Abstract
Fluorination is a reaction that is useful in improving the chemical stability and changing the binding affinity of biologically active compounds. The protocol described here can be used to replace aliphatic, C(sp3)-H hydrogen in small molecules with fluorine. Notably, isolated methylene groups and unactivated benzylic sites are accessible. The method uses readily available manganese porphyrin and manganese salen catalysts and various fluoride ion reagents, including silver fluoride (AgF), tetrabutylammonium fluoride and triethylamine trihydrofluoride (TREAT·HF), as the source of fluorine. Typically, the reactions afford 50–70% yield of mono-fluorinated products in one step. Two representative examples, the fragrance component celestolide and the nonsteroidal anti-inflammatory drug ibuprofen, are described; they produced useful isolated quantities (250–300 mg, ~50% yield) of fluorinated material over periods of 1–8 h. The procedures are performed in a typical fume hood using ordinary laboratory glassware. No special precautions to rigorously exclude water are required.
INTRODUCTION
The use of fluorinated drug compounds, herbicides and pesticides has become extremely important in the pharmaceutical and agrochemical industries1–3. The introduction of fluorine into a molecular scaffold can lead to profound alterations in a number of pharmacokinetic parameters, such as biological activity, target affinity, phase-I metabolism and molecular transport. In addition, radioactive 18F-labeled imaging agents, such as 2-[18F]fluoro-2-deoxyglucose, have found wide application in positron emission tomography (PET)4–7. Among various methods for incorporating fluorine atoms, substitution of the ubiquitous hydrogen atom by fluorine is of crucial importance and practical value2,3. As the van der Waals radius of fluorine is only slightly larger than that of hydrogen, fluorine substitution exerts only minor steric effects on molecular interactions with a protein3. In addition, fluorine can actively participate in hydrogen bonding and electrostatic interactions. For this reason, fluorinated derivatives often show stronger binding to protein targets than the parent molecules do2,3. The C-H bonds of these bioactive molecules are often the sites of hydroxylation by cytochrome P450 enzymes during phase-I metabolism. Thus, fluorine substitution for hydrogen generally increases the metabolic stability of the molecule3. C-H fluorination reactions, especially late-stage fluorination methods during the structure-activity relationship stage of development, can potentially provide facile access to fluorinated derivatives of currently known pharmaceutical or agrochemical structures, enabling the discovery of new agents, the diversification of old ones or novel lead compounds for therapeutic and PET imaging applications2,3. However, it has proven to be very challenging to develop synthetic methods for C-H fluorination that do not require special laboratory conditions and equipment. Further, these methods usually involve the use of fluorine gas and can lead to product mixtures that are difficult to separate8.
Although chemists have developed a variety of new and useful methods for the fluorination of organic molecules over the past 5 years9, one-step C(sp3)-H fluorination reactions remain rare. The first catalytic C-H fluorination reaction was developed by Sanford and co-workers10 using electrophilic fluorination reagents (F+) such as N-fluoropyridinium salts and Selectfluor with palladium catalysts10. Very recently, several other C-H fluorination reactions have been developed on the basis of F+ reagents as well as nucleophilic fluoride sources11–14. Lectka’s group described a poly-component metal-catalyzed C-H fluorination using Selectfluor as the fluorine source11. Sanford and coworkers12 have reported palladium-catalyzed C-H fluorination of a variety of 8-methylquinoline derivatives using AgF as the nucleophilic fluoride source.
