significant heterogeneity between studies was detected even within a particular region.
In conclusion, the prevalence of EcO157 in cattle at the global level seems to be 5.68%. The random effects pooled prevalence estimates of it in Africa, Northern America, Oceania, Europe, Asia and Latin America-Caribbean are likely to be 31.20%, 7.35%, 6.85%, 5.15%, 4.69% and 1.65%, respectively, although between studies heterogeneity was evidenced in most of these world regions. Excluding the regional, other factors that might have roles in the variation of EcO157 prevalence estimates include kind of screening specimen matrix, type of cattle, and probably method of pre-enrichment applied for the organism. Their precise roles in varying the detection level of the organism need to be investigated in laboratory to suggest a better methodology to undertake any prevalence study for EcO157 in future.
Acknowledgments
The authors would like to acknowledge WHO for HINARI Program which have enabled developing countries to gain access to biomedical literature database. They would like to thank Himel Barua to locate some full text articles. They would like to thank many other authors who supplied their paper by email.
Supporting Information
PRISMA checklist.
(DOC)
A pretested data extraction spreadsheet.
(XLS)
Description of studies reporting prevalence of E. coli O157 in cattle.
(DOC)
List of papers included in this meta-analysis.
(DOC)
List of excluded full text paper with proper justification.
(DOC)
Funding Statement
There was no external funding source. The research was conducted as departmental extension work. Therefore, The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gyles CL (2007) Shiga toxin-producing Escherichia coli: an overview. J Anim Sci 85: E45–E62. [DOI] [PubMed] [Google Scholar]
- 2. Dorn CR, Angrick EJ (1991) Serotype O157:H7 Escherichia coli from bovine and meat sources. J Clin Microbiol 29: 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blanco M, Blanco JE, Blanco J, Gonzalez EA, Mora A, et al. (1996) Prevalence and characteristics of Escherichia coli serotype O157:H7 and other verotoxin-producing E. coli in healthy cattle. Epidemiol Infect 117: 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heuvelink A, Schulten S, Hoenderken R, Bijker P, de Boer E (1996) Verocytotoxin-producing Escherichia coli O157 in Dutch veal calves and beef cattle. Tijdschr Diergeneeskd 121: 642–646. [PubMed] [Google Scholar]
- 5. Besser TE, Hancock DD, Pritchett LC, McRae EM, Rice DH, et al. (1997) Duration of detection of fecal excretion of Escherichia coli O157:H7 in cattle. J Infect Dis 175: 726–729. [DOI] [PubMed] [Google Scholar]
- 6. Toth I, Schmidt H, Kardos G, Lancz Z, Creuzburg K, et al. (2009) Virulence genes and molecular typing of different groups of Escherichia coli O157 strains in cattle. Appl Environ Microbiol 75: 6282–6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gregory L, Armstrong, Hollingsworth J, Morris JG (1996) Emerging foodborne pathogens: Escherichia coli O157:H7 as a model of entry of a new pathogen into the food supply of the developed world, Epidemiol Rev. 18: 29–51. [DOI] [PubMed] [Google Scholar]
- 8. Fey PD, Wickert RS, Rupp ME, Safranek TJ, Hinrichs SH (2000) Prevalence of non-O157:H7 Shiga toxin-producing Escherichia coli in diarrheal stool samples from Nebraska. Emerging Infect Dis 6: 530–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karmali MA (1989) Infection by verotoxin-producing Escherichia coli . Clin Microbiol Rev 2: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mead PS, Griffin PM (1998) Escherichia coli O157:H7. Lancet 352: 1207–1212. [DOI] [PubMed] [Google Scholar]
- 11. Public Health Agency of Canada (2000) Waterborne outbreak of gastroenteritis associated with a contaminated municipal water supply, Walkerton, Ontario, May–June 2000. Can Commun Dis Rep 26: 170–173. [PubMed] [Google Scholar]
- 12. Pennington H (2010) Escherichia coli O157. Lancet 376: 1428–1435. [DOI] [PubMed] [Google Scholar]
- 13. Cooley M, Carychao D, Crawford-Miksza L, Jay MT, Myers C, et al. (2007) Incidence and tracking of Escherichia coli O157:H7 in a major produce production region in California. PLoS ONE 2(11): e1159 10.1371/journal.pone.0001159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin YL, Chou C, Pan T (2001) Screening procedure from cattle feces and the prevalence of Escherichia coli O157:H7 in Taiwan dairy cattle. J Microbiol Immunol Infect 34: 17–24. [PubMed] [Google Scholar]
- 15. Fox JT, Shi X, Nagaraja TG (2008) Escherichia coli O157 in the rectoanal mucosal region of cattle. Foodborne Pathog Dis 5: 69–77. [DOI] [PubMed] [Google Scholar]
- 16. Hussein HS, Bollinger LM (2005) Prevalence of shiga toxin-producing Escherichia coli in beef cattle. J Food Prot 68: 2224–2241. [DOI] [PubMed] [Google Scholar]
- 17. Woerner DJ, Ransom R, Sofos JN, Dewell GA, Smith GC, et al. (2006) Determining the prevalence of Escherichia coli O157 in cattle and beef from the feedlot to the cooler. J Food Prot 69: 2824–2827. [DOI] [PubMed] [Google Scholar]
