Abstract
Objective
Endovascular interventions for critical limb ischemia (CLI) continue to have variable reported results. The purpose of this study is to determine the effect of disease level and distribution on the outcomes of tibial interventions.
Methods
A retrospective analysis of all tibial interventions done for CLI between 2006 and 2009 was performed. Outcomes of isolated tibial (group I) and multilevel interventions (group II) (femoropopliteal and tibial) were compared.
Results
Endovascular interventions were utilized to treat 136 limbs in 123 patients for CLI: 54 isolated tibial (85% tissue loss), and 82 multilevel (80% tissue loss). Mean age and baseline comorbidities were comparable. The mean ankle-brachial index (ABI) was significantly lower prior to intervention in group II (0.53 vs 0.74; P < .001) but was similar postintervention (0.86 vs 0.88; P = NS). Wound healing or improvement was achieved in 69% in group I and in 87% in group II (P = .05). Mean overall follow-up was 12.6 ± 5.3 months. Time to healing was significantly longer in group I: 11.5 ± 8.8 months vs 7.7 ± 6.6 months (P = .03). Limb salvage was achieved in 81% of group I and 95% of group II (P = .05). The rate of reintervention was similar (13% vs 18%, P = NS), so was the rate of late surgical conversion (0% vs 6%; P = NS). Limb loss resulted from lack of conduit or initial target vessel for bypass and high-risk systemic comorbidities. Overall mortality rates were similar among both groups. An isolated tibial intervention was a predictor of limb loss at 1 year on multivariate analysis and resulted in a lower rate of limb salvage at 1 year compared with multilevel interventions. Additionally, despite comparable primary patency rates, there was improved secondary patency with multilevel interventions compared with the isolated tibial interventions. Predictors of limb loss in patients treated with isolated tibial intervention included multiple synchronous tibial revascularization (P = .005) and advanced coronary artery disease requiring revascularization (P = .005).
Conclusions
Adequate rates of limb salvage can be achieved in patients undergoing multilevel interventions for CLI, and improved patency is seen with multilevel compared to isolated tibial interventions. Patients with isolated tibial disease appear to have a higher incidence of limb loss secondary to poor initial pedal runoff, more extensive distal disease, and severe comorbidities precluding surgical bypass. Other therapeutic strategies should be considered in these patients, including primary amputation or pedal bypass when applicable.
Patients presenting with critical limb ischemia (CLI) (rest pain and tissue loss, Rutherford category 4, 5, 6) have been traditionally treated with surgical bypass, however, advances in endovascular techniques, including subintimal angioplasty, as well as technological advances have allowed the successful treatment of more complex patterns of disease. This has resulted in a paradigm shift in the treatment of CLI, and multiple series have reported on successful percutaneous treatment of CLI at the femoral and popliteal levels.1–3
Recommendations for the treatment of infrapopliteal disease remain mixed, as the updated TransAtlantic Intersociety Consensus (TASC) II guidelines state that for the endovascular treatment of infrapopliteal disease, angioplasty may be indicated for limb salvage and that the treatment of tibial artery occlusion should be reserved for cases in which in-line flow into the pedal vasculature can be established.4 Additionally, while a diffuse disease distribution may be more challenging to treat percutaneously and may represent a more advanced plaque burden, improved outcomes have been previously suggested for multilevel compared with isolated tibial interventions in terms of patency.5 In an attempt to better define treatment algorithms based on lesion extent and distribution, this study sought to compare multilevel to isolated tibial interventions and determine predictors of failure.
METHODS
Patient population
All patients treated with an infrainguinal endovascular revascularization, which included tibial artery endovascular interventions (TAEI) between September 2006 and January 2009, were retrospectively identified from a physician database. Indications for treatment included rest pain (Rutherford category 4) and/or tissue loss (Rutherford category 5/6). Patients who presented with acute ischemia or who were treated for claudication were excluded. Patient characteristics, comorbidities, intervention sites, and complications were recorded. Limbs were grouped based on the level of intervention performed: limbs treated with isolated TAEI constituted group I (group I) and those undergoing multilevel interventions group II (group II). Clinical outcomes, including primary patency, primary-assisted patency, secondary patency, limb salvage, and wound healing rates were determined for both groups.