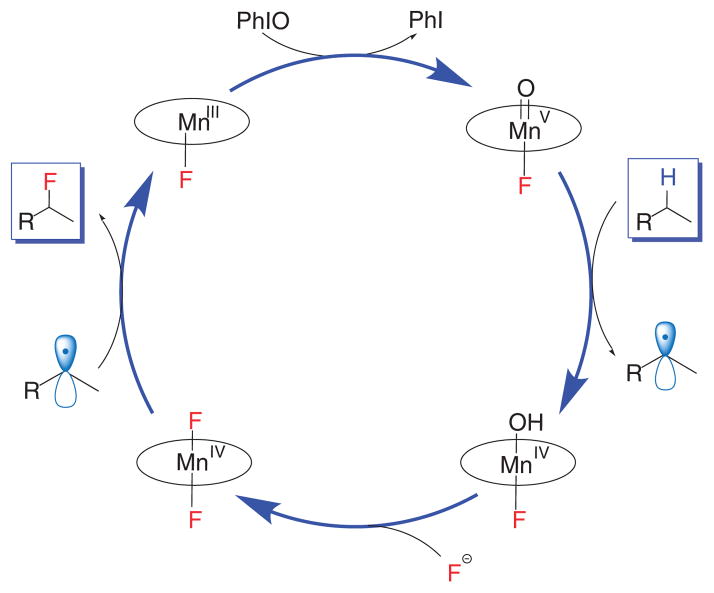
The recent discovery of an efficient process for the one-step conversion of unactivated aliphatic carbon-hydrogen bonds into carbon-fluorine bonds has added a potentially powerful tool to the synthetic chemist’s toolbox15. The reaction used a manganese porphyrin catalyst, using fluoride ions from the simple, easy-to-use reagents AgF and tetrabutylammonium fluoride. Mechanistic examinations have revealed that the reaction proceeds through a catalytic cycle involving a novel trans-difluoro manganese(IV) complex, which has been isolated and structurally characterized. This F-Mn(IV)-F species has been shown to transfer a fluorine atom efficiently to short-lived substrate radicals generated by a reactive oxoMn(V) intermediate (Fig. 1). We have also extended this protocol to manganese salen catalysts, resulting in a process that has enabled efficient transformation of benzylic C-H bonds to C-F bonds16.
Figure 1.
General catalytic cycle for manganese porphyrin/salen–catalyzed C-H fluorination.
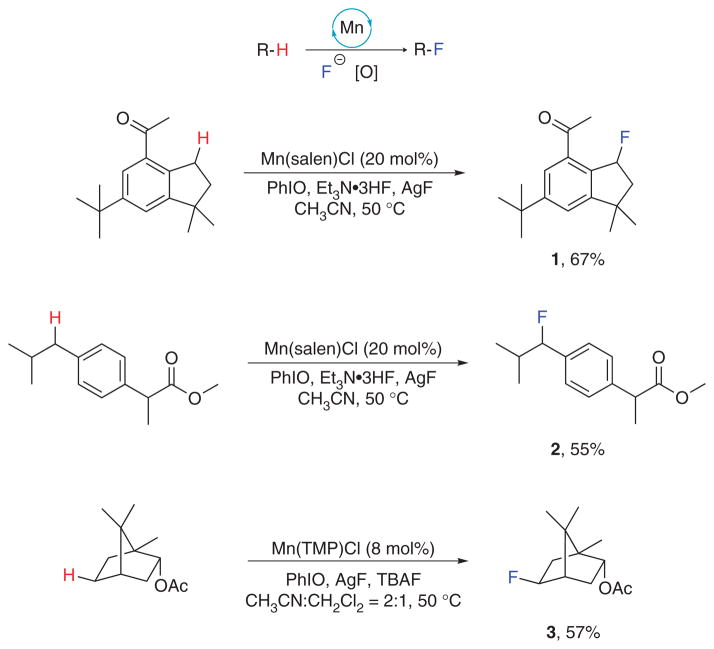
These manganese porphyrin– and manganese salen–catalyzed C-H fluorination reactions are carried out in common organic solvents (acetonitrile or acetonitrile/dichloromethane), and the catalysts are commercially available or can be easily prepared from bench-stable, commercially available reagents. Various functional groups including ethers, tertiary alcohols, amides, imides, esters, ketones, halogens and heterocycles are all well tolerated. The methods can also be applied to molecules with structures of biological or pharmaceutical importance such as vitamin E acetate, terpenoids, steroids, tetrahydronaphthalene, indan, tetrahydroquinoline and dibenzocycloheptene16. High regioselectivity has also been achieved. For example, the monoterpene, bornyl acetate, was converted exclusively to exo-5-fluoro-bornyl acetate (compound 3; Fig. 2). The steroid, 5α-androstan-17-one, was fluorinated selectively in the A ring, and vitamin E acetate was fluorinated only at the benzylic position15.