- 18. Gunn GJ, McKendrick IJ, Ternent HE, Thomson-Carter F, Foster G, et al. (2007) An investigation of factors associated with the prevalence of verocytotoxin producing Escherichia coli O157 shedding in Scottish beef cattle. Vet J 174: 554–564. [DOI] [PubMed] [Google Scholar]
- 19. Reinstein SJ, Fox T, Shi X, Alam MJ, Renter DG, et al. (2009) Prevalence of Escherichia coli O157:H7 in organically- and naturally-raised beef cattle. Appl Environ Microbiol 75: 5421–5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sargeant JM, Amezcua MR, Rajic A, Waddell L (2007) Pre-harvest interventions to reduce the shedding of E. coli O157 in the faeces of weaned domestic ruminants: a systematic review. Zoonoses Public Health 54: 260–277. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Controlled Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 23. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) the PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097 10.1371/journal.pmed1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chapman PA, Siddons CA, Malo ATC, Harkin MA (1997) A 1-year study of Escherichia coli O157 in cattle, sheep, pigs and poultry. Epidemiol Infect 119: 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chapman PA, Siddons CA, Wright DJ, Norman P, Fox J, et al. (1993) Cattle as a possible source of verocytotoxin-producing Escherichia coli O157 infections in man. Epidemiol Infect 111: 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Foster G, Hopkins GF, Gunn GJ, Ternent HE, Thomson-Carter F, et al. (2003) A comparison of two pre-enrichment media prior to immunomagnetic separation for the isolation of E. coli O157 from bovine faeces. J Appl Microbiol 95: 155–159. [DOI] [PubMed] [Google Scholar]
- 28. Laven RA, Ashmore A, Stewart CS (2003) Escherichia coli in the rumen and colon of slaughter cattle, with particular reference to E. coli O157. Vet J 165: 78–83. [DOI] [PubMed] [Google Scholar]
- 29. Low JC, McKendrick IJ, McKechnie C, Fenlon D, Naylor SW, et al. (2005) Rectal carriage of enterohemorrhagic Escherichia coli O157 in slaughtered cattle. Appl Environ Microbiol 71: 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mechie SC, Chapman PA, Siddons CA (1997) A fifteen month study of Escherichia coli O157:H7 in a dairy herd. Epidemiol Infect 118: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Milnes AS, Stewart I, Clifton-Hadley FA, Davies RH, Newell DG, et al. (2008) Intestinal carriage of verocytotoxigenic Escherichia coli O157, Salmonella, thermophilic Campylobacter and Yersinia enterocolitica, in cattle, sheep and pigs at slaughter in Great Britain during 2003. Epidemiol Infect 136: 739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ogden ID, MacRae M, Strachan NJC (2004) Is the prevalence and shedding concentrations of E. coli O157 in beef cattle in Scotland seasonal? FEMS Microbiol Lett 233: 297–300. [DOI] [PubMed] [Google Scholar]
- 33. Omisakin F, MacRae M, Ogden ID, Strachan NJC (2003) Concentration and prevalence of Escherichia coli O157 in cattle feces at slaughter. Appl Environ Microbiol 69: 2444–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paiba GA, Gibbens JC, Pascoe SJS, Wilesmith JW, Kidd SA, et al. (2002) Faecal carriage of verocytotoxin-producing Escherichia coli O157 in cattle and sheep at slaughter in Great Britain. Vet Rec 150: 593–598. [DOI] [PubMed] [Google Scholar]
- 35. Paiba GA, Wilesmith JW, Evans SJ, Pascoe SJS, Smith RP, et al. (2003) Prevalence of faecal excretion of verocytotoxigenic Escherichia coli O157 in cattle in England and Wales. Vet Rec 153: 347–353. [DOI] [PubMed] [Google Scholar]
- 36. Pearce MC, Chase-Topping ME, McKendrick IJ, Mellor DJ, Locking ME, et al. (2009) Temporal and spatial patterns of bovine Escherichia coli O157 prevalence and comparison of temporal changes in the patterns of phage types associated with bovine shedding and human E. coli O157 cases in Scotland between 1998–2000 and 2002–2004. BMC Microbiol 9: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Richards MS, Corkish JD, Sayers AR, McLaren IM, Evans SJ, et al. (1998) Studies of the presence of verocytotoxic Escherichia coli O157 in bovine faeces submitted for diagnostic purposes in England and Wales and on beef carcases in abattoirs in the United Kingdom. Epidemiol Infect 120: 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ennis C, McDowell D, Bolton DJ (2012) The prevalence, distribution and characterization of Shiga toxin-producing Escherichia coli (STEC) serotypes and virulotypes from a cluster of bovine farms. J Appl Microbiol 113: 1238–1248. [DOI] [PubMed] [Google Scholar]
- 39. Kerr P, Finlay D, Thomson-Carter F, Ball HJ (2001) A comparison of a monoclonal antibody-based sandwich ELISA and immunomagnetic bead selective enrichment for the detection of Escherichia coli O157 from bovine faeces. J Appl Microbiol 91: 933–936. [DOI] [PubMed] [Google Scholar]