Endovascular approach
All procedures were performed by vascular surgeons using fixed-imaging under local anesthesia with conscious sedation. Contralateral retrograde common femoral access was most commonly performed, whereas ipsilateral antegrade access or transbrachial access was selectively used. Interventions were performed under systemic heparinization (100 U/kg). For complete occlusions, it has been our practice to cross femoropopliteal lesions in a subintimal plane given our higher success rate compared with intraluminal techniques, while an intraluminal recanalization was our first-line approach for tibial occlusions given the concerns of perforation and difficult reentry with subintimal techniques. Balloon diameter was selected based on the angiographic measurements of the nondiseased arterial segment proximal and distal to the lesion. Stenting of the origin of the superficial femoral artery, the retro and infrageniculate popliteal, and the tibials was generally avoided. In addition to angioplasty and stenting, debulking of some tibial lesions was performed with laser atherectomy (Spectranetics Corporation, Colorado Springs, Colo) at the discretion of the operating surgeon. All interventions were performed with the intention to treat all levels of disease with the maximal tibial runoff in an attempt to obtain in-line flow to the foot.
Definitions and classifications
Primary patency was defined as the absence of restenosis or occlusion in the treated arterial segment, and primary patency was lost if there was a need for repeat endovascular intervention, surgical bypass, or progression of tissue loss requiring amputation. Restenosis was determined on nonnvasive testing, which confirmed recurrent disease (ankle-brachial index [ABI] decrease >0.15, dampened pulse volume recordings [PVRs] or evidence of stenoses by duplex ultrasound scan), regardless of symptom status, and was confirmed on repeat angiography only in patients with recurrent symptoms or failure of wound healing. In patients with noncompressible ABI, reliance on toe pressures and PVR tracings was the norm. The duplex ultrasound criteria utilized for the detection of a hemodynamically significant restenosis in an arterial segment previously treated with angioplasty were a peak systolic velocity (PSV) of >300 cm/s or a velocity ratio (Vr) >3.0. The criteria utilized for the detection of a significant (>80%) in-stent femoropopliteal stenosis were PSV >275 cm/s and a Vr >3.5.6 Although there are no standard duplex criteria for the classification of tibial stenoses, we have utilized a PSV of ≥300 cm/s and a peak stenotic velocity/prestenotic velocity ratio of 3.5 as indicators of a severe stenosis. Noninvasive vascular laboratory surveillance was routinely performed on all patients at 1 month, 3 months, and 6 months postprocedure. Patients were then evaluated at 6-month intervals.
Assisted-primary patency was achieved via secondary endovascular interventions to treat restenoses involving the originally treated arterial segment. Additional procedures to treat lesions proximal or distal to the initially treated segment were also considered secondary interventions to achieve primary-assisted patency. Secondary patency was achieved utilizing reinterventions on occluded but previously treated arterial segments. Patients with initial isolated tibial interventions who required later femoropopliteal interventions were counted as part of the isolated tibial group, but lost primary patency at the time of femoropopliteal intervention in favor of primary-assisted patency.
Preintervention angiograms were reviewed and a TASC classification was assessed for the level of intervention. Because the updated TASC II guidelines do not include the tibial runoff, the TASC I guidelines were utilized to assign a TASC classification to infrapopliteal lesions.7
Attempts were made to standardize the wound care regimen. General principles included sharp excisional debridement at each outpatient visit as needed, along with topical outpatient wound care. If the wound appeared infected with signs of inflammation, systemic antibiotics were administered as well as topical antibiotic therapy. Once the infection was cleared, routine wound care was resumed. Our preferred wound measurement is the wound surface area (measured as minor axis × major axis × 0.8). However, exact measurements were not always documented, and changes in wound size were often obtained from the follow-up notes by the treating surgeon describing the wound as smaller/improved, larger/worse, or unchanged.
