Abstract
Background
Valproic acid (VPA) has been shown to improve survival in animal models of hemorrhagic shock at 300mg/kg. Our aim was to identify the ideal dose through a dose escalation, split dosing, and dose de-escalation regimen.
Materials and Methods
Rats were subjected to sublethal 40% hemorrhage and treated with vehicle, or VPA (300 mg/kg, 400 mg/kg, or 450 mg/kg) after 30 minutes of shock. Acetylated histones and activated proteins from the PI3K/Akt/GSK-3β survival pathway at different time points were quantified by Western blot. In a similar model, a VPA dose of 200 mg/kg followed 2 hours later by another 100 mg/kg was administered. Finally, animals were subjected to a lethal 50% hemorrhage and VPA was administered in a dose de-escalation manner (starting at 300 mg/kg) until a significant drop in percent survival was observed.
Results
Larger doses of VPA resulted in greater acetylation of histone 3 and increased activation of PI3K pathway proteins. Dose-dependent differences were significant in histone acetylation, but not in the activation of the survival pathway proteins. Split-dose administration of VPA resulted in similar results to a single full dose. Survival was as follows: 87.5% with 300 mg/kg and 250 mg/kg VPA, 50% with 200 mg/kg VPA, and 14% with vehicle treated animals.
Conclusions
While higher doses of VPA result in greater histone acetylation and activation of prosurvival protein signaling, doses as low as 250 mg/kg VPA confer the same survival advantage in lethal hemorrhagic shock. Also, VPA can be given in a split-dose fashion without a reduction in its cytoprotective effectiveness.
Keywords: Hemorrhagic shock, valproic acid, histone deacetylase inhibitors, dose optimization
1. Introduction
Hemorrhage remains a leading cause of preventable death following civilian and combat trauma. Conventional treatment involves prompt surgical control of bleeding and aggressive fluid resuscitation to reverse hypotension [1]. However, while fluid resuscitation restores circulatory volume, it also leads to hemodilution, increased coagulopathy, edema, and upregulation of proinflammatory mediators and their receptors, which in turn can exacerbate the lethal triad of acidosis, coagulopathy and hypothermia [2, 3]. Damage control resuscitation, which involves permissive hypotension and use of blood products instead of crystalloids, is a better alternative, but is limited by availability of these products in remote and austere locations such as the battlefield. Moreover, these resuscitation methods lack any specific properties that can correct the shock-induced changes at a cellular level. For these reasons, we are investigating pharmacological alternatives to fluid resuscitation that are portable, protect against cellular damage induced by hemorrhagic shock, and are capable of maintaining survival until the injured patients can be transferred to a higher level facility (“bridge to definitive care”).
One promising pharmacological alternative is valproic acid (VPA), which is widely prescribed as an anti-epilepsy drug [4], but in higher doses can act as a histone deacetylase inhibitor (HDACI) [5]. While hemorrhagic shock can disrupt cellular “acetylation homeostasis” [6] by suppressing histone acetyltransferase (HATs) activity, leading to excessive histone deacetylation and suppressed gene transcription, administration of VPA reverses these changes [7]. Our group has shown that VPA treatment prolongs survival in highly lethal models of hemorrhagic shock in swine and rats [8, 9]. Additionally, VPA administration reduces cellular levels of pro-apoptotic caspase 3 in liver [10], deactivates inflammatory ERK, JNK, and p38 mitogen-activated protein kinase (MAPK) pathway [11], stabilizes intestinal tight junctions [12], and attenuates systemic effects of ischemia-reperfusion injury in small and large animal models [13, 14]. These data strongly suggests that treatment with HDACI can create a pro-survival phenotype in a wide variety of lethal insults such as hemorrhagic shock, sepsis, and traumatic brain injury [15, 16, 17]
The dose of VPA (250–300 mg/kg) shown to cause HDAC inhibition and survival improvement is 6–8 fold higher than what is currently approved by the FDA for the treatment of seizures and mood disorders (20–60 mg/kg). When given as an HDACI in cancer patients, total VPA doses of >300 mg/kg have been administered in clinical trials, but in 5–6 divided doses. Typically, the single maximum IV dose is kept under 75 mg/kg to avoid side effects [18]. As massive blood loss is rapidly lethal, splitting the total dose over many days is not a practical option; therefore, we have typically used a single administration of VPA (300 mg/kg) to treat controlled hemorrhagic shock in animal models, and have even used a dose as high as 400 mg/kg in a model of ongoing blood loss and massive resuscitation (large volume of distribution) [19]. Single administration of VPA in this large dose (300 mg/kg) has consistently been shown to sufficiently correct the histone acetylation profile, and activate numerous pro-survival mechanisms [17]. However, it remains unknown whether 300 mg/kg of VPA is the optimal dose, if larger doses would increase efficacy, or if smaller doses would suffice. High dose VPA has many potential side effects such as pancreatitis, hepatic injury, and CNS depression. It is therefore logical to use the lowest possible dose that can produce the desired effect; therefore, we designed a three-part dose study to identify the optimal dose. Experiment 1, a dose escalation study, examines whether VPA doses greater than 300 mg/kg result in any additional activation of key pro-survival pathways. We measured alterations in the PI3-AKT pathway as the endpoint because it is a well-known survival pathway that is affected by VPA treatment. Experiment 2 examines whether a given dose of VPA administered in a split fashion (two doses over a period of 2 hrs) has the same effect on protein activation as a single bolus dose. Experiment 3, a dose de-escalation study, determines whether doses lower than 300 mg/kg produce survival advantage in the setting of lethal hemorrhagic shock.
2. Materials and Methods
Experiments were designed and performed in accordance with the statutes from The Guide for the Care and Use of Laboratory Animals (National Research Council, 1996 edition). This study complies with the Animal Welfare Act and other Federal regulations and was approved by our Institutional Animal Care and Use Committee.
2.1. Surgical Procedure
Rats had access to food and water ad libitum before and after surgery. Male Sprague-Dawley rats (200– 333g, Charles River Laboratories) were anesthetized with 5% isoflurane. 1% bupivacaine was injected at the operative site for local anesthesia. A veterinary multi-channel anesthesia delivery system (Kent Scientific Corporation, Torrington, CT) was used to administer isoflurane at 0.7–2% inspiratory fraction. Both femoral vessels were then isolated and aseptically cannulated with PE50 polyethylene catheters (Clay Adams, Sparks, MD) primed with heparinized saline (100 USP units/ml). The venous line was used for sampling, hemorrhage, and administration of drug treatment. The arterial catheter was attached to a pressure transducer for continuous blood pressure monitoring (Ponemah Physiology Platform, Gould Instrument Systems, Valley View, OH).