Figure 2.
General reaction scheme and the representative fluorides synthesized using the described protocol.
Here we present detailed protocols for C-H fluorination using this novel manganese-based catalytic system. As examples of the present synthetic approach, we describe in detail the fluorination procedures of a common fragrance component, celestolide (to form compound 1; Fig. 2), and a widely used, anti-inflammatory drug, ibuprofen ester (to form compound 2; Fig. 2). Yields listed in this figure are on the basis of smaller-scale reactions as reported in refs. 15 and 16. Yields for larger scale as reported in this protocol are slightly lower (55 and 49%, respectively). Celestolide is a good example for this approach because it contains a carbonyl group, which might be reactive under traditional fluorination conditions, such as C-H hydroxylation followed by treatment with diethylaminosulfur trifluoride (DAST). The fluorination of bornyl acetate can be achieved in a similar manner, with 8 mol% Mn(TMP)Cl as the catalyst in place of Mn(salen)Cl.
Experimental design
Scale of reactions
Normally, the protocol described here can be applied to reactions ranging from 0.8-mmol to 2-mmol scales. Higher yields were observed at small scale.
Choice of catalyst
Manganese porphyrins can be used for aliphatic C-H fluorinations. Manganese salen complexes are the better choice for benzylic C-H fluorinations.
Choice of fluoride source
For aliphatic C-H fluorination, the combination of the tetrabutylammonium fluoride with AgF should be used. For benzylic C-H fluorination, the combination of TREAT·HF with AgF can be used.
MATERIALS
REAGENTS
! CAUTION All chemicals must be handled with care, and thus a lab coat, gloves and eye protection should always be used. All operations must be performed in a laboratory fume hood. Fluoride reagents should be handled with caution, as they are toxic.
(R,R)-(-)-N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamino-manganese(III) chloride (Mn(salen)Cl; Aldrich, cat. no. 404446)
Methyl 2-(4-isobutylphenyl)propanoate (Ibuprofen methyl ester; Acros, cat. no. SEW06433EA)
4-Acetyl-6-tert-butyl-1,1-dimethylindan (Celestolide; TCI, cat. no. A2209)
Silver fluoride (AgF; Aldrich, cat. no. 226866)
Triethylamine trihydrofluoride (TREAT·HF; Aldrich, cat. no. 344648)
Acetonitrile (CH3CN; see Reagent Setup)
(Diacetoxyiodo)benzene (PhI(OAc)2; Aldrich, cat. no. 178721)
Iodosylbenzene (PhIO; see Reagent Setup)
Ethyl acetate (Aldrich, cat. no. 319902)
Dichloromethane (DCM; Aldrich, cat. no. 650463)
Hexanes (Aldrich, cat. no. 227064)
EQUIPMENT
Dual nitrogen–vacuum manifold with vacuum line
Vacuum pump
Schlenk tube flask with 2-mm polytetrafluoroethylene (PTFE) stopcock (50 ml, 14/20; VWR, cat. no. 60003-100)
Teflon-coated magnetic stir bars (3 × 12.7 mm; VWR, cat. no. 58947-140)
Teflon-coated magnetic stir bars (10 × 70 mm; VWR, cat. no. 74950-300)
Rubber septa (14/20; VWR, cat. no. 890-916)