Statistical analysis
An independent statistician performed all advanced statistical analyses. Count data were summarized as frequencies and continuous variables as means ± standard deviations. One-year cumulative primary patency, primary-assisted patency, secondary patency and limb salvage were calculated by the Kaplan-Meier approach. Multivariable Cox proportional hazards regression was used to develop predictive models.
RESULTS
Endovascular interventions were performed on 136 limbs in 123 patients, all for CLI. Overall the mean age was 74 ± 11.3 years, and 62% of patients were male. Demographics and baseline comorbidities were comparable between the two groups (Table I). The overall mean follow-up was 12.6 ± 5.3 months. A total of four patients were lost to follow-up.
Table I.
Demographic data and comorbidities of patients undergoing TAEI
| Group I (n) | Group II (n) | P value | |
|---|---|---|---|
| Mean age (years) | 72.6 ± 11.3 (73, 49–91) | 74.9 ± 11.4 (77.5, 50–95) | NS |
| Male | 65% (32) | 60% (44) | .28 |
| Diabetes mellitus | 65% (32) | 66% (49) | .51 |
| Chronic renal insufficiency | 55% (27) | 51% (37) | .92 |
| ESRD/dialysis | 20% (10) | 19% (14) | .63 |
| Hypertension | 94% (46) | 92% (67) | .87 |
| Statin use | 60% (29) | 66% (46) | .74 |
| Prior CABG | 32% (15) | 36% (26) | .56 |
| Coronary artery disease | 68% (32) | 69% (50) | .68 |
| Congestive heart failure | 36% (17) | 40% (27) | .88 |
| History of MI | 38% (17) | 39% (24) | 1 |
| COPD | 19% (9) | 19% (12) | .87 |
| Cancer | 13% (6) | 10% (7) | .97 |
| History of tobacco use | 49% (23) | 53% (36) | .67 |
| Smoking status | |||
| Never | 51% (24) | 46% (31) | |
| Former | 38% (18) | 43% (29) | |
| Current | 11% (5) | 12% (8) |
CABG, Coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; MI, myocardial infarction; TAEI, tibial artery endovascular interventions.
All patients had a tibial artery intervention with or without a more proximal intervention. There were 54 limbs treated in group I, 85% of which were for tissue loss (Rutherford category 5/6 disease); 82 limbs were treated in group II, 79% for tissue loss (P = .58) (Table II). Preintervention, the mean ABI was significantly lower in group II (0.53 vs 0.74; P < .001) but was similar between the two groups postintervention (0.86 vs 0.88; P = NS). The lesion TASC classifications for limbs treated in both groups were similar and are presented in Table III.
Table II.
Indications for intervention, and wound type and location among group I and group II
| Group I (n) | Group II (n) | P value | |
|---|---|---|---|
| Indication: | NS | ||
| Gangrene | 46% (25) | 28% (23) | |
| Ulcer | 39% (21) | 51% (42) | |
| Rest pain | 15% (8) | 21% (17) | |
| Wound type | NS | ||
| Gangrene | 54% (25) | 65% (42) | |
| Ulcer | 46% (21) | 35% (23) | |
| Wound location | NS | ||
| Forefoot | 80% (37) | 75% (49) | |
| Heel | 15% (7) | 19% (12) | |
| Ankle | 2.2% (1) | 6% (4) | |
| Missing | 2.2% (1) |
Table III.