2.2. Sub-lethal Hemorrhagic Shock Protocol for Experiment 1 (Dose Escalation) and Experiment 2 (Split-dose Study)
Rats were subjected to a sub-lethal hemorrhage of 40% total blood volume over 10 minutes. The hemorrhage volume was calculated using the formula: total blood volume (ml) = rat’s weight (g)×0.006 (mL/g) + 0.77 (26). Blood was withdrawn from the venous catheter using adjustable syringe pumps (Kent Scientific Corporation). A baseline (BL) arterial blood gas (ABG) measurement was taken prior to hemorrhage, and 30 minutes after hemorrhage for a post shock (PS) measurement. Blood samples were analyzed using a Stat Profile Critical Care Xpress machine (Nova Biomedical, Waltham, MA). Rats were then randomly assigned to receive either saline vehicle, 300 mg/kg, 400 mg/kg, or 450 mg/kg dose of VPA (Calbiochem San Diego, CA) given over a span of 10 minutes. Cannulas were removed, vessels ligated, skin closed, and animals returned to their cage to recuperate before sacrifice at 1h, 6h, or 16h (n=3/timepoint, resulting in n=9 per group for each of the four groups) after the end of VPA treatment (therefore,) and organ tissues were harvested and flash frozen. Sham (instrumentation, no hemorrhage) rats served as controls.
In the split-dose experiment, rats (n=5) were subjected to a sub-lethal hemorrhage of 40% total blood volume, followed by 30 minutes of unresuscitated shock. At the end of the shock period, a 200 mg/kg dose of VPA was administered intravenously over 10 minutes. Two hours later, an additional 100 mg/kg of VPA was administered intravenously. At this stage, cannulas were removed, vessels ligated, skin closed, and animals returned to their cage to recuperate before sacrifice four hours after the first VPA dose. Serum and organ tissues were collected and flash frozen for later use. Also, serum and organ samples from sham rats (n=3) were collected as controls.
2.3. Lethal Hemorrhagic Shock Protocol for Experiment 3 (Dose De-escalation Study)
Rats were subjected to a lethal hemorrhage of 50% total blood volume over 30 minutes. 40% total blood volume was withdrawn over 10 minutes; the remaining 10% was removed over the next 20 minutes. Blood was drawn from the venous catheter using adjustable syringe pumps (Kent Scientific Corporation, Torrington, Connecticut). ABG measurements were taken prior to hemorrhage, after the 40% hemorrhage, and after 50% hemorrhage was completed. Blood samples were analyzed using a Stat Profile Critical Care Xpress machine (Nova Biomedical, Waltham, MA). Groups were tested in the following order: vehicle (saline of equal volume), 300 mg/kg VPA, 250 mg/kg VPA, and 200 mg/kg VPA. The sample size consisted of 8 animals per group, except for the vehicle group, which had 14 animals. After treatment, cannulas were removed, vessels ligated, skin closed, and animals returned to their cage for monitoring. Time between end of treatment and death was recorded, with survival for 24h as the endpoint of the experiment.
2.4. Western Blotting for the Dose Escalation and the Split-dose Study
Liver nuclear extracts were prepared using 50 mg of tissue and a subcellular proteome extraction kit (Calbiochem San Diego, CA). Whole cell lysate was prepared using 50 mg of tissue and an extraction kit (Millipore Temecula, CA). Prior to loading on a 15% or a 12% polyacrylamide gel, respectively, nuclear and whole cell extracts were balanced by spectrophotometry using the Bradford assay to ensure equal loading. Once separated by SDS-PAGE gel electrophoresis, proteins were then electrotransfered onto a nitrocellulose membrane. Membranes were blocked for 30 minutes with 5% dry milk powder dissolved in phosphate buffered saline infused with 0.05% Tween-20 (PBST). Then, membranes were incubated in a primary antibody solution according to the manufacturer's directions overnight at 4° C. Primary antibodies were purchased from and diluted as follows: histone 3 acetylated at lysine 9 (AcH3K9; 1:5000) from Millipore (Temecula, CA); β-actin (1:3000) from Sigma-Aldrich (St. Louis, MO); phospho-PI3K, PI3K, phospho-AKT, AKT, phospho-GSK-3β, GSK-3β (1:1000) all from Cell Signaling Technology (Danvers, MA). Application of secondary antibodies (1:3000 in PBST and 5% milk) linked to horseradish peroxidase in 5% milk and PBST to the membrane allowed detection of the primary antibody when visualized with Western Lighting Chemiluminescence Reagent Plus (PerkinElmer LAS, Inc., Boston, MA). Films were developed in a dark room and evaluated using a VersaDoc Imaging System (Bio-Rad Laboratories) scanning machine.
2.5. Statistical Analysis
Statistical analysis was performed using the IBM SPSS Statistics 20 software (Armonk, NY). In the dose escalation and the split-dose studies, mean differences in physiological parameters, VPA serum levels, and mean relative densities of western blot results were assessed using one way analysis of variance (ANOVA) followed by a Bonferoni post hoc testing. Summary statistics were used to describe continuous variables, while proportions were calculated for categorical variables. In the dose de-escalation study, mean differences in survival times were assessed using ANOVA followed by Bonferoni post hoc testing for multiple comparisons. Fisher’s exact test was used to evaluate differences in percent survival. The survival curve was plotted using the GraphPad Prism statistical software (LaJolla, CA). Statistical significance was defined as p<0.05.
3. Results
3.1. Dose Escalation Study: Physiology
There were no significant differences in physiologic parameters at baseline or post-shock (Table) between vehicle and treatment groups. As expected, all rats survived the sub-lethal (40%) hemorrhage. However, rats receiving VPA (300 mg/kg, 400 mg/kg, 450 mg/kg) had a significantly higher average mean arterial pressure (MAP; 84.0 ±8.8 mmHg, 92.6 ±4.7 mmHg, and 94.1 ±6.5 mmHg respectively) at 1 hour after treatment compared to the vehicle group (57.4 ± 2.8 mmHg, p<0.05 in each case). There were no statistically significant differences in the post-treatment MAP between the various VPA doses groups. Similarly, there were no significant differences between the various VPA groups in terms of hemoglobin, pH, lactate, or base deficit at baseline, post-shock or post-treatment.
Table 1. Dose Escalation Study Selected Physiological Values.
