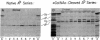Abstract
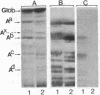
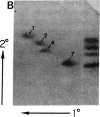
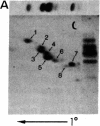
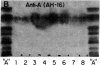
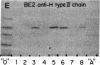
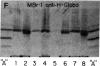
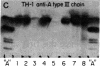
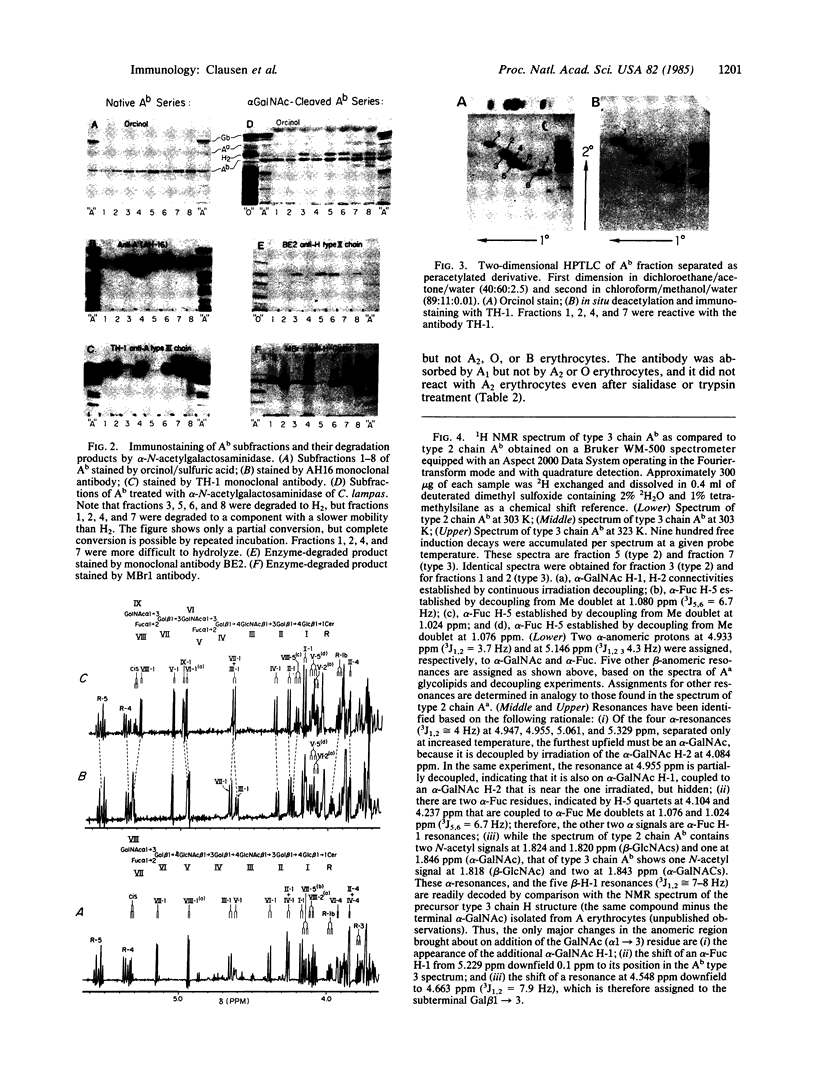
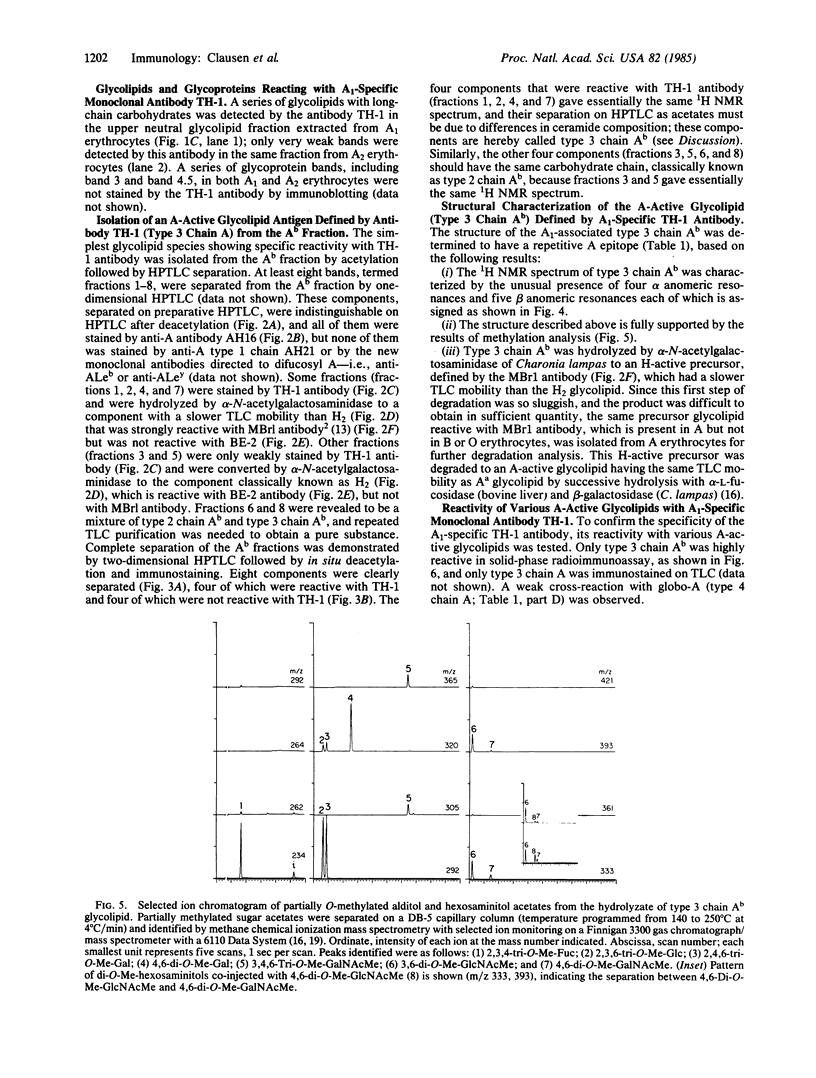
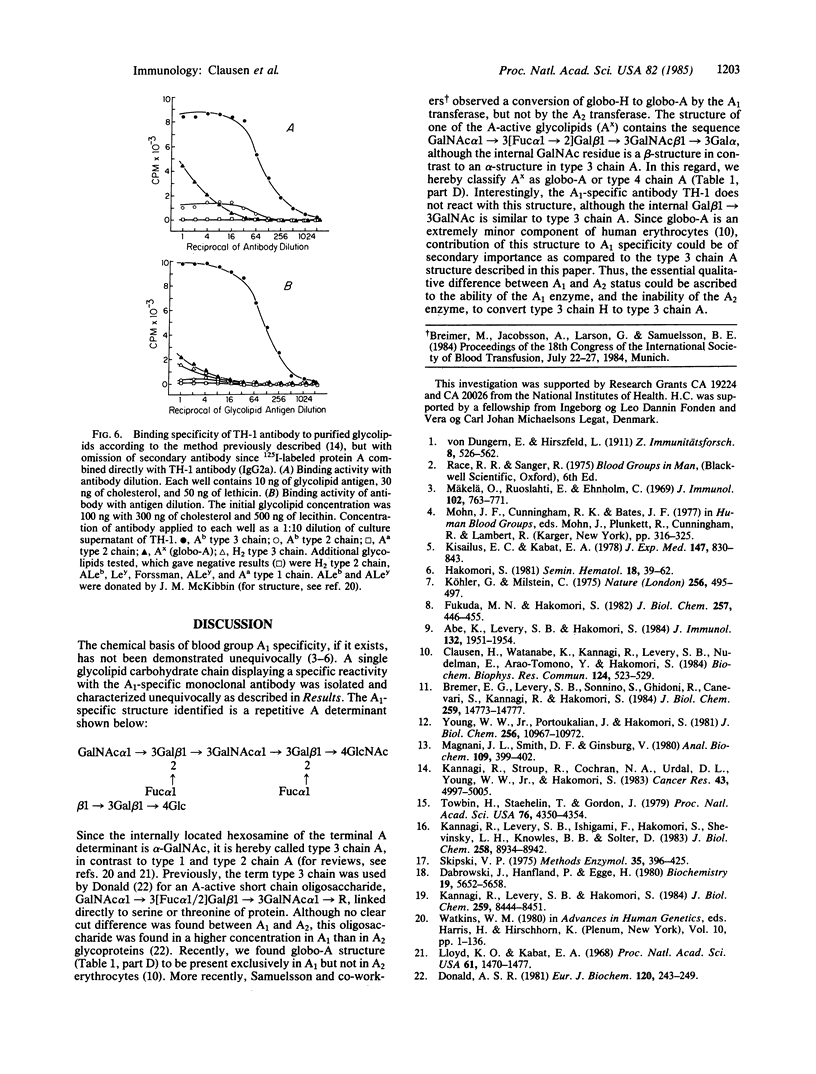
The IgG2a monoclonal antibody TH-1, which reacts specifically with blood group A1 but with neither A2 nor O erythrocytes, has been established. The antibody reacted only with A1 erythrocytes in hemagglutination and antibody absorption assays; it did not react with A2 erythrocytes, even after trypsin or sialidase treatment. This antibody detected, on TLC immunostaining, a series of glycolipids from A1 erythrocytes but virtually none or very weak bands from A2 erythrocytes. It did not react with type 1 or type 2 chain A, or with globo-A. The simplest reactive component was isolated from a previously assigned Ab fraction by HPTLC of acetylated compounds. The structure of the reactive component was characterized by 1H NMR spectroscopy, methylation analysis, and enzymatic degradation, as shown below: (Formula: see text). The structure is essentially a repetitive A epitope attached to type 2 chain and is hereby called type 3 chain A. The determinant can be carried on extended and/or branched structures, but it was not detectable in glycoproteins. The structure was characteristic of A1 erythrocytes and