Lesion classification and hemodynamic data
| Group I (n = 54) |
Group II (n = 81) |
P value | |
|---|---|---|---|
| Lesion characterization | |||
| TASC II | |||
| Femoral and popliteal | |||
| A | NA | 4.2% (3) | |
| B | NA | 33.3% (27) | |
| C | NA | 31.9% (26) | |
| D | NA | 30.6% (25) | |
| TASC I | |||
| Tibial lesions | |||
| A | 0% (0) | 1% (1) | |
| B | 6% (3) | 5% (4) | |
| C | 41% (22) | 42% (34) | |
| D | 54% (29) | 52% (42) | |
| Hemodynamic data | |||
| ABI | |||
| Preprocedure | 0.74 ± 0.23 | 0.53 ± 0.24 | <.001 |
| Postprocedure | 0.88 ± 0.25 | 0.86 ± 0.20 | .63 |
| Toe pressure (mm Hg) | |||
| Preprocedure | 34.9 ± 26.5 | 21.5 ± 23.6 | .2 |
| Postprocedure | 55.1 ± 45.3 | 64.9 ± 38.3 | .57 |
ABI, Ankle-brachial index; TASC, TransAtlantic Intersociety Consensus.
Overall periprocedural complications included groin hematoma (2.2%), pseudoaneurysm formation (0.7%), and transient acute renal failure (0.7%). The overall 30-day mortality rate was 2.5%. The rates of morbidity and mortality between the two groups were similar (P = NS) (Table IV). A total of three patients died within 30 days of their procedure. One patient died in group I from multiple medical problems unrelated to her procedure and was readmitted 2 weeks postprocedure with pneumonia and sepsis. She went on to expire from multisystem organ failure. In group II, one patient died from complications of a groin hematoma after antegrade access requiring operative repair and developed multisystem organ failure, with ultimate withdrawal of support and death. The other death in group II was secondary to overwhelming sepsis related to a peripherally-inserted central catheter line placed 3 weeks postprocedure. Twenty patients died during the study from unrelated causes, namely coronary disease and malignancy.
Table IV.
Perioperative data and complications
| All interventions (n) |
Multilevel interventions (n) |
Isolated tibial interventions (n) |
P value | |
|---|---|---|---|---|
| Hematoma | 2.2% (3) | 2.5% (2) | 1.8% (1) | NS |
| Pseudoaneurysm | 0.7% (1) | 1.2% (1) | 0% (0) | NS |
| Vessel thrombosis | 0% (0) | 0% (0) | 0% (0) | — |
| Renal failure | 0.7% (1) | 1.2% (1) | 0% (0) | NS |
| MI | 0% (0) | 0% (0) | 0% (0) | — |
| Bleeding requiring surgery | 2.2% (3) | 2.5% (2) | 1.8% (1) | NS |
| Death | 2.5% (3) | 2.7% (2) | 2.1% (1) | NS |
| Mean length of stay (d) | 3.0 ± 5.3 (1, 0–40) | 3.1 ± 5.7 (1, 0–40) | 2.8 ± 4.7 (1, 0–18) | NS |
| ICU admission | 3.7% (5) | 3.7% (3) | 3.7% (2) | NS |
Wound healing
Of the limbs treated for tissue loss, there was a trend toward better wound healing in the multilevel intervention group (group II). Wounds were healed or improved at last follow-up in 69% of the limbs treated in group I compared with 87% in the limbs treated in group II (P = .055). In addition, the mean time to complete healing was significantly longer in group I; 14.6 ± 8.1 months compared with 9.8 ± 7.2 months in group II (P = .02) (Table V).
Table V.
Wound healing
| Wound healing | All patients (n = 136) | Isolated tibial interventions (n = 54) |
Multilevel interventions (n = 82) |
P value |
|---|---|---|---|---|
| Healed or improved | 80% (99) | 69% (33) | 87% (66) | .055 |
| Healed | 46.0% (57) | 45.8% (22) | 46.0% (35) | NS |
| Improved | 33.9% (42) | 22.9% (11) | 40.8% (31) | <.05 |
| Worse | 4.0% (5) | 4.2% (2) | 3.9% (3) | NS |
| Amputation | 10.5% (13) | 18.7% (9) | 5.3% (4) | .05 |
| No change | 5.6% (7) | 8.3% (4) | 3.9% (3) | |
| Time to healing (mo) | ||||
| Healed (n = 57) | 11.6 ± 7.9 (11.5, 0–30.2) | 14.6 ± 8.1 (13.9, 0–30.2) | 9.8 ± 7.2 (9.0, 0.25–27.0) | .02 |
A logistic regression model was also constructed to determine the effect of wound type, wound location, and TASC I tibial lesion classification on wound healing. The only significant finding identified was a trend toward increased wound healing in the multilevel intervention group with heel wounds compared with forefoot lesions (P = .064) (Table VI).