Selected variables from the sub-lethal hemorrhagic shock protocol are shown at baseline (BL) and following hemorrhagic and un-resuscitated shock (post-shock, PS). As treatment animals have not yet received VPA at PS, weight, mean arterial pressure (MAP), pH, and lactate (LAC) values from the 300 mg/kg, 400 mg/kg, and 450 mg/kg group have been combined together. Data is expressed as mean ± SEM. All blood samples for ABG were arterial. At BL and PS, there were no significant differences (p>0.05) between vehicle and treatment animals in terms of weight, MAP, pH, and LAC.
| Baseline | Post-Shock | |||
|---|---|---|---|---|
| Vehicle (n=9) | VPA (n=27) | Vehicle (n=9) | VPA (n=27) | |
| Weight (g) | 296.55 ± 15.43 | 275.21 ± 5.66 | - | - |
| MAP (mmHg) | 97.88 ± 2.29 | 95.71 ± 1.67 | 47.66 ± 4.01 | 54.11 ± 1.43 |
| pH | 7.44 ± 0.01 | 7.43 ± 0.01 | 7.36 ± 0.02 | 7.38 ± 0.01 |
| LAC (mmol/L) | 0.81 ± 0.10 | 0.85 ± 0.10 | 5.33 ± 0.40 | 4.19 ± 0.30 |
Data presented as group means ± SEM. VPA= valproic acid; MAP=mean arterial pressure; LAC=serum lactate
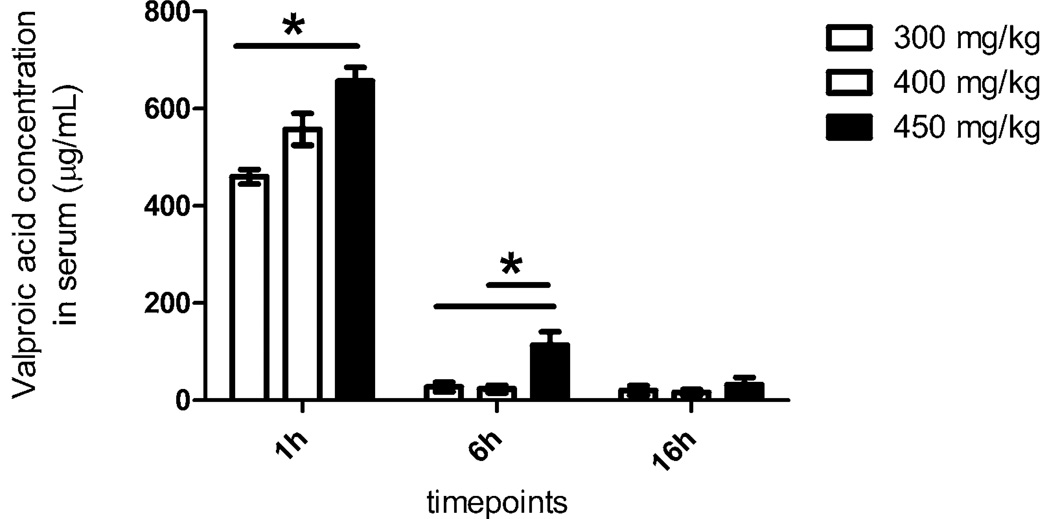
3.2. Dose Escalation Study: Serum VPA Levels
Serum levels one hour post administration were the highest, with a rapid drop by the 6 hour point (Figure 1). The 450 mg/kg dose had the highest serum levels at all the measurement time points.
Figure 1. Dose Escalation Study VPA Levels Circulating in Serum at Time of Sacrifice.
Prior to sacrifice at 1 h, 6h, or 16h, blood samples were collected in Vacutainer and serum tubes; (BD, Franklin Lakes, NJ). Serum samples were sent to the Massachusetts General Hospital Core Lab facility to measure the levels of circulating VPA in the blood. At 1h, VPA in circulation from a 450 mg/kg dose was significantly higher than that from a 300 mg/kg dose (p<0.05). At 6h, the level of VPA in circulation from a 450 mg/kg dose was significantly higher than the 300 mg/kg and the 400 mg/kg dose (p<0.05). By 16h, any significant differences in circulating VPA levels have disappeared. * denotes a significant difference (p<0.05).
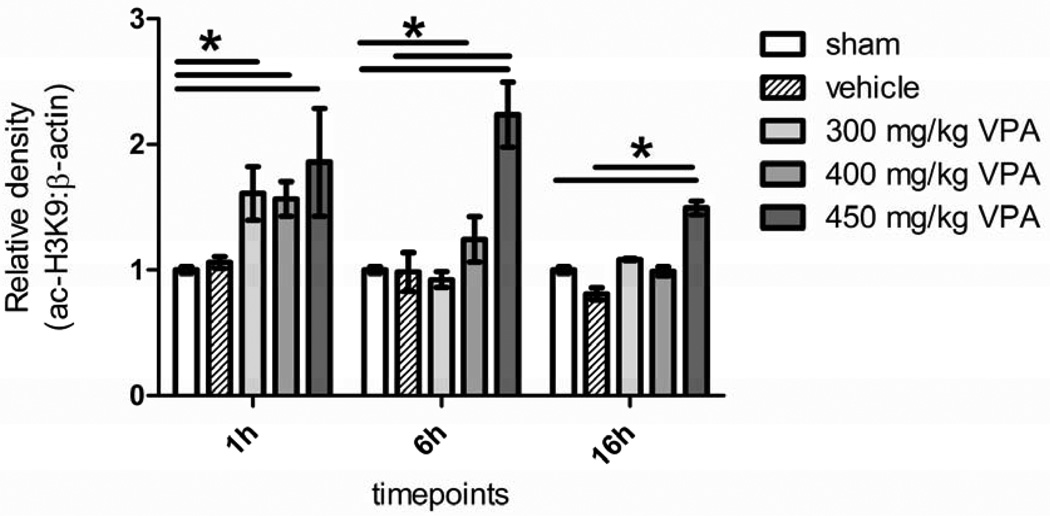
3.3. Dose Escalation Study: Histone Acetylation Levels
All the groups receiving VPA showed an increased level of acetylation at histone 3 lysine 9 in comparison to the sham and vehicle groups, with higher doses generally resulting in greater acetylation. At 1 hour, all the three doses showed significantly elevated degrees of acetylation compared to sham, but by 6h, histone acetylation in the 300 mg/kg group had returned to the baseline. In the 400 mg/kg group, acetylation decreased between 1h and 6h; however, in the 450 mg/kg group, histone acetylation actually increased between 1 and 6 hours, and was significantly higher than the sham and vehicle groups. By 16h, histone acetylation in the 400 mg/kg group had fallen to baseline levels, but was still high in the group that had received the 450 mg/kg dose (Figure 2).
Figure 2. Dose Escalation Study Effect of Hemorrhage and VPA on Acetylation of Histone 3 Lysine 9 in Liver Tissue.
Rat liver was harvested 1, 6, and 16 hours after hemorrhagic shock and administration of treatment. Sham (no hemorrhagic shock, no treatment) served as a control, vehicle rats received saline, and VPA rats received doses of 300 mg/kg, 400 mg/kg, or 450 mg/kg. Data are shown as mean densitometry ratio of acetylated H3k9: β-actin. * denotes a significant difference (p<0.05).