Syringe (VWR, cat. no. BD309597)
Needle (VWR, cat. no. BD305156)
Microliter syringe (100 μl; Hamilton, cat. no. 7638-01)
Small hub removable needles (10 inch; Hamilton, cat. no. 7731-03)
Magnetic stir plate with heating functionality
Water bath
Weighing balance
Spatula
Plastic pipette
Disposable glass pipette
Weighing paper
Silica thin-layer chromatography (TLC) plates and spotter (EMD, Silica Gel 60, F25)
TLC developing tank
RediSep Rf disposable flash columns (40 g; VWR, cat. no. 10432-872)
Screw-thread vials (4 ml, 10-425; VWR, cat. no. 82028-426)
Polyethylene vials (20 ml; VWR, cat. no. 66022-274)
Polypropylene caps with White Silicone Septa (VWR, cat. no. 82028-434)
Flash chromatography system (Teledyne, CombiFlash Rf 200)
Test tubes
Büchner funnel with vacuum adaptor (14/20, 60 ml; VWR, cat. no. 80068-472)
Round-bottom flask
Büchner filter funnel (240 mm; VWR, 89038-134)
Filtering flask (2 liter; VWR, cat. no. 89090-856)
Rotary evaporator
Solvent system
Access to NMR and mass spectrometry instruments/apparatus
REAGENT SETUP
PhIO
Prepare PhIO by the hydrolysis of PhI(OAc)2 in excess 4N NaOH solution (~5 equivalents) for 4 h in a beaker. After suction filtration and washing with a large amount of water until the filtrate becomes neutral, solid, yellow PhIO is obtained. Leave the PhIO solid in the funnel overnight with suction pump connected for complete dryness. Grind the solid PhIO into powder and store it in a refrigerator. PhIO should be used within 2 months.
▲ CRITICAL Choose a large magnetic stir bar (e.g., 10 × 70 mm), as the solution will become a slurry within 1 h of hydrolysis.
CH3CN
Purify and dry CH3CN through a Grubbs-type solvent purification system17. The dry solvent can be stored in a sealed vial and used within 1 h.
EQUIPMENT SETUP
Gas chromatography–mass spectrometry (GC-MS)
Perform GC-MS analyses on an Agilent 7890A gas chromatograph equipped with an Agilent 5975 mass selective detector. A representative method for monitoring the reaction is as follows: (i) set the oven temperature at 50 °C upon injection; (ii) hold the temperature at 50 °C for 2 min; (iii) increase the temperature to 250 °C for 20 min; and (iv) hold the temperature at 250 °C for 2 min.
Schlenk flask
Before use, dry the Schlenk flask in an oven overnight at 100 °C, and cool it in a desiccator.
PROCEDURE
Reaction setup ● TIMING 15–20 min
▲ CRITICAL Detailed instructions are provided for the fluorination of celestolide. The fluorination of ibuprofen follows the same steps, differing only in small details. These are summarized under the appropriate headings in the ANTICIPATED RESULTS section.
-
1|
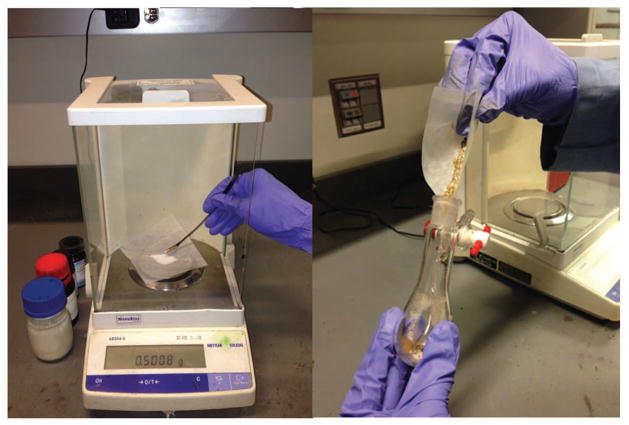
Weigh 500 mg of 4-acetyl-6-tert-butyl-1,1-dimethylindan (Celestolide), 260 mg (20 mol%) of Mn(salen)Cl and 780 mg (3 equivalents) of AgF into a 25-ml Schlenk tube flask. Place a Teon-coated magnetic stir bar in the flask. Cap the flask with a rubber septum (Fig. 3).