Table VI.
Effect of wound type, wound location, and TASC I classification as predictors of wound healing
| Characteristic | Odds ratio |
95% CI |
P value |
|---|---|---|---|
| Ulcer vs gangrene | 1.26 | 0.54–2.93 | .59 |
| Heel vs forefoot (overall) | 1.54 | 0.59–4.07 | .38 |
| Heel vs forefoot (multilevel intervention) | 3 | 0.94–9.57 | .06 |
| Heel vs forefoot (tibial intervention) | 0.37 | 0.07–2.01 | .25 |
| TASC I C/D vs A/B lesions | 0.72 | 0.13–4.02 | .7 |
| TASC I D vs A/B/C lesions | 0.72 | 0.33–1.53 | .39 |
TASC, TransAtlantic Intersociety Consensus.
Limb salvage
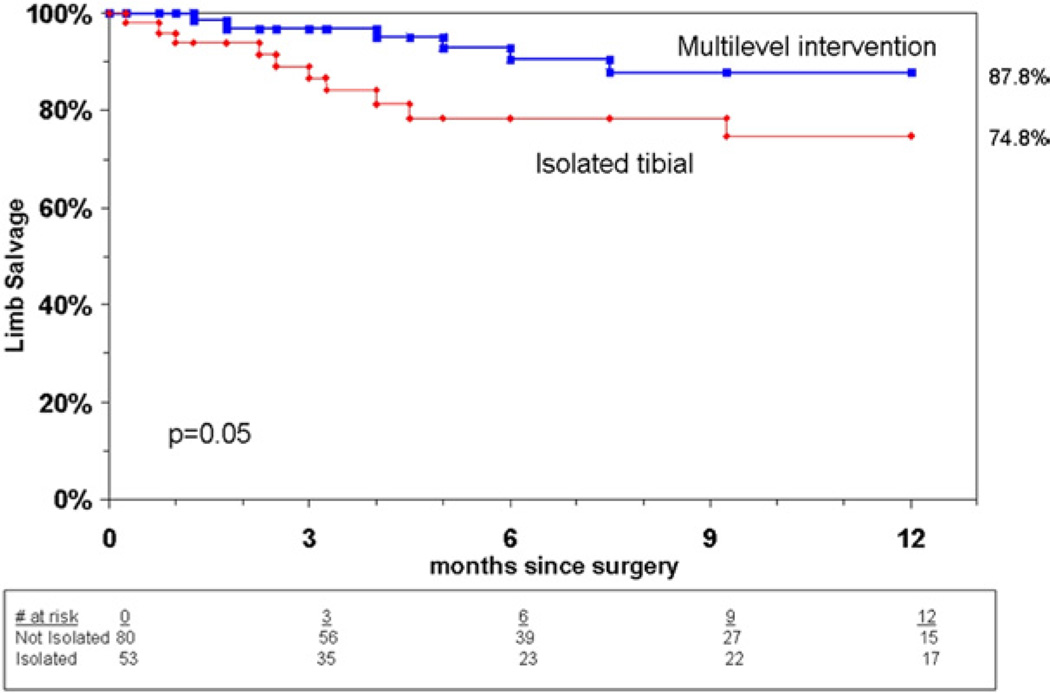
There were a total of 13 major amputations: 11 below the knee and two above the knee. The overall limb salvage at the latest follow-up was significantly higher in group II and was achieved in 95% of limbs compared with a limb salvage rate of 81% in group I (P = .05). In addition, isolated tibial interventions were predictive of limb loss, on multivariate analysis. Life table analysis also showed a significantly lower rate of limb salvage at 9 months and 1 year in group I compared with group II (P = .05) (Fig 1). Predictors of limb loss in patients in group I included multiple synchronous tibial revascularization (P = .005) and advanced coronary disease requiring revascularization (P = .005). There was an association between renal insufficiency and limb loss, although this did not reach statistical significance (P = .06). Limb salvage was also analyzed by wound type, location, and extent of tibial lesions treated (TASC I). Wound type and/or location were not predictive of limb loss. Surprisingly, treatment of more extensive TASC C and D lesions was found to be predictive of limb salvage compared with TASC A and B lesions treated, although a limited number of patients (three in group I, five in group II) were classified as TASC A or B (Table VII). This has to be interpreted with caution given the small number of patients with TASC A and B lesions and the likelihood of a type II error. Other variables evaluated that had no significant effect on limb loss included comorbidities such as diabetes and hyperlipidemia, as well as antiplatelet therapy.