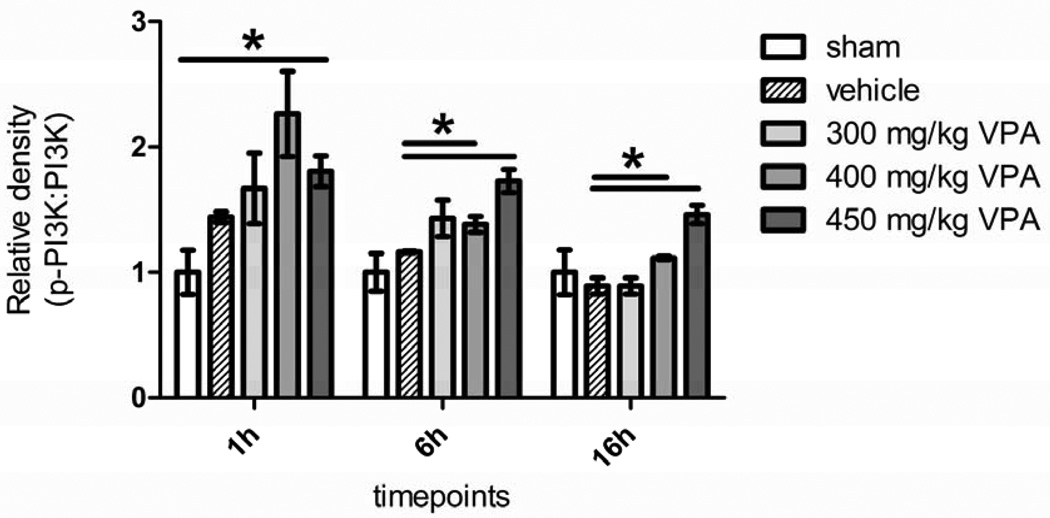
3.4. Dose Escalation Study: Pro-Survival Pathway Activation of PI3K, Akt, and GSK-3β
Activation of PI3K peaked early (1 hour) in the VPA treated animals and trended down by the 6 hour time point (Figure 3). However, PI3K activation in the 400 and the 450 mg/kg groups was still statistically higher compared to the vehicle group. By 16h, phosphorylation levels had fallen further, but significant differences persisted between the high dose VPA (400 and the 450 mg/kg) groups and the vehicle control (Figure 3).
Figure 3. Dose Escalation Study Effect of Hemorrhage and VPA on Phosphorylation of PI3K in Liver Tissue.
Rat liver was harvested 1, 6, and 16 hours after hemorrhagic shock and administration of treatment. Sham (no hemorrhagic shock, no treatment) served as a control, vehicle rats received saline, and VPA rats received doses of 300 mg/kg, 400 mg/kg, or 450 mg/kg. Data are shown as mean relative density of phosphorylated PI3K: PI3K. * denotes a significant difference (p<0.05).
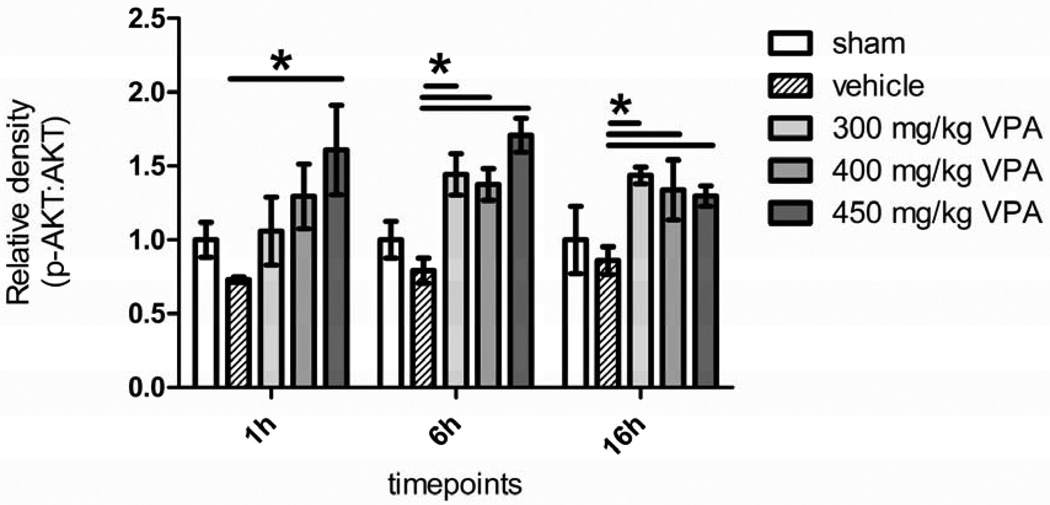
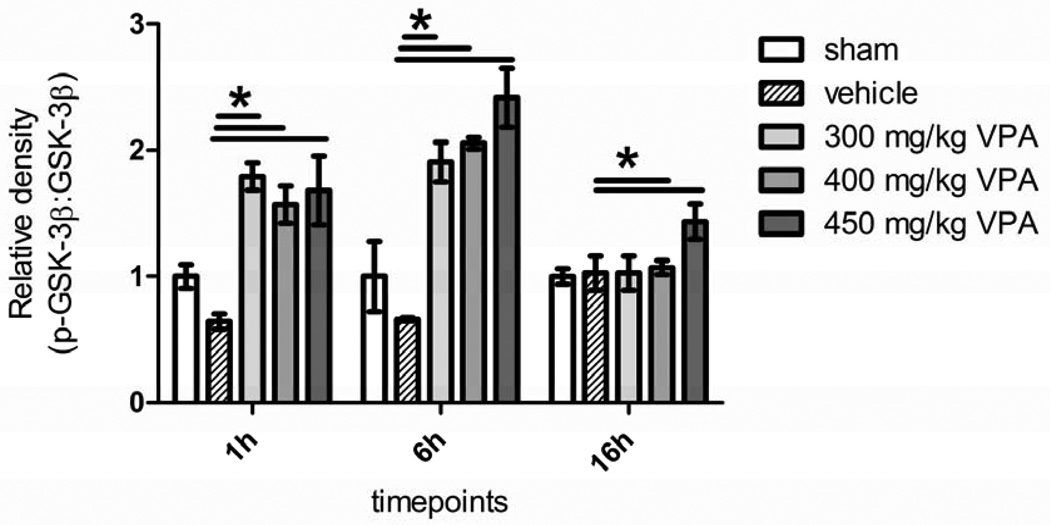
Activated PI3K, phosphorylates Akt which in turn prosphorylates GSK-3β to exert a pro-survival effect. The activation of Akt was seen in all the three VPA groups and peaked later than PI3K, but there were no significant differences between the three doses (Figure 4). A similar pattern was seen for GSK-3β, where administration of VPA increased phosphorylation significantly in all the VPA groups compared to the vehicle within an hour, with an increase at the 6h time point; however, there were no significant differences between the three doses (Figure 5).