▲ CRITICAL STEP As AgF is light sensitive, weighing of AgF should be carried out quickly and in an area of low light intensity. The color of AgF will become dark if the weighing step takes too much time.
-
2|
Connect the Schlenk flask to the Schlenk line. Evacuate the flask for 2 min and back-fill it with nitrogen for 1 min. Repeat the pump–back-fill cycle three times (Fig. 4).
▲ CRITICAL STEP As AgF is light sensitive, all operations should be done in dark places. Normally, turning off the light of the hood and wrapping the bottom of the flask with aluminum foil is sufficient to avoid the decomposition of AgF.
-
3|
Add 130 μl of TREAT·HF into a 4-ml vial. Cap it and add 1.0 ml of dry and degassed CH3CN (from the solvent system) via syringe through the septum into the vial. Swirl the vial to obtain a clear solution. Flush N2 into the vial for 4 min. During all of these processes, the vial should be under a positive pressure of N2.
▲ CRITICAL TREAT·HF is used as received. This procedure is intended for substrates that are solids. Liquid substrates can be dissolved in CH3CN and dispensed via syringe.
-
4|
Transfer the CH3CN solution of TREAT·HF into the Schlenk flask using a 1-ml syringe with a needle (Fig. 5). Wash the syringe with additional 0.5 ml of CH3CN, and then transfer it into the reaction vessel as well. During the transfer, keep the Schlenk flask and the vial under positive N2 pressure.
? TROUBLESHOOTING
-
5|
Submerge the bottom portion of the Schlenk flask in a preheated water bath at 50 °C. Set the speed of stirring to ~600 r.p.m.
▲ CRITICAL STEP Avoid stirring too vigorously because the reaction mixture, especially AgF, will spill onto the wall of the flask.
? TROUBLESHOOTING
Figure 3.
Reaction setup. Left, weighing reagents on the balance. Right, transfer of the reagents to the reaction flask.
Figure 4.
Deaeration of the reaction flask.
Figure 5.
Addition of liquids by syringe through the septum.
Fluorination of 4-acetyl-6-tert-butyl-1,1-dimethylindan ● TIMING 6–8 h
▲ CRITICAL During the fluorination, the Schlenk flask should be connected to the N2 flow. Control the on/off of N2 flow with stopcock.
-
6|
Weigh 450 mg of PhIO (1 equivalent) in the 4-ml vial.
-
7|
Add the 1 equivalent of PhIO into the Schlenk flask in small portions within 1 h. The detailed procedure for each addition is as follows: (i) connect the Schlenk flask to N2 by turning the stopcock; (ii) take a small portion of PhIO using the bottom part of a disposable glass pipette; (iii) quickly open the rubber septa and add the PhIO into the reaction mixture using the glass pipette; (iv) cap the flask with rubber septa and keep the N2 flow for 1 more minute before closing the N2 by turning the stopcock (Fig. 6).
▲ CRITICAL STEP Every portion is ~70–80 mg. The time interval between the addition of each portion is about 4 min.
? TROUBLESHOOTING
-
8|
Repeat Step 7 until all of the PhIO has been added.
▲ CRITICAL STEP As the reaction progresses, the solvent volume may decrease. Add 0.5 ml of CH3CN into the reaction mixture for every 2 equivalent of added PhIO.