Fig 1.
Cumulative limb salvage by type of intervention.
Table VII.
Effect of wound type, wound location, and TASC I classification as predictors of limb loss
| Characteristic | Hazard ratio |
95% CI | P value |
|---|---|---|---|
| Ulcer vs gangrene | 0.81 | 0.27–2.49 | .72 |
| Heel vs forefoot | 1.54 | 0.41–5.82 | .52 |
| TASC I C/D vs A/B lesions | 0.16 | 0.03–0.74 | .019 |
| TASC I D vs A/B/C lesions | 0.94 | 0.35–2.50 | .89 |
TASC, TransAtlantic Intersociety Consensus.
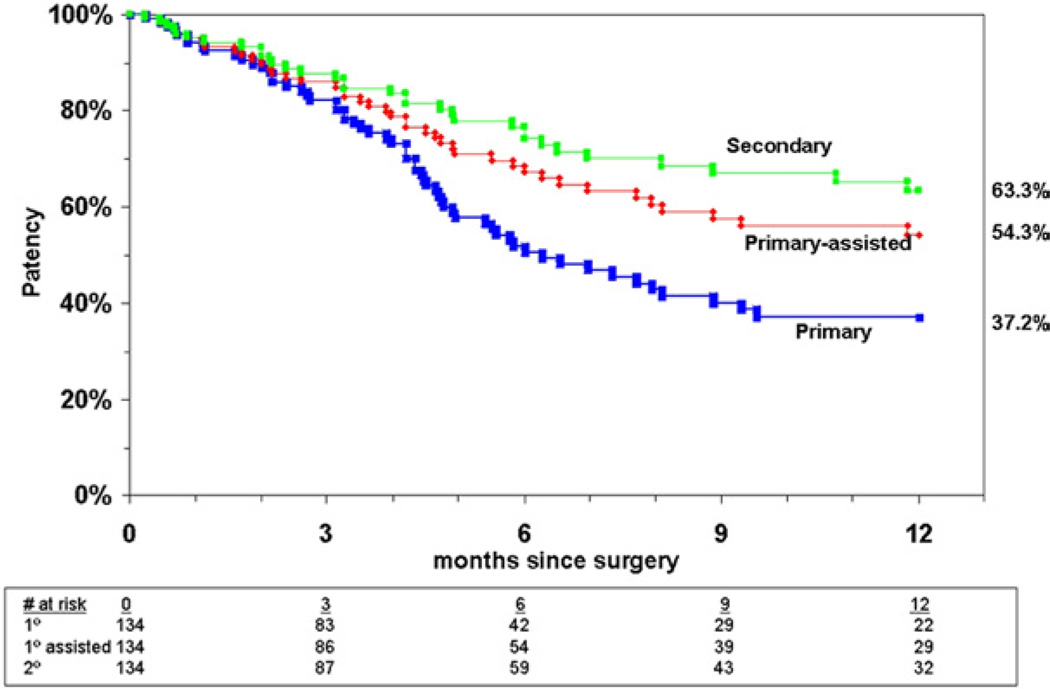
Patency rates