- 40. Lynch MJ, Fox EM, O′Connor L, Jordan K, Murphy M (2012) Surveillance of verocytotoxigenic Escherichia coli in Irish bovine dairy herds. Zoonoses Public Health 59: 264–271. [DOI] [PubMed] [Google Scholar]
- 41. Madden RH, Murray KA, Gilmour A (2007) Carriage of four bacterial pathogens by beef cattle in Northern Ireland at time of slaughter. Lett Appl Microbiol 44: 115–119. [DOI] [PubMed] [Google Scholar]
- 42. McEvoy JM, Doherty AM, Sheridan JJ, Thomson-Carter FM, Garvey P, et al. (2003) The prevalence and spread of Escherichia coli O157:H7 at a commercial beef abattoir. J Appl Microbiol 95: 256–266. [DOI] [PubMed] [Google Scholar]
- 43. Minihan D, O′Mahony M, Whyte P, Collins JD (2003) An investigation on the effect of transport and lairage on the faecal shedding prevalence of Escherichia coli O157 in cattle. J Vet Med B Infect Dis Vet Public Health 50: 378–382. [DOI] [PubMed] [Google Scholar]
- 44. Thomas KM, McCann MS, Collery MM, Logan A, Whyte P, et al. (2012) Tracking verocytotoxigenic Escherichia coli O157, O26, O111, O103 and O145 in Irish cattle. Int J Food Microbiol 153: 288–296. [DOI] [PubMed] [Google Scholar]
- 45. Albonetti S, Trevisani M, Alonso-Alveraz S, Rosmini R (2004) Detection of Escherichia coli serotypes 0157 in beef carcasses and faecal material. Vet Res Commun 28: 249–251. [DOI] [PubMed] [Google Scholar]
- 46. Alonso S, Azucena M, Blanco M, Blanco JE, Dahbi G, et al. (2007) Fecal carriage of Escherichia coli O157:H7 and carcass contamination in cattle at slaughter in northern Italy. Int Microbiol 10: 109–116. [PubMed] [Google Scholar]
- 47. Bonardi S, Foni E, Brindani F, Bacci C, Chiapponi C, et al. (2004) Detection and characterization of verocytotoxin-producing Escherichia coli (vtec) O157 and non-O157 in cattle at slaughter. New Microbiol 27: 255–261. [PubMed] [Google Scholar]
- 48. Bonardi SE, Bottarellia MA, Pacciarinib ML, Ansuinib A, Vellinic G, et al. (1999) Isolation of Verocytotoxin-producing Escherichia coli O157:H7 from cattle at slaughter in Italy. Vet Microbiol 67: 203–211. [DOI] [PubMed] [Google Scholar]
- 49. Bonardi S, Maggi E, Pizzin G, Morabito S, Caprioli A (2001) Faecal carriage of Verocytotoxin-producing Escherichia coli O157 and carcass contamination in cattle at slaughter in northern Italy. Int J Food Microbiol 66: 47–53. [DOI] [PubMed] [Google Scholar]
- 50. Conedera G, Chapman PA, Marangon S, Tisato E, Dalvit P, et al. (2001) A field survey of Escherichia coli O157 ecology on a cattle farm in Italy. Int J Food Microbiol 66: 85–93. [DOI] [PubMed] [Google Scholar]
- 51. Sisti M, Benedetti C, Lonzi A, Schiavano GF, Pianetti A, et al. (2004) Isolation of Escherichia coli O157 from human and bovine faeces in the Urbino area, Italy. Int J Hyg Environ Health 207: 577–583. [DOI] [PubMed] [Google Scholar]
- 52. Andral B, Aspan A, Perelle S, Fach P (2004) PCR detection of virulence genes and molecular epidemiology of STEC O157 isolates from French abattoirs. Vet Rec 155: 365–368. [DOI] [PubMed] [Google Scholar]
- 53. Fremaux B, Raynaud S, Beutin L, Rozand CV (2006) Dissemination and persistence of Shiga toxin-producing Escherichia coli (STEC) strains on French dairy farms. Vet Microbiol 117: 180–191. [DOI] [PubMed] [Google Scholar]
- 54. Pradel N, Livrelli V, Champs CD, Palcoux JB, Reynaud A, et al. (2000) Prevalence and characterization of Shiga toxin-producing Escherichia coli isolated from cattle, food, and children during a one-year prospective study in France. J Clin Microbiol 38: 1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vernozy-Rozand C, Feng P, Montet MP, Ray-Gueniot S, Villard L, et al. (2000) Detection of Escherichia coli O157:H7 in heifers' faecal samples using an automated immunoconcentration system. Lett Appl Microbiol 30: 217–222. [DOI] [PubMed] [Google Scholar]
- 56. Aslantas Ö, Erdog S, Cantekin Z, Gulacti I, Gulsum A, et al. (2006) Isolation and characterization of verocytotoxin-producing Escherichia coli O157 from Turkish cattle. Int J Food Microbiol 106: 338–342. [DOI] [PubMed] [Google Scholar]
- 57. Cabalar M, Boynukara B, Gulhan T, Ekin IH (2001) Prevalence of rotavirus, Escherichia coli K99 and O157: H7 in healthy dairy cattle herds in Van, Turkey. Turk J Vet Anim Sci 25: 191–196. [Google Scholar]
- 58. Ongor H, Kalin R, Cetinkaya B (2007) Investigation of Escherichia coli O157 and some virulence genes in samples of meat and faeces from clinically healthy cattle in Turkey. Vet Rec 161: 392–394. [DOI] [PubMed] [Google Scholar]
- 59. Yilmaz A, Gun H, Yilmaz H (2002) Frequency of Escherichia coli O157:H7 in Turkish cattle. J Food Prot 65: 1637–1640. [DOI] [PubMed] [Google Scholar]
- 60. Johnsen G, Wasteson Y, Heir E, Berget OI, Herikstad H (2001) Escherichia coli O157:H7 in faeces from cattle, sheep and pigs in the southwest part of Norway during 1998 and 1999. Int J Food Microbiol 65: 193–200. [DOI] [PubMed] [Google Scholar]