Figure 4. Dose Escalation Study Effect of Hemorrhage and VPA on Phosphorylation of Akt in Liver Tissue.
Rat liver was harvested 1, 6, and 16 hours after hemorrhagic shock and administration of treatment. Sham (no hemorrhagic shock, no treatment) served as a control, vehicle rats received saline, and VPA rats received doses of 300 mg/kg, 400 mg/kg, or 450 mg/kg. Data are shown as mean relative density of phosphorylated Akt: Akt. * denotes a significant difference (p<0.05).
Figure 5. Dose Escalation Study Effect of Hemorrhage and VPA on Phosphorylation of GSK-3β in Liver Tissue.
Rat liver was harvested 1, 6, and 16 hours after hemorrhagic shock and administration of treatment. Sham (no hemorrhagic shock, no treatment) served as a control, vehicle rats received saline, and VPA rats received doses of 300 mg/kg, 400 mg/kg, or 450 mg/kg. Data are shown as mean relative density of phosphorylated GSK-3β. * denotes a significant difference (p<0.05).
3.5. Split Dose Study: Activation of Akt
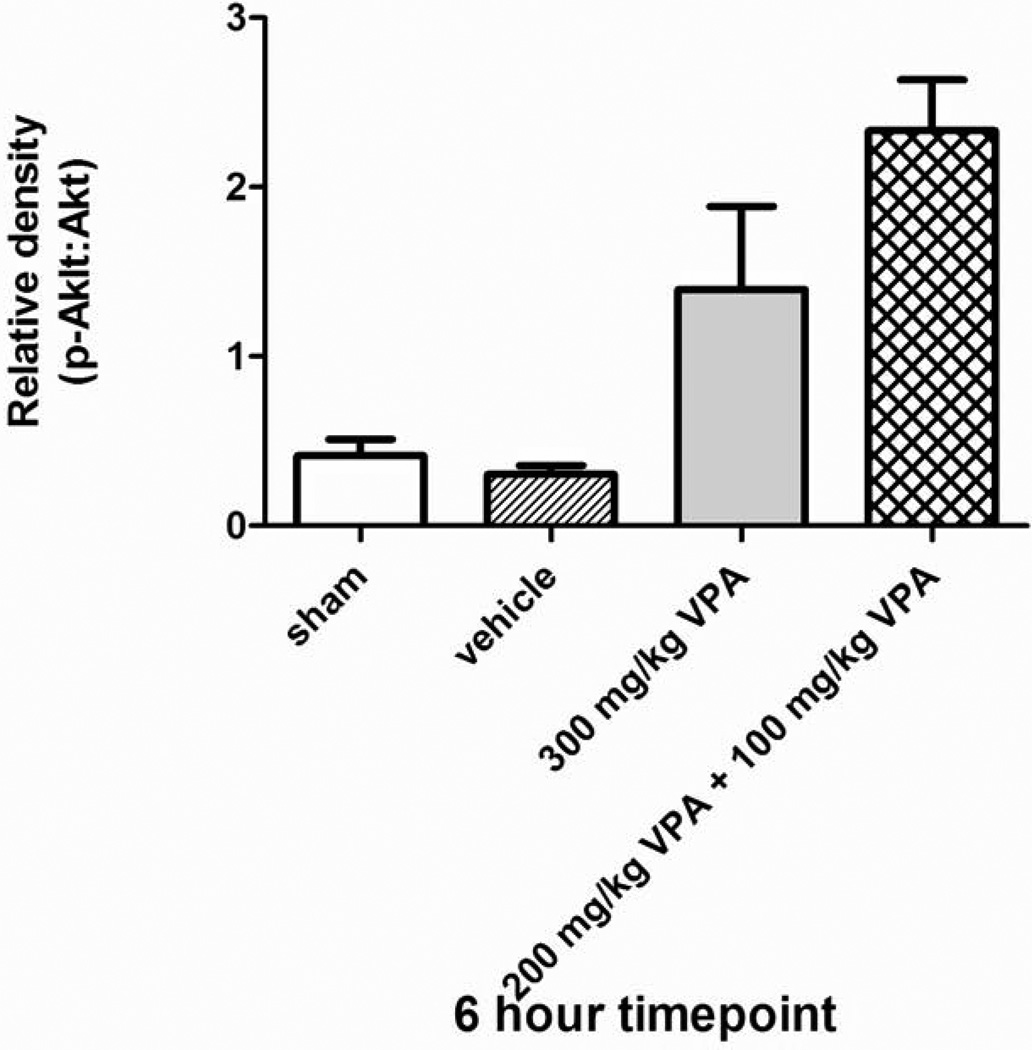
The efficacy of a split-dose of VPA in activating survival proteins was measured by the degree of Akt phosphorylation in the liver tissue after sub-lethal hemorrhage. There was no statistically significant difference in Akt activation between a 300 mg/kg dose and a 200 mg/kg initial dose + 100 mg/kg booster (Figure 6). In fact, split dose regimen resulted in a higher (albeit not statistically significant) activation ratio.
Figure 6. Split-dose Study Effect of Hemorrhage and VPA on Phosphorylation of Akt in Liver Tissue.
Rat liver was harvested 6 hours after hemorrhagic shock and administration of treatment. Sham (no hemorrhagic shock, no treatment) served as a control, vehicle rats received saline, and VPA rats received either a 300 mg/kg dose or a split dose of 200 mg/kg followed by 100 mg/kg. Data are shown as mean relative density of phosphorylated Akt: Akt. * denotes a significant difference (p<0.05).