-
9|
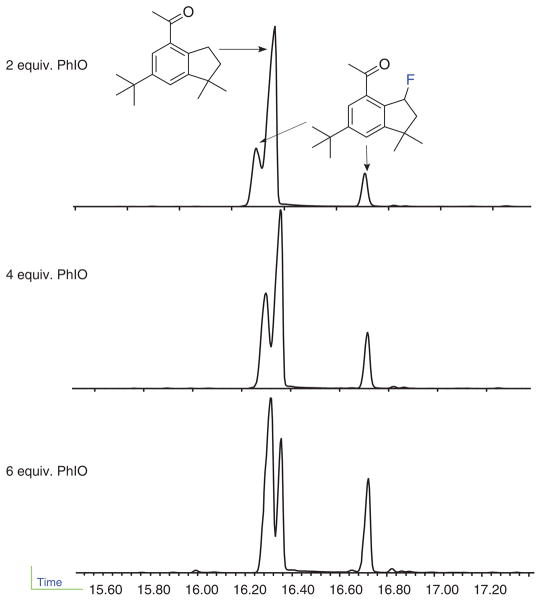
After the addition of 4 equivalents of PhIO, for every 0.5–1 equivalents of newly added PhIO, use a microliter syringe to take an aliquot (~5 μl) of the reaction mixture. Dilute the aliquot with 1 ml of DCM and pass through a 3-cm plug of silica gel, eluting with excess DCM (~10 ml). The resulting solution is used for monitoring the reaction by GC/MS as described in Equipment Setup (Fig. 7).
▲ CRITICAL STEP Stop the stirring when taking the aliquot to avoid clogging of the syringe.
-
10|
The reaction would be stopped when there is no further increase in yield with newly added PhIO. The flask is removed from the bath and allowed to cool to room temperature (25 °C).
▲ CRITICAL STEP The stop point differs from substrate to substrate, typically ranging from 4–9 equivalents of PhIO. For celestolide, the stopping point was around 5 equivalents of PhIO.
Figure 6.
Addition of iodosylbenzene to the reaction mixture.
Figure 7.
Monitoring the reaction by GC-MS. The fluorinated celestolide shows two peaks in the GC trace owing to partial loss of HF.
Purification of the product ● TIMING 1–1.5 h
-
11|
Dilute the reaction mixture with DCM (10 ml), and then filter the reaction mixture through a thin (2 cm) pad of silica gel using a Büchner funnel attached to a round-bottomed flask. Wash the reaction vessel and funnel with excess DCM (50 ml), which is also collected in the round-bottomed flask.
▲ CRITICAL STEP This step will remove the insoluble species in the reaction mixture. It is common that the color of the filtrate is red owing to the presence of catalyst, which will be removed in the later purification steps. ■ PAUSE POINT The filtrate can be stored overnight in a sealed round-bottomed flask in the cold (0 to −20 °C).
-
12|
Concentrate the solution under reduced pressure using a rotary evaporator at a temperature of 25 °C.
-
13|
Purify the crude product by flash chromatography on silica gel (RediSep Rf normal-phase flash column, 40 g) using a mixture of hexane and ethyl acetate (Fig. 8). Retention times are as follows: PhI at 5 min, substrate at 15 min, product at 16 min and by-product at 26 min.
-
14|
Collect the fractions that contain the pure product, as determined by GC-MS, into a round-bottomed flask and remove the solvent using a rotary evaporator at a temperature of 25–30 °C.
? TROUBLESHOOTING
-
15|
Check structure and purity of the product by NMR and GC-MS by using the methods mentioned in the Equipment Setup section.
? TROUBLESHOOTING
Figure 8.
Separation of reaction mixture by flash column chromatography.