- 61. Vold L, Klungseth Johansen B, Kruse H, Skjerve E, et al. (1998) Occurrence of shigatoxinogenic Escherichia coli O157 in Norwegian cattle herds. Epidemiol Infect 120: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cobeljic M, Dimic B, Opacic D, Lepsanovic Z, Stojanovic V, et al. (2005) The prevalence of Shiga toxin-producing Escherichia coli in domestic animals and food in Serbia. Epidemiol Infect 133: 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nastasijevic I, Mitrovic R, Buncic S (2009) The occurrence of Escherichia coli O157 in/on faeces, carcasses and fresh meats from cattle. Meat Sci 82: 101–105. [DOI] [PubMed] [Google Scholar]
- 64. Sanchez S, Martínez R, García A, Blanco J, Echeita A, et al. (2010) Shiga toxin-producing Escherichia coli O157:H7 from extensive cattle of the fighting bulls breed. Res Vet Sci 88: 208–210. [DOI] [PubMed] [Google Scholar]
- 65. Albihn A, Eriksson E, Wallen C, Aspán A (2003) Verotoxinogenic Escherichia coli (VTEC) O157:H7-a nationwide Swedish survey of bovine faeces. Acta Vet Scand 44: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Boqvist S, Aspan A, Eriksson E (2009) Prevalence of verotoxigenic Escherichia coli O157:H7 in fecal and ear samples from slaughtered cattle in Sweden. J Food Prot 72: 1709–1712. [DOI] [PubMed] [Google Scholar]
- 67. Al-Saigh H, Zweifel C, Blanco J, Blanco JE, Blanco M, et al. (2004) Fecal shedding of Escherichia coli O157, Salmonella, and Campylobacter in Swiss cattle at slaughter. J Food Prot 67: 679–684. [DOI] [PubMed] [Google Scholar]
- 68. Kuhnert P, Dubosson CR, Roesch M, Homfeld E, Doherr MG, et al. (2005) Prevalence and risk-factor analysis of Shiga toxigenic Escherichia coli in faecal samples of organically and conventionally farmed dairy cattle. Vet Microbiol 109: 37–45. [DOI] [PubMed] [Google Scholar]
- 69. Heuvelink AE, Van den Biggelaar FLAM, Boer ED, Herbes RG, Melchers WJ, et al. (1998) Isolation and characterization of verocytotoxin-producing Escherichia coli O157 strains from Dutch cattle and sheep. J Clin Microbiol 36: 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Heuvelink AE, Van den biggelaar FLAM, Zwartkruis-nahuis JTM, Herbes RG, Huyben R, et al. (1998) Occurrence of verocytotoxin-producing Escherichia coli O157 on Dutch dairy farms. J Clin Microbiol 36: 3480–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tutenel AV, Pierard D, Uradzinski J, Jozwik E, Pastuszczak M, et al. (2002) Isolation and characterization of enterohaemorrhagic Escherichia coli O157:H7 from cattle in Belgium and Poland. Epidemiol Infect 129: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cizek A, Alexa P, Literak I, Hamrik J, Novak P, et al. (1999) Shiga toxin-producing Escherichia coli O157 in feedlot cattle and Norwegian rats from a large-scale farm.". Lett Appl Microbiol 28: 435–439. [DOI] [PubMed] [Google Scholar]
- 73. Nielsen EM, Tegtmeiera C, Andersen HJ, Grønbæk C, Andersen JS (2002) Influence of age, sex and herd characteristics on the occurrence of Verocytotoxin-producing Escherichia coli O157 in Danish dairy farms. Vet Microbiol 88: 245–257. [DOI] [PubMed] [Google Scholar]
- 74. Lahti E, Keskimäki M, Rantala L, Hyvönen P, Siitonen A, et al. (2001) Occurrence of Escherichia coli O157 in Finnish cattle. Vet Microbiol 79: 239–251. [DOI] [PubMed] [Google Scholar]
- 75. Montenegro MA, Bülte M, Trumpf T, Aleksic S, Reuter G, et al. (1990) Detection and characterization of fecal verotoxin-producing Escherichia coli from healthy cattle. J Clin Microbiol 28: 1417–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Alam MJ, Zurek L (2006) Seasonal prevalence of Escherichia coli O157:H7 in beef cattle feces. J Food Prot 69: 3018–3020. [DOI] [PubMed] [Google Scholar]
- 77. Arthur TM, Keen JE, Bosilevac JM, Brichta-Harhay DM, Kalchayanand N, et al. (2009) Longitudinal study of Escherichia coli O157:H7 in a beef cattle feedlot and role of high-level shedders in hide contamination. Appl Environ Microbiol 75: 6515–6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Branham LA, Carr MA, Scott CB, Callaway TR (2005) E. coli O157 and Salmonella spp. in white-tailed deer and livestock. Curr Issues Intest Microbiol 6: 25–29. [PubMed] [Google Scholar]
- 79. Brichta-Harhay DM, Arthur TM, Bosilevac JM, Guerini MN, Kalchayanand N, et al. (2007) Enumeration of Salmonella and Escherichia coli O157:H7 in ground beef, cattle carcass, hide and faecal samples using direct plating methods. J Appl Microbiol 103: 1657–1668. [DOI] [PubMed] [Google Scholar]
- 80. Callaway TR, Edrington TS, Brabban AD, Keen JE, Anderson RC, et al. (2006) Fecal prevalence of Escherichia coli O157, Salmonella, Listeria, and Bacteriophage Infecting E. coli O157:H7 in feedlot cattle in the Southern plains region of the United States. Foodborne Pathog Dis 3: 234–244. [DOI] [PubMed] [Google Scholar]
- 81. Cernicchiaro N, Pearl DL, McEwen SA, Harpster L, Homan HJ, et al. (2012) Association of wild bird density and farm management factors with the prevalence of E. coli O157 in dairy herds in Ohio (2007–2009). Zoonoses Public Health 59: 320–329. [DOI] [PubMed] [Google Scholar]