3.6. Dose De-escalation Study: Survival as an Endpoint
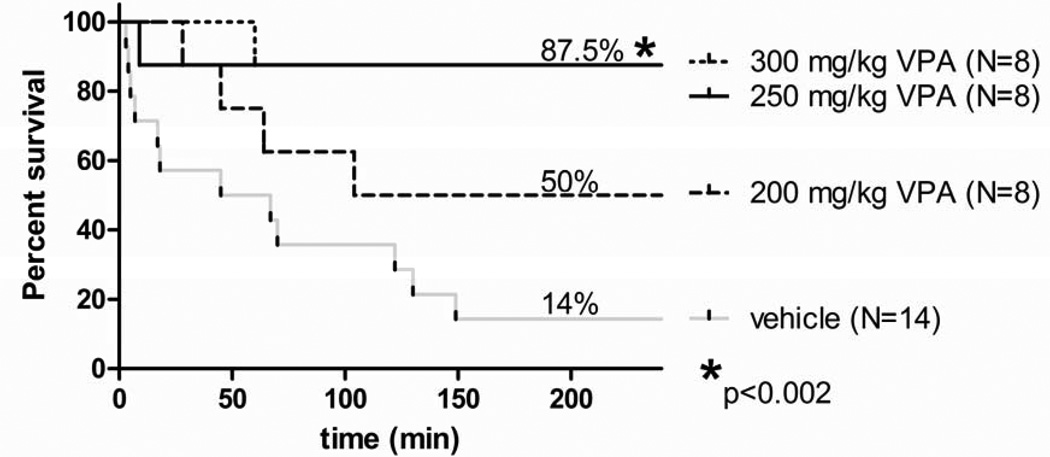
Using 24 hour survival as the primary endpoint, we found that 87.5%, 87.5%, 50%, and 14% of the animals survived in the 300 mg/kg, 250 mg/kg, 200 mg/kg and vehicle control, respectively. The 300 mg/kg and the 250 mg/kg doses were equally effective in improving the survival rate compared to the vehicle treatment (p<0.002). Also, there were statistically significant differences in mean survival time between the vehicle group and the 300 mg/kg group, and the 250 mg/kg group (p<0.001 and p<0.002, respectively). Survival times for the vehicle, 300 mg/kg, 250 mg/kg, and 200 mg/kg groups were 215 ±135, 1268 ±173, 1261 ± 179, and 750 ± 261 minutes, respectively.
4. Discussion
In a series of 3 complementary experiments, we have shown that higher doses of VPA (400 and 450 mg/kg) are not clearly superior to the 300 mg/kg dose in activating the pro-survival pathways. The 300 mg/kg dose may be administered in a split fashion without losing efficacy, and doses as low as 250 mg/kg are just as beneficial in prolonging survival following lethal blood loss.
We have previously demonstrated that treatment with large doses of valproic acid (300 mg/kg) improves survival in rodent and swine model of hemorrhagic and septic shock [9, 11, 20, 21], gut ischemia/reperfusion [22], and traumatic brain injury [23]. The optimal dose of VPA, however, was unknown. In an earlier study, we noted that when 200 or 250 mg/kg of VPA was added to normal saline resuscitation (3×volume), it did not increase histone acetylation in a rodent model of 40% hemorrhage [24]. However, in that study, VPA was not given alone but added to fluids and the effect on survival was not measured. Additionally, non-nuclear proteins were not assayed and most importantly, tissues were harvested immediately after treatment (given over 45 minutes), which was too early for peak acetylation, as demonstrated by later studies. In a swine model of 60% blood loss, liver injury, and massive resuscitation, we administered a dose of 400mg/kg with a significant improvement in survival and robust activation of the PI3-Akt pathway [19]. The peak plasma levels of VPA in that experiment were 990µg/ml immediately at the end of VPA infusion, and 560 µg/ml after 2 hours. In a much more severe swine model with ongoing hemorrhage from liver and spleen we administered a dose of 300 mg/kg and achieved a peak serum level of 503.5µg/ml [25]. These serum levels in the large animal models were in line with this rodent study. However, none of those studies were designed to identify the optimal VPA dose. This current study is an attempt to determine the lowest efficacious dose of VPA in the setting of hemorrhagic shock.
The FDA first approved VPA in 1978 as a treatment for epilepsy, bipolar disorder, and migraines [26]. With many decades of wide spread use its characteristics are well established, making it a suitable candidate for potential use in trauma practice. In fact, the FDA recently approved our application for a phase I dose escalation study in healthy human volunteers and trauma patients. Although, we expect that this phase I trial will establish safety of larger doses, it will not answer the question about the lowest possible effective dose. An animal study with multiple tissue sampling time points is the most logical way to tackle this question. VPA has a relatively good therapeutic window with mood stabilizing effects seen when plasma concentrations are around 0.35 mM, [27]; and toxicities reported when the concentrations exceed 1.4 mM [28]. Recently, because of its tumor-suppressing properties, VPA has been tested in phase 1 dose-escalation clinical trials, where doses of 300 mg/kg are given over 5–6 days with a single maximum tolerable dose (MTD) of 60 mg/kg/day. Patients who received higher doses (>60 mg/kg) experienced neurological symptoms including somnolence, dizziness, and nausea being the most common [18]. These are reversible symptoms and clearly acceptable if the drug is given as a life saving strategy. Other rare, but more serious side effects include pancreatitis [29] and hepatic injury [30] occasionally seen in long term users of the drug. Whether a single large dose can cause the same injury pattern in unclear. A single dose of 500 mg/kg of VPA in fasting rats was shown by others to cause zone specific hepatic steatosis [31].
To determine whether VPA doses greater than 300 mg/kg have any additional benefits in treating hemorrhagic shock, we started with a dose escalation study. As we had previously shown that a 300 mg/kg dose improves survival to 75% in lethal hemorrhage [9], it was not practical to use survival as an endpoint for this experiment; instead, we selected the activation of well know pro-survival proteins as a surrogate marker of efficacy. We used a sub-lethal model of shock to ensure that all animals survive, and the tissue analysis is not affected by a “survival bias”. We chose the PI3K/Akt pathway as our gauge for cellular health because these proteins are involved in averting apoptotic events and activating pro-survival components. When activated by cytokines (like IL-4 and IL-13) and macrophage colony-stimulating factors (MCSFs), phosphatidylinositol 3-kinase (PI3K) phosphorylates its substrate, phosphatidylinsolitol, which then activates the downstream effector Akt through phosphorylation [32]. Akt sits at the crossroads between many regulatory functions, including glucose metabolism, protein translation, and the cell cycle [33]. Akt inhibits Bax, a pro-apoptotic protein in the Bcl-2 family, via phosphorylation at the Ser-128 site [34]. This prevents Bax from translocating into the mitochondria, where it inserts into the outer mitochondrial membrane, opening mitochondrial voltage-dependant anion channels for cytochrome c release, resulting in activation of apoptosis-triggering caspases [35]. Activated Akt also activates NF-κB, a transcription factor for many pro-survival genes, including Bcl-2, an inhibitor of Bax [36] and FLIP, an inhibitor of caspase-8 [37]. We have also studied GSK-3β, another downstream target of Akt, which is constitutively active. Cells with active forms of GSK-3 have increased levels of apoptosis [38]. Once inactivated by phosphorylation of amino acid residue Ser-9, GSK-3β promotes a pro-survival phenotype, conferring inflammatory protection by curbing cytokine and interleukin signaling [39]. Also, active GSK-3β tags β-catenin with phosphorylation, marking it for ubiquitination and proteasome-mediated degradation [40, 41]. Once GSK-3β is inactivated, β-catenin is able to translocate to the nucleus where it binds with members of the lymphoid enhancer factor-1 (LEF-1)/T factor (TCF) family [42] to recruit transcription coactivators that upregulate cyclin D1 and c-myc expression. Both genes code for products that promote cell growth and proliferation [43, 44]. Thus, phosphorylated PI3K, Akt, and GSK-3β serve as a measure of a pro-survival and anti-apoptotic phenotype in cells.