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
TABLE 1.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 4 | Reaction reagents stick on the wall of the flask during the transfer | N2 flows too fast | Decrease the flow of N2 Wash the wall of the flask with 0.5 ml CH3CN |
| 5 | The septum pops out | The flask is placed too deeply in the bath | Regulate the height of the flask to let the bath just submerge the reaction mixture |
| 7 | PhIO powder sticks to the side wall of the flask | N2 flows too fast and some CH3CN evaporates and condenses on the flask wall | Decrease the flow of N2 or plug the pipette deeper in the flask during the addition of the oxidant Use 0.5 ml of CH3CN and wash the wall of the flask every 2 equivalents of added PhIO |
| 14 | High GC-MS yield but very low isolated yield | Pure fluorination products are unstable in glassware | Concentrate the volume of solution to about 5 ml and transfer the concentrated solution to a 20 ml polyethylene vial for further evaporation Use PTFE RBF during the evaporation step |
| Fluorination products are unstable on silica gel | Change the method to shorten the time of chromatography Use other materials as stationary phase, such as florisil |
||
| 15 | Pure product is verified in GC-MS, but the NMR shows a mixture of compounds | The fluorination product is unstable in CDCl3 | Use acetone-d6 or benzene-d6 instead |
● TIMING
Steps 1–5, reaction setup: 15–20 min
Steps 6–10, fluorination of 4-acetyl-6-tert-butyl-1,1-dimethylindan: 6–8 h
Steps 11–15, purification of the product: 1–1.5 h
ANTICIPATED RESULTS
Preparation of 4-acetyl-6-tert-butyl-3-fluoro-1,1-dimethylindan (F-celestolide)
By following the procedure depicted above, and by using 4-acetyl-6-tert-butyl-1,1-dimethylindan (500 mg), Mn(salen)Cl (260 mg), TREAT·HF (126 μl) and AgF (780 mg) in CH3CN (1 ml), the reaction mixture was stirred at 50 °C under N2 atmosphere with PhIO added in small portions. 5 equivalents of PhIO was added to achieve the maximum yield as determined by GC-MS. The reaction time was about 5 h. Flash column chromatography on silica gel (method: 0% ethyl acetate/hexanes - 15% ethyl acetate/hexanes) provided the title compound as a yellow solid (295 mg, 55%). 1H NMR (500 MHz, CDCl3) 1.34 (s, 3H), 1.37 (s, 12H), 2.39–2.06 (m, 2H), 2.65 (s, 3H), 6.44 (ddd, J = 53.9, 5.9, 1.5 Hz, 1H), 7.43 (t, J = 1.5 Hz, 1H), 7.77 (d, J = 1.7 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 28.6, 29.0, 31.4, 31.5, 35.2, 42.7, 48.4, 93.9, 123.6, 125.7, 134.8, 135.2, 154.2, 155.9, 199.9; 19F NMR –158.6 p.p.m.; MS (EI) m/z calculated C17H23FO [M]+ : 262.2, found 262.2.
Preparation of methyl 2-(4-(1-fluoro-2-methylpropyl)phenyl)propanoate (F-ibuprofen methyl ester)
By following the procedure described above, and by using ibuprofen methyl ester (500 mg), Mn(salen)Cl (288 mg), TREAT·HF (140 μl) and AgF (865 mg) in CH3CN (1.4 ml), the reaction mixture was stirred at 50 °C under N2 atmosphere with PhIO added in small portions. 4 equivalents of PhIO were added to reach the maximum yield, as determined by GC-MS. The reaction time was about 4 h. Flash column chromatography on silica gel (method: 0% ethyl acetate/hexanes – 20% ethyl acetate/hexanes) provided the title compound as colorless oil (265 mg, 49%). 1H NMR (500 MHz, CDCl3) 0.77 (d, J = 6.9 Hz, 3H), 0.94 (d, J = 6.8 Hz, 3H), 1.42 (dd, J = 7.3, 4.7 Hz, 3H), 2.01 (dh, J = 16.8, 6.7 Hz, 1H), 3.58 (s, 3H), 3.66 (q, J = 7.2 Hz, 1H), 5.00 (dd, J = 47.0, 6.9 Hz, 1H), 7.23 – 7.13 (m, 4H); 13C NMR (125 MHz, CDCl3) δ 17.6, 18.4, 18.6, 34.2, 34.4, 45.2, 52.1, 99.3, 126.46, 126.52, 127.5, 138.3, 140.7, 175.0; 19F NMR –179.0 p.p.m.; MS (EI) m/z calculated C14H19FO2 [M]+ : 238.1, found 238.1.