- 82. Cho S, Bender JB, Diez-gonzalez F, Fossler CP, Hedberg CW, et al. (2006) Prevalence and characterization of Escherichia coli O157 isolates from Minnesota dairy farms and county fairs. J Food Prot 69: 252–259. [DOI] [PubMed] [Google Scholar]
- 83. Dodson K, LeJeune J (2005) Escherichia coli O157:H7, Campylobacter jejuni, and Salmonella prevalence in cull dairy cows marketed in northeastern Ohio. J Food Prot 68: 927–931. [DOI] [PubMed] [Google Scholar]
- 84. Dunn JR, Keen JE, Vecchio RD, Wittum TE, Thompson RA (2004) Escherichia coli O157:H7 in a cohort of weaned, preconditioned range beef calves. J Food Prot 67: 2391–2396. [DOI] [PubMed] [Google Scholar]
- 85. Elder RO, Keen JE, Siragusa GR, Barkocy-Gallagher GA, Koohmaraie M, et al. (2000) Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc Natl Acad Sci USA 97: 2999–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Faith NG, Shere JA, Brosch R, Arnold KW, Ansay SE, et al. (1996) Prevalence and clonal nature of Escherichia coli O157:H7 isolated on dairy farms in Wisconsin. Abstracts of the General Meeting of the American Society for Microbiology 95: 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fox JT, Renter DG, Sanderson MW, Nutsch AL, Shi X, et al. (2008) Associations between the presence and magnitude of Escherichia coli O157 in feces at harvest and contamination of preintervention beef carcasses. J Food Prot 71: 1761–1767. [DOI] [PubMed] [Google Scholar]
- 88. Garber L, Wells S, Schroeder-tucker L, Ferris K (1999) Factors associated with fecal shedding of verotoxin-producing Escherichia coli O157 on dairy farms. J Food Prot 62: 307–312. [DOI] [PubMed] [Google Scholar]
- 89. Hancock DD, Besser TE, Kinsel ML, Tarr PI, Rice DH, et al. (1994) The prevalence of Escherichia coli O157.H7 in dairy and beef cattle in Washington State. Epidemiol Infect 113: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hancock DD, Besser TE, Rice DH, Ebel ED, Herriott DE, et al. (1998) Multiple sources of Escherichia coli O157 in feedlots and dairy farms in the northwestern USA. Prev Vet Med 35: 11–19. [DOI] [PubMed] [Google Scholar]
- 91. Hancock DD, Besser TE, Rice DH, Herriott DE, Tarr PI (1997) A longitudinal study of Escherichia coli O157 in fourteen cattle herds. Epidemiol Infect 118: 193–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hancock DD, Rice DH, Herriott DE, Besser TE, Ebel E, et al. (1997) Effects of farm manure-handling practices on Escherichia coli O157 prevalence in cattle. J Food Prot 60: 363–366. [DOI] [PubMed] [Google Scholar]
- 93. Hyatt DR, Galland JC, Gillespie JR (2001) Usefulness of a commercially available enzyme immunoassay for Shiga-like toxins I and II as a presumptive test for the detection of Escherichia coli O157:H7 in cattle feces. J Vet Diagn Invest 13: 71–73. [DOI] [PubMed] [Google Scholar]
- 94. Jacob ME, Almes KM, Shi X, Sargeant JM, Nagaraja TG (2011) Escherichia coli O157:H7 genetic diversity in bovine fecal samples. J Food Prot 74: 1186–1188. [DOI] [PubMed] [Google Scholar]
- 95. Jacob ME, Renter DG, Nagaraja TG (2010) Animal and truckload-level associations between Escherichia coli O157:H7 in feces and on hides at harvest and contamination of preevisceration beef carcasses. J Food Prot 73: 1030–1037. [DOI] [PubMed] [Google Scholar]
- 96. Keen JE, Durso LM, Meehan TP (2007) Isolation of Salmonella enterica and Shiga-toxigenic Escherichia coli O157 from feces of animals in public contact areas of United States zoological parks. Appl Environ Microbiol 73: 362–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Keen JE, Wittum TE, Dunn JR, Bono JL, Durso LM (2006) Shiga-toxigenic Escherichia coli O157 in agricultural fair livestock, United States. Emerg Infect Dis 12: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Laegreid WW, Elder RO, Keen JE (1999) Prevalence of Escherichia coli O157:H7 in range beef calves at weaning. Epidemiol Infect 123: 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. LeJeune JT, Besser TE, Rice DH, Berg JL, Stilborn RP, et al. (2004) Longitudinal study of fecal shedding of Escherichia coli O157:H7 in feedlot cattle: predominance and persistence of specific clonal types despite massive cattle population turnover. Appl Environ Microbiol 70: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. LeJeune JT, Hancock D, Wasteson Y, Skjerve E, Urdahl AM (2006) Comparison of E. coli O157 and Shiga toxin-encoding genes (stx) prevalence between Ohio, USA and Norwegian dairy cattle. Int J Food Microbiol 109: 19–24. [DOI] [PubMed] [Google Scholar]
- 101. Lejeune JT, Kauffman MD (2005) Effect of sand and sawdust bedding materials on the fecal prevalence of Escherichia coli O157:H7 in dairy cows. Appl Environ Microbiol 71: 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. McDonough PL, Rossiter CA, Rebhun RB, Stehman SM, Lein DH, et al. (2000) Prevalence of Escherichia coli O157:H7 from cull dairy cows in New York state and comparison of culture methods used during preharvest food safety investigations. J Clin Microbiol 38: 318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Murinda SE, Nguyen LT, Ivey SJ, Gillespie BE, Almeida RA, et al. (2002) Prevalence and molecular characterization of Escherichia coli O157:H7 in bulk tank milk and fecal samples from cull cows: a 12-month survey of dairy farms in east Tennessee. J Food Prot 65: 752–759. [DOI] [PubMed] [Google Scholar]