Although the 450 mg/kg dose of VPA produced statistically higher phosphorylation of PI3K, Akt, and GSK-3β at 1 hour compared to the vehicle, this was not superior to 300 and 400 mg/kg doses; thus, higher doses of VPA do not necessarily lead to a more robust activation of pro-survival proteins. Therefore, it is reasonable to administer 300 mg/kg of VPA in future hemorrhagic shock models, lethal or otherwise.
In the split-dose study, we examined the benefits of splitting the 300 mg/kg dose into two smaller doses. Theoretically, if the resulting protein activation were equivalent, a split dose would be advantageous over the single dose because it would limit the peak circulating VPA levels, and potentially offer a better safety profile. Since VPA has a turnover rate in plasma of 0.3–4 hours in rats [45], the second “booster” dose was given two hours later. We found no significant difference in phosphorylated Akt levels between split-dose and single dose VPA administration.
Thirdly, we performed a dose de-escalation study to find the lowest optimal dose, because, VPA high concentrations can be potentially toxic [46]. In addition to central nervous system depression, cases of prolonged hypotension [47] and hepatotoxicity [48] due to VPA overdose have also been reported. In this experiment, we subjected the animals to lethal blood loss and used survival as the primary endpoint. Both the 300 mg/kg and 250 mg/kg doses resulted in an identical survival rate of 87.5%. Even the 200 mg/kg VPA dose improved survival from 14% in the vehicle group to 50%. This, however, did not reach significance die to the small sample size. Taken together these data suggest that the “pro-survival” dose of VPA lies between 200–250 mg/kg.
Limitations of this study include a relatively small (but statistically adequate) sample size. We were unable to analyze tissue samples for comparative analyses in the more severe hemorrhage model (experiment 3) because the majority of vehicle treated rats (controls) died before 3 hours. Even the earliest circulating markers, such as CINC-1, are not significantly elevated in the serum until four or more hours after VPA treatment [49]. However, the underlying mechanisms have previously been studied in numerous previous experiments. Due to logistical constraints, we limited the test doses and pathways examined in this study. Higher doses may have different effects on different pathways. Despite these limitations, our data suggests that while higher doses of VPA result in the greater histone acetylation and activation of pro-survival protein signaling, there is no significant increase in activation between the 300 mg/kg, and the highest dose tested, 450 mg/kg. In fact, doses as low as 250 mg/kg produce the same survival advantage in lethal hemorrhagic shock as the 300 mg/kg dose. Also, VPA can be administered in a split- dose regimen without a reduction in its effectiveness. This knowledge is an important step in transitioning this novel approach from animal models to clinical use.
Figure 7. Dose De-escalation Study Effect of VPA on Survival after Lethal Hemorrhage.
Sprague-Dawley rats were subjected to lethal hemorrhage. Survival rates were recorded for 24h. Kaplan–Meier curves were used for the comparison of survival rates between the Veh control and VPA groups. The symbol * indicates a significant difference from the control group.
Acknowledgements
Funded by a grant (N000140910378) from the Office of Naval Research (PI: HBA), and NIH grant R01 GM084127 (PI: HBA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Advanced Trauma Life Support ATLS Student Course Manual. 9th Edition. Chicago, IL: 2012. American College of Surgeons Committee on Trauma. [Google Scholar]
- 2.Lee CC, Chang IJ, Yen ZS, et al. Effect of different resuscitation fluids on cytokine response in a rat model of hemorrhagic shock. Shock. 2005;24:177. doi: 10.1097/01.shk.0000171870.42900.15. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Koustova E, Shults C, Sailhamer EA, Alam HB. Differential effect of resuscitation on Toll-like receptors in a model of hemorrhagic shock without a septic challenge. Resuscitation. 2007;74:526. doi: 10.1016/j.resuscitation.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 4.Glister C, Satchell L, Michael AE, Bicknell AB, Knight PG. The antiepileptic drug valproic acid (VPA) inhibits steroidogenesis in bovine theca and granulosa cells in vitro. PLoS One. 2012;7:e49553. doi: 10.1371/journal.pone.0049553. doi: 10.1371/journal.pone.0049553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Göttlicher M. Valproic acid: an old drug newly discovered as inhibitor of histone deacetylases. Ann Hematol. 2004;83(Suppl 1):S91. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- 6.Saha RN, Pahan K. HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell Death Differ. 2006;13:539. doi: 10.1038/sj.cdd.4401769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin T, Alam HB, Chen H, et al. Cardiac histones are substrates of histone deacetylase activity in hemorrhagic shock and resuscitation. Surgery. 2006;139:365. doi: 10.1016/j.surg.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Alam HB, Shuja F, Butt MU, et al. Surviving blood loss without blood transfusion in a swine poly-trauma model. Surgery. 2009;146:325. doi: 10.1016/j.surg.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Shults C, Sailhamer EA, Li Y, et al. Surviving blood loss without fluid resuscitation. J Trauma. 2008;64:629. doi: 10.1097/TA.0b013e3181650ff3. [DOI] [PubMed] [Google Scholar]
- 10.Butt MU, Sailhamer EA, Li Y, et al. Pharmacologic resuscitation: cell protective mechanisms of histone deacetylase inhibition in leth al hemorrhagic shock. J Surg Res. 2009;156:290. doi: 10.1016/j.jss.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Fukudome EY, Kochanek AR, Li Y, et al. Pharmacologic resuscitation promotes survival and attenuates hemorrhageinduced activation of extracellular signal-regulated kinase 1/2. J Surg Res. 2010;163:118. doi: 10.1016/j.jss.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Liu B, Dillon ST, et al. Identification of a novel potential biomarker in a model of hemorrhagic shock and valproic acid treatment. J Surg Res. 2010;159:474. doi: 10.1016/j.jss.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Kim K, Li Y, Jin G, et al. Effect of Valproic Acid on Acute Lung Injury in a Rodent Model of Intestinal Ischemia Reperfusion. Resuscitation. 2012;83:243. doi: 10.1016/j.resuscitation.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Causey MW, Miller S, Hoffer Z, et al. Beneficial effects of histone deacetylase inhibition with severe hemorrhage and ischemia-reperfusion injury. J Surg Res. 2013 doi: 10.1016/j.jss.2013.03.087. In press. [DOI] [PubMed] [Google Scholar]