Acknowledgments
C-H fluorination of hydrocarbons and method development were supported by the Center for Catalytic Hydrocarbon Functionalization, an Energy Frontier Research Center, US Department of Energy, Office of Science, Basic Energy Sciences, under award no. DE SC0001298. Fluorination of biomolecules and mechanistic analyses were supported by the US National Science Foundation (CHE-1148597). Purchase of the GC-mass spectrometer was supported by the National Institutes of Health (2R37 GM036298). Partial support of this work, including the purchase of flash chromatographic equipment, was provided by Merck, Inc.
Footnotes
AUTHOR CONTRIBUTIONS
W.L., X.H. and J.T.G. designed the experiments; W.L. and X.H. conducted the experiments; W.L., X.H. and J.T.G. analyzed the data and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Bohm HJ, et al. Fluorine in medicinal chemistry. Chembiochem. 2004;5:637–643. doi: 10.1002/cbic.200301023. [DOI] [PubMed] [Google Scholar]
- 2.Muller K, Faeh C, Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 3.Purser S, Moore PR, Swallow S, Gouverneur V. Fluorine in medicinal chemistry. Chem Soc Rev. 2008;37:320–330. doi: 10.1039/b610213c. [DOI] [PubMed] [Google Scholar]
- 4.Ametamey SM, Honer M, Schubiger PA. Molecular imaging with PET. Chem Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 5.Lee E, Hooker JM, Ritter T. Nickel-mediated oxidative fluorination for PET with aqueous F-18 fluoride. J Am Chem Soc. 2012;134:17456–17458. doi: 10.1021/ja3084797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee E, et al. A fluoride-derived electrophilic late-stage fluorination reagent for PET imaging. Science. 2011;334:639–642. doi: 10.1126/science.1212625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlyer DJ. PET tracers and radiochemistry. Ann Acad Med Singapore. 2004;33:146–154. [PubMed] [Google Scholar]
- 8.Rozen S. Elemental fluorine and HOF-CH3CN in service of general organic chemistry. Eur J Org Chem. 2005;2005:2433–2447. [Google Scholar]
- 9.Ritter T, Furuya T, Kamlet AS. Catalysis for fluorination and trifluoromethylation. Nature. 2011;473:470–477. doi: 10.1038/nature10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hull KL, Anani WQ, Sanford MS. Palladium-catalyzed fluorination of carbon-hydrogen bonds. J Am Chem Soc. 2006;128:7134–7135. doi: 10.1021/ja061943k. [DOI] [PubMed] [Google Scholar]
- 11.Bloom S, et al. A polycomponent metal-catalyzed aliphatic, allylic, and benzylic fluorination. Angew Chem Int Ed Engl. 2012;51:10580–10583. doi: 10.1002/anie.201203642. [DOI] [PubMed] [Google Scholar]
- 12.McMurtrey KB, Racowski JM, Sanford MS. Pd-catalyzed C-H fluorination with nucleophilic fluoride. Org Lett. 2012;14:4094–4097. doi: 10.1021/ol301739f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloom S, et al. Iron(II)-catalyzed benzylic fluorination. Org Lett. 2013;15:1722–1724. doi: 10.1021/ol400424s. [DOI] [PubMed] [Google Scholar]
- 14.Amaoka Y, Nagatomo M, Inoue M. Metal-free fluorination of C(sp3)-H bonds using a catalytic N-oxyl radical. Org Lett. 2013;15:2160–2163. doi: 10.1021/ol4006757. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, et al. Oxidative aliphatic C-H fluorination with fluoride ion catalyzed by a manganese porphyrin. Science. 2012;337:1322–1325. doi: 10.1126/science.1222327. [DOI] [PubMed] [Google Scholar]
- 16.Liu W, Groves JT. Manganese-catalyzed oxidative benzylic C-H fluorination by fluoride ions. Angew Chem Int Ed Engl. 2013;52:6024–6027. doi: 10.1002/anie.201301097. [DOI] [PubMed] [Google Scholar]
- 17.Pangborn AB, Giardello MA, Grubbs RH, Rosen RK, Timmers FJ. Safe and convenient procedure for solvent purification. Organometallics. 1996;15:1518–1520. [Google Scholar]