- 104. Murinda SE, Nguyen LT, Nam HM, Almeida RA, Headrick SJ, et al. (2004) Detection of sorbitol-negative and sorbitol-positive Shiga toxin-producing Escherichia coli, Listeria monocytogenes, Campylobacter jejuni, and Salmonella spp. in dairy farm environmental samples. Foodborne Pathog Dis 1: 97–104. [DOI] [PubMed] [Google Scholar]
- 105. Ransom JR, Belk KE, Bacon RT, Sofos JN, Scanga JA, et al. (2002) Comparison of sampling methods for microbiological testing of beef animal rectal/colonal feces, hides, and carcasses. J Food Prot 65: 621–626. [DOI] [PubMed] [Google Scholar]
- 106. Renter DG, Sargeant JM, Hungerford LL (2004) Distribution of Escherichia coli O157:H7 within and among cattle operations in pasture-based agricultural areas. Am J Vet Res 65: 1367–1376. [DOI] [PubMed] [Google Scholar]
- 107. Sargeant JM, Sandersonb MW, Smithc RA, Griffin DD (2003) Escherichia coli O157 in feedlot cattle feces and water in four major feeder-cattle states in the USA. Prev Vet Med 61: 127–135. [DOI] [PubMed] [Google Scholar]
- 108. Smith D, Blackford M, Younts S, Moxley R, Gray J, et al. (2001) Ecological relationships between the prevalence of cattle shedding Escherichia coli O157:H7 and characteristics of the cattle or conditions of the feedlot pen. J Food Prot 64: 1899–1903. [DOI] [PubMed] [Google Scholar]
- 109. Walker C, Shi X, Sanderson M, Sargeant J, Nagaraja TG (2010) Prevalence of Escherichia coli O157:H7 in gut contents of beef cattle at slaughter. Foodborne Pathog Dis 7: 249–255. [DOI] [PubMed] [Google Scholar]
- 110. Wells JG, Shipman LD, Greene KD, Sowers EG, Green JH, et al. (1991) Isolation of Escherichia coli serotype O157 H7 and other shiga-like-toxin-producing Escherichia coli from dairy cattle. J Clin Microbiol 29: 985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Woerner DR, Ransom JR, Sofos JN, Dewell GA, Smith GC, et al. (2006) Determining the prevalence of Escherichia coli O157 in cattle and beef from the feedlot to the cooler. J Food Prot 69: 2824–2827. [DOI] [PubMed] [Google Scholar]
- 112. Besser TE, Hancock DD, Pritchett LC, McRae EM, Rice DH, et al. (1997) Duration of detection of fecal excretion of Escherichia coli O157:H7 in cattle. J Infect Dis 175: 726–729. [DOI] [PubMed] [Google Scholar]
- 113. Greenquist MA, Drouillard JS, Sargeant JM, Depenbusch BE, Shi X, et al. (2005) Comparison of rectoanal mucosal swab cultures and fecal cultures for determining prevalence of Escherichia coli O157:H7 in feedlot cattle. Appl Environ Microbiol 71: 6431–6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Oot RA, Raya RR, Callaway TR, Edrington TS, Kutter EM, et al. (2007) Prevalence of Escherichia coli O157 and O157:H7-infecting bacteriophages in feedlot cattle feces. Lett Appl Microbiol 45: 445–453. [DOI] [PubMed] [Google Scholar]
- 115. Cernicchiaro N, Pearl DL, Ghimire S, Gyles CL, Johnson RP, et al. (2009) Risk factors associated with Escherichia coli O157:H7 in Ontario beef cow-calf operations. Prev Vet Med 92: 106–115. [DOI] [PubMed] [Google Scholar]
- 116. Cristancho L, Johnson RP, McEwen SA, Gyles CL (2008) Escherichia coli O157:H7 and other Shiga toxin-producing E. coli in white veal calves. Vet Microbiol 126: 200–209. [DOI] [PubMed] [Google Scholar]
- 117. Schurman RD, Hariharan H, Heaney SB, Rahn K (2000) Prevalence and characteristics of shiga toxin-producing Escherichia coli in beef cattle slaughtered on Prince Edward Island. J Food Prot 63: 1583–1586. [DOI] [PubMed] [Google Scholar]
- 118. Van Donkersgoed J, Graham T, Gannon V (1999) The prevalence of verotoxins, Escherichia coli O157:H7, and Salmonella in the feces and rumen of cattle at processing. Can Vet J 40: 332–338. [PMC free article] [PubMed] [Google Scholar]
- 119. Vidovic S, Korber DR (2006) Prevalence of Escherichia coli O157 in Saskatchewan cattle: characterization of isolates by using random amplified polymorphic DNA PCR, antibiotic resistance profiles, and pathogenicity determinants. Appl Environ Microbiol 72: 4347–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Callaway TR, Anderson RC, Tellez G, Rosario C, Nava GM, et al. (2004) Prevalence of Escherichia coli O157 in cattle and swine in central Mexico. J Food Prot 67: 2274–2276. [DOI] [PubMed] [Google Scholar]
- 121. Ezawa A, Gocho F, Saitoh M, Tamura T, Kawata K, et al. (2004) A three-year study of enterohemorrhagic Escherichia coli O157 on a farm in Japan. J Vet Med Sci 66: 779–784. [DOI] [PubMed] [Google Scholar]
- 122. Fukushima H, Seki R (2004) High numbers of Shiga toxin-producing Escherichia coli found in bovine faeces collected at slaughter in Japan. FEMS Microbiol Lett 238: 189–197. [DOI] [PubMed] [Google Scholar]