- 15.Jin G, Duggan M, Imam A, et al. Pharmacologic resuscitation for hemorrhagic shock combined with traumatic brain injury. J Trauma Acute Care Surg. 2012;73:1461. doi: 10.1097/TA.0b013e3182782641. [DOI] [PubMed] [Google Scholar]
- 16.Hwabejire JO, Jin G, Imam AM, et al. Pharmacological Modulation of Cerebral Metabolic Derangement and Excitotoxicity in a Porcine Model of Traumatic Brain Injury and Hemorrhagic Shock. Surgery. 2013;154:234–243. doi: 10.1016/j.surg.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Alam HB. Creating a pro-survival and anti-inflammatory phenotype by modulation of acetylation in models of hemorrhagic and septic shock. Adv Exp Med Biol. 2012;710:107. doi: 10.1007/978-1-4419-5638-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atmaca A, Al-Batran SE, Maurer A, et al. Valproic acid (VPA) in patients with refractory advanced cancer: a dose escalating phase I clinical trial. Br J Cancer. 2007;97:177. doi: 10.1038/sj.bjc.6603851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alam HB, Shuja F, Butt MU, et al. Surviving blood loss without blood transfusion in a swine poly-trauma model. Surgery. 2009;146:325. doi: 10.1016/j.surg.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Alam HB, Hamwi KB, Duggan M, et al. Hemostatic and pharmacologic resuscitation: results of a long-term survival study in a swine polytrauma model. J Trauma. 2011;70:636. doi: 10.1097/TA.0b013e31820d0dcc. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Li Y, Liu B, et al. Synergistic effects of hypertonic saline and valproic acid in a lethal rat two-hit model. J Trauma Acute Care Surg. 2013;74:991. doi: 10.1097/TA.0b013e31828583e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim K, Li Y, Jin G, et al. Effect of valproic acid on acute lung injury in a rodent model of intestinal ischemia reperfusion. Resuscitation. 2012;83:243. doi: 10.1016/j.resuscitation.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin G, Duggan M, Imam A, et al. Pharmacologic resuscitation for hemorrhagic shock combined with traumatic brain injury. J Trauma Acute Care Surg. 2012;73:1461. doi: 10.1097/TA.0b013e3182782641. [DOI] [PubMed] [Google Scholar]
- 24.Lin T, Chen H, Koustova E, et al. Histone deacetylase as therapeutic target in a rodent model of hemorrhagic shock: effect of different resuscitation strategies on lung and liver. Surgery. 2007;141:784. doi: 10.1016/j.surg.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Alam HB, Hamwi KB, Duggan M, et al. Hemostatic and pharmacologic resuscitation: results of a long-term survival study in a swine polytrauma model. J Trauma. 2011;70:636. doi: 10.1097/TA.0b013e31820d0dcc. [DOI] [PubMed] [Google Scholar]
- 26. [Last Accessed June, 2013];U.S. Food and Drug Administration Information for healthcare professionals: risk of neural tube birth defects following prenatal exposure to valproate. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm192649.htm.
- 27.Bowden CL, Brugger AM, Swann AC, et al. Efficacy of divalproex vs lithium and placebo in the treatment of mania. The Depakote Mania Study Group. JAMA. 1994;271:918. [PubMed] [Google Scholar]
- 28.McElroy SL, Keck PE Jr, editors. Textbook of Psychopharmacology. Washington DC: American Psychiatric Press; 1995. [Google Scholar]
- 29.Nørgaard M, Jacobsen J, Ratanajamit C, et al. Valproic acid and risk of acute pancreatitis: a population-based case-control study. Am J Ther. 2006;13:113. doi: 10.1097/00045391-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 30.eHealthMe. [Last Accessed June, 2013];From FDA reports: Valproic acid and Hepatitis. http://www.ehealthme.com/ds/valproic+acid/hepatitis. [Google Scholar]
- 31.Olson MJ, Handler JA, Thurman RG. Mechanism of zone-specific hepatic steatosis caused by valproate: inhibition of ketogenesis in periportal regions of the liver lobule. Mol Pharmacol. 1986;30:520. [PubMed] [Google Scholar]
- 32.Antoniv TT, Ivashkiv LB. Interleukin-10-induced gene expression and suppressive function are selectively modulated by the PI3K-Akt-GSK3 pathway. Immunology. 2011;132:567. doi: 10.1111/j.1365-2567.2010.03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duronio V. The life of a cell: apoptosis regulation by the PI3K/PKB pathway. Biochem J. 2008;415:333. doi: 10.1042/BJ20081056. [DOI] [PubMed] [Google Scholar]
- 34.Gardai SJ, Hildeman DA, Frankel SK, et al. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem. 2004;279:21085. doi: 10.1074/jbc.M400063200. [DOI] [PubMed] [Google Scholar]
- 35.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hundal RS, Gómez-Muñoz A, Kong JY, et al. Oxidized low density lipoprotein inhibits macrophage apoptosis by blocking ceramide generation, thereby maintaining protein kinase B activation and Bcl-XL levels. J Biol Chem. 2003;278:24399. doi: 10.1074/jbc.M209179200. [DOI] [PubMed] [Google Scholar]
- 37.Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3- Kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 39.Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem. 1997;272:24735. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- 41.Maniatis T. A ubiquitin ligase complex essential for the NF-kappaB, Wnt/Wingless, and Hedgehog signaling pathways. Genes Dev. 1999;13:505. doi: 10.1101/gad.13.5.505. [DOI] [PubMed] [Google Scholar]
- 42.Behrens J, von Kries JP, Kühl M, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 43.Shtutman M, Zhurinsky J, Simcha I, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci. 1999;96:5522. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He TC, Sparks AB, Rago C. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 45.Nau H. Species differences in pharmacokinetics and drug teratogenesis. Environ Health Perspect. 1986;70:113. doi: 10.1289/ehp.8670113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilimowska J, Florek E, Piekoszewski W. Disposition of valproic acid in self-poisoned adults. Basic Clin Pharmacol Toxicol. 2006;99:22. doi: 10.1111/j.1742-7843.2006.pto_417.x. [DOI] [PubMed] [Google Scholar]
- 47.Ota KS. Probable valproate sodium-associated hypotension. Am J Geriatr Pharmacother. 2010;8:281. doi: 10.1016/j.amjopharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Eikel D, Lampen A, Nau H. Teratogenic effects mediated by inhibition of histone deacetylases: evidence from quantitative structure activity relationships of 20 valproic acid derivatives. Chem Res Toxicol. 2006;19:272. doi: 10.1021/tx0502241. [DOI] [PubMed] [Google Scholar]
- 49.Fukudome EY, Li Y, Kochanek AR, et al. Pharmacologic resuscitation decreases circulating cytokine-induced neutrophil chemoattractant-1 levels and attenuates hemorrhage-induced acute lung injury. Surgery. 2012;152:254. doi: 10.1016/j.surg.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]