present in only trace amounts in A2 erythrocytes. The precursor H (Fuc alpha 1----2Gal beta 1----3GalNAc alpha 1----3[Fuc alpha 1----2]Gal beta 1----4GlcNAc beta 1----R; type 3 chain H) was present in greater quantity in A2 erythrocytes than in A1 erythrocytes, but it was absent in both O and B erythrocytes. The A1 transferase apparently can transfer alpha-GalNAc to type 3 chain H, while the A2 transferase may not have this ability.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Levery S. B., Hakomori S. The antibody specific to type 1 chain blood group A determinant. J Immunol. 1984 Apr;132(4):1951–1954. [PubMed] [Google Scholar]
- Bremer E. G., Levery S. B., Sonnino S., Ghidoni R., Canevari S., Kannagi R., Hakomori S. Characterization of a glycosphingolipid antigen defined by the monoclonal antibody MBr1 expressed in normal and neoplastic epithelial cells of human mammary gland. J Biol Chem. 1984 Dec 10;259(23):14773–14777. [PubMed] [Google Scholar]
- Clausen H., Watanabe K., Kannagi R., Levery S. B., Nudelman E., Arao-Tomono Y., Hakomori S. Blood group A glycolipid (Ax) with globo-series structure which is specific for blood group A1 erythrocytes: one of the chemical bases for A1 and A2 distinction. Biochem Biophys Res Commun. 1984 Oct 30;124(2):523–529. doi: 10.1016/0006-291x(84)91585-7. [DOI] [PubMed] [Google Scholar]
- Dabrowski J., Hanfland P., Egge H. Structural analysis of glycosphinoglipids by high-resolution 1H nuclear magnetic resonance spectroscopy. Biochemistry. 1980 Nov 25;19(24):5652–5658. doi: 10.1021/bi00565a030. [DOI] [PubMed] [Google Scholar]
- Donald A. S. A-active trisaccharides isolated from A1 and A2 blood-group-specific glycoproteins. Eur J Biochem. 1981 Nov;120(2):243–249. doi: 10.1111/j.1432-1033.1981.tb05695.x. [DOI] [PubMed] [Google Scholar]
- Fukuda M. N., Hakomori S. Structures of branched blood group A-active glycosphingolipids in human erythrocytes and polymorphism of A- and H-glycolipids in A1 and A2 subgroups. J Biol Chem. 1982 Jan 10;257(1):446–455. [PubMed] [Google Scholar]
- Hakomori S. Blood group ABH and Ii antigens of human erythrocytes: chemistry, polymorphism, and their developmental change. Semin Hematol. 1981 Jan;18(1):39–62. [PubMed] [Google Scholar]
- Kannagi R., Levery S. B., Hakomori S. Hybrid type glycolipids (lacto-ganglio series) with a novel branched structure. Their presence in undifferentiated murine leukemia cells and their dependence on differentiation. J Biol Chem. 1984 Jul 10;259(13):8444–8451. [PubMed] [Google Scholar]
- Kannagi R., Levery S. B., Ishigami F., Hakomori S., Shevinsky L. H., Knowles B. B., Solter D. New globoseries glycosphingolipids in human teratocarcinoma reactive with the monoclonal antibody directed to a developmentally regulated antigen, stage-specific embryonic antigen 3. J Biol Chem. 1983 Jul 25;258(14):8934–8942. [PubMed] [Google Scholar]
- Kannagi R., Stroup R., Cochran N. A., Urdal D. L., Young W. W., Jr, Hakomori S. Factors affecting expression of glycolipid tumor antigens: influence of ceramide composition and coexisting glycolipid on the antigenicity of gangliotriaosylceramide in murine lymphoma cells. Cancer Res. 1983 Oct;43(10):4997–5005. [PubMed] [Google Scholar]
- Kisailus E. C., Kabat E. A. Immunochemical studies on blood groups LXVI. Competitive binding assays of A1 and A2 blood group substances with insolubilized anti-A serum and insolubilized A agglutinin from Dolichos biflorus. J Exp Med. 1978 Mar 1;147(3):830–843. doi: 10.1084/jem.147.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lloyd K. O., Kabat E. A. Immunochemical studies on blood groups. XLI. Proposed structures for the carbohydrate portions of blood group A, B, H, Lewis-a, and Lewis-b substances. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1470–1477. doi: 10.1073/pnas.61.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani J. L., Smith D. F., Ginsburg V. Detection of gangliosides that bind cholera toxin: direct binding of 125I-labeled toxin to thin-layer chromatograms. Anal Biochem. 1980 Dec;109(2):399–402. doi: 10.1016/0003-2697(80)90667-3. [DOI] [PubMed] [Google Scholar]
- Mäkelä O., Ruoslahti E., Ehnholm C. Subtypes of human ABO blood groups and subtype-specific antibodies. J Immunol. 1969 Mar;102(3):763–771. [PubMed] [Google Scholar]
- Skipski V. P. Thin-layer chromatography of neutral glycosphingolipids. Methods Enzymol. 1975;35:396–425. doi: 10.1016/0076-6879(75)35178-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. W., Jr, Portoukalian J., Hakomori S. Two monoclonal anticarbohydrate antibodies directed to glycosphingolipids with a lacto-N-glycosyl type II chain. J Biol Chem. 1981 Nov 10;256(21):10967–10972. [PubMed] [Google Scholar]