- 123. Kijima-Tanaka M, Ishihara K, Kojima A, Morioka A, Nagata R, et al. (2005) A national surveillance of Shiga toxin-producing Escherichia coli in food-producing animals in Japan. J Vet Med B Infect Dis Vet Public Health 52: 230–237. [DOI] [PubMed] [Google Scholar]
- 124. Kobayashi H, Miura A, Hayashi H, Ogawa T, Endo T, et al. (2003) Prevalence and characteristics of eae-positive Escherichia coli from healthy cattle in Japan. Appl Environ Microbiol 69: 5690–5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Miyao Y, Kataokaa T, Nomotoa T, Kaib A, Itohb T, et al. (1998) Prevalence of verotoxin-producing Escherichia coli harbored in the intestine of cattle in Japan. Vet Microbiol 61: 137–143. [DOI] [PubMed] [Google Scholar]
- 126. Sasaki Y, Tsujiyama Y, Kusukawa M, Murakami M, Katayama S, et al. (2011) Prevalence and characterization of Shiga toxin-producing Escherichia coli O157 and O26 in beef farms. Vet Microbiol 150: 140–145. [DOI] [PubMed] [Google Scholar]
- 127. Widiasih DA, Ido N, Omoe K, Sugii S, Shinagawa K (2004) Duration and magnitude of faecal shedding of Shiga toxin-producing Escherichia coli from naturally infected cattle. Epidemiol Infect 132: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Shinagawa K, Kanehira M, Omoe K, Matsuda I, Hu DL, et al. (2010) Frequency of Shiga toxin-producing Escherichia coli in cattle at a breeding farm and at a slaughterhouse in Japan. Vet Microbiol 76: 305–309. [DOI] [PubMed] [Google Scholar]
- 129. Chattopadhyay UK, Dutta S, Deb A. Pal D (2001) Verotoxin-producing Escherichia coli-an environment-induced emerging zoonosis in and around Calcutta. Int J Environ Health Res 11: 107–112. [DOI] [PubMed] [Google Scholar]
- 130. Manna SK, Brahmane MP, Manna C, Batabyal K, Das R (2006) Occurrence, virulence characteristics and antimicrobial resistance of Escherichia coli O157 in slaughtered cattle and. Lett Appl Microbiol 43: 405–409. [DOI] [PubMed] [Google Scholar]
- 131. Wani SA, Bhat MA, Samanta, Nishikawa Y, Buchh AS (2003) Isolation and characterization of Shiga toxin-producing Escherichia coli (STEC) and enteropathogenic Escherichia coli (EPEC) from calves and lambs with diarrhoea in India. Lett Appl Microbiol 37: 121–126. [DOI] [PubMed] [Google Scholar]
- 132. Jo MY, Kimb JH, Lima JH, Kanga MH, Koha HB, et al. (2004) Prevalence and characteristics of Escherichia coli O157 from major food animals in Korea. Int J Food Microbiol 95: 41–49. [DOI] [PubMed] [Google Scholar]
- 133. Kang SJ, Ryua SJ, Chaea JS, Eoa SK, Woo GJ, et al. (2004) Occurrence and characteristics of enterohemorrhagic Escherichia coli O157 in calves associated with diarrhoea. Vet Microbiol 98: 323–328. [DOI] [PubMed] [Google Scholar]
- 134. Panutdaporn N, Chongsa-nguan M, Nair GB, Ramamurthy T, Yamasaki S, et al. (2004) Genotypes and phenotypes of Shiga toxin producing-Escherichia coli isolated from healthy cattle in Thailand. J Infect 48: 149–160. [DOI] [PubMed] [Google Scholar]
- 135. Vuddhakul V, Patararungrong N, Pungrasamee P, Jitsurong S, Morigaki T, et al. (2000) Isolation and characterization of Escherichia coli O157 from retail beef and bovine feces in Thailand. FEMS Microbiol Lett 182: 343–347. [DOI] [PubMed] [Google Scholar]
- 136. Islam MA, Mondol AS, Boer ED, Beumer RR, Zwietering MH, et al. (2008) Prevalence and genetic characterization of shiga toxin-producing Escherichia coli isolates from slaughtered animals in Bangladesh. Appl Environ Microbiol 74: 5414–5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhou Z, Nishikawa Y, Zhu P, Hong S, Hase A, et al. (2002) Isolation and characterization of Shiga toxin-producing Escherichia coli O157:H7 from beef, pork and cattle fecal samples in Changchun, China. J Vet Med Sci 64: 1041–1044. [DOI] [PubMed] [Google Scholar]
- 138. Leung PH, Yam WC, Ng WWS, Peiris JSM (2001) The prevalence and characterization of verotoxin-producing Escherichia coli isolated from cattle and pigs in an abattoir in Hong Kong. Epidemiol Infect 126: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Tahamtan Y, Hayati M, Namavari MM (2010) Prevalence and distribution of the stx1, stx2 genes in Shiga toxin producing E. coli (STEC) isolates from cattle. Iran J Microbiol 2: 9–14. [PMC free article] [PubMed] [Google Scholar]
- 140. Osaili TM, Alaboudi AR, Rahahlah M (2013) Prevalence and antimicrobial susceptibility of Escherichia coli O157: H7 on beef cattle slaughtered in Amman abattoir. Meat Sci 93: 463–468. [DOI] [PubMed] [Google Scholar]
- 141. Nguyen TD, Vo TT, Vu-Khac H (2011) Virulence factors in Escherichia coli isolated from calves with diarrhea in Vietnam. J Vet Sci 12: 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chinen I, Otero JL, Miliwebsky ES, Rold ML, Baschkier A, et al. (2003) Isolation and characterisation of Shiga toxin-producing Escherichia coli O157:H7 from calves in Argentina. Res Vet Sci 74: 283–286. [DOI] [PubMed] [Google Scholar]
- 143. Fernandez D, Irino K, Sanz ME, Padola NL, Parma AE (2010) Characterization of Shiga toxin-producing Escherichia coli isolated from dairy cows in Argentina. Lett Appl Microbiol 51: 377–382. [DOI] [PubMed] [Google Scholar]
- 144. Masana MO, Leotta GA, Castillo LLD, Dastek BA, Palladino PM, et al. (2010) Prevalence, characterization, and genotypic analysis of Escherichia coli O157:H7/NM from selected beef exporting abattoirs of Argentina. J Food Prot 73: 649–656. [DOI] [PubMed] [Google Scholar]
- 145. Meichtri L, Miliwebsky E, Gioffre A, Chinen I, Baschkier A, et al. (2004) Shiga toxin-producing Escherichia coli in healthy young beef steers from Argentina: prevalence and virulence properties. Int J Food Microbiol 96: 189–198. [DOI] [PubMed] [Google Scholar]
- 146. Tanaro JD, Leotta GA, Lound LH, Galli L, Piaggio MC, et al. (2010) Escherichia coli O157 in bovine feces and surface water streams in a beef cattle farm of Argentina. Foodborne Pathog Dis 7: 475–477. [DOI] [PubMed] [Google Scholar]
- 147. Cerqueira AM, Guth BE, Joaquim RM, Andrade JR (1999) High occurrence of Shiga toxin-producing Escherichia coli (STEC) in healthy cattle in Rio de Janeiro State, Brazil. Vet Microbiol 70: 111–121. [DOI] [PubMed] [Google Scholar]
- 148. Irino K, Kato MAMF, Vaz TMI, Ramos II, Souza MAC, et al. (2005) Serotypes and virulence markers of Shiga toxin-producing Escherichia coli (STEC) isolated from dairy cattle in Sao Paulo State, Brazil. Vet Microbiol 105: 29–36. [DOI] [PubMed] [Google Scholar]
- 149. Moreira CN, Pereirac MA, Broda CS, Rodriguesd DP, Carvalhalc JB, et al. (2003) Shiga toxin-producing Escherichia coli (STEC) isolated from healthy dairy cattle in southern Brazil. Vet Microbiol 93: 179–183. [DOI] [PubMed] [Google Scholar]
- 150. Borif CF, Monreal Z, Martinez J, Arellano C, Prado V (1997) Detection and characterization of enterohaemorrhagic Escherichia coli in slaughtered cattle. Zentralbl Veterinarmed B 44: 273–279. [DOI] [PubMed] [Google Scholar]
- 151. Rivera FP, Sotelo E, Morales I, Menacho F, Medina AM, et al. (2012) Detection of Shiga toxin-producing Escherichia coli (STEC) in healthy cattle and pigs in Lima, Peru. J Dairy Sci 95: 1166–1169. [DOI] [PubMed] [Google Scholar]
- 152. Narvaez-Bravo CA, Carruyo-Núñez G, Moreno M, Rodas-González A, Hoet AE, et al. (2007) Isolation of Escherichia coli O157:H7 from feces in dual purpose cattle at Miranda Municipality, Zulia State, Venezuela. Revista Cientifica-Facultad De Ciencias Veterinarias 17: 239–245. [Google Scholar]
- 153. Akanbi BO, Mbah IP, Kerry PC (2011) Prevalence of Escherichia coli O157:H7 on hides and faeces of ruminants at slaughter in two major abattoirs in Nigeria. Lett Appl Microbiol 53: 336–340. [DOI] [PubMed] [Google Scholar]
- 154. Ojo OE, Ajuwape ATP, Otesile EB, Owoade AA, Oyekunle MA, et al. (2010) Potentially zoonotic shiga toxin-producing Escherichia coli serogroups in the faeces and meat of food-producing animals in Ibadan, Nigeria. Int J Food Microbiol 142: 214–221. [DOI] [PubMed] [Google Scholar]
- 155. Ateba CN, Mbewe M (2011) Detection of Escherichia coli O157:H7 virulence genes in isolates from beef, pork, water, human and animal species in the northwest province, South Africa: public health implications. Res Microbiol 162: 240–248. [DOI] [PubMed] [Google Scholar]
- 156. Ateba CN, Mbewe M, Bezuidenhout CC (2008) Prevalence of Escherichia coli O157 strains in cattle, pigs and humans in North West province, South Africa. South African Journal of Science 104: 7–8. [Google Scholar]
- 157. Barlow RS, Mellor GE (2010) Prevalence of enterohemorrhagic Escherichia coli serotypes in Australian beef cattle. Foodborne Pathog Dis 7: 1239–1245. [DOI] [PubMed] [Google Scholar]
- 158. Cobbold R, Desmarchelier P (2000) A longitudinal study of Shiga-toxigenic Escherichia coli (STEC) prevalence in three Australian dairy herds. Vet Microbiol 71: 125–137. [DOI] [PubMed] [Google Scholar]
- 159. Fegan N, Higgs G, Duffy LL, Barlow RS (2009) The effects of transport and lairage on counts of Escherichia coli O157 in the feces and on the hides of individual cattle. Foodborne Pathog Dis 6: 1113–1120. [DOI] [PubMed] [Google Scholar]
- 160. Fegan N, Vanderlinde P, Higgs G, Desmarchelier P (2004) The prevalence and concentration of Escherichia coli O157 in faeces of cattle from different production systems at slaughter. J Appl Microbiol 97: 362–370. [DOI] [PubMed] [Google Scholar]
- 161. Jeon SJ, Elzo M, DiLorenzo N, Lamb GC, Jeong KC (2013) Evaluation of Animal Genetic and Physiological Factors That Affect the Prevalence of Escherichia coli O157 in Cattle. PLoS ONE 8(2): e55728 10.1371/journal.pone.0055728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. British Medical Journal 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOC)
A pretested data extraction spreadsheet.
(XLS)
Description of studies reporting prevalence of E. coli O157 in cattle.
(DOC)
List of papers included in this meta-analysis.
(DOC)
List of excluded full text paper with proper justification.
(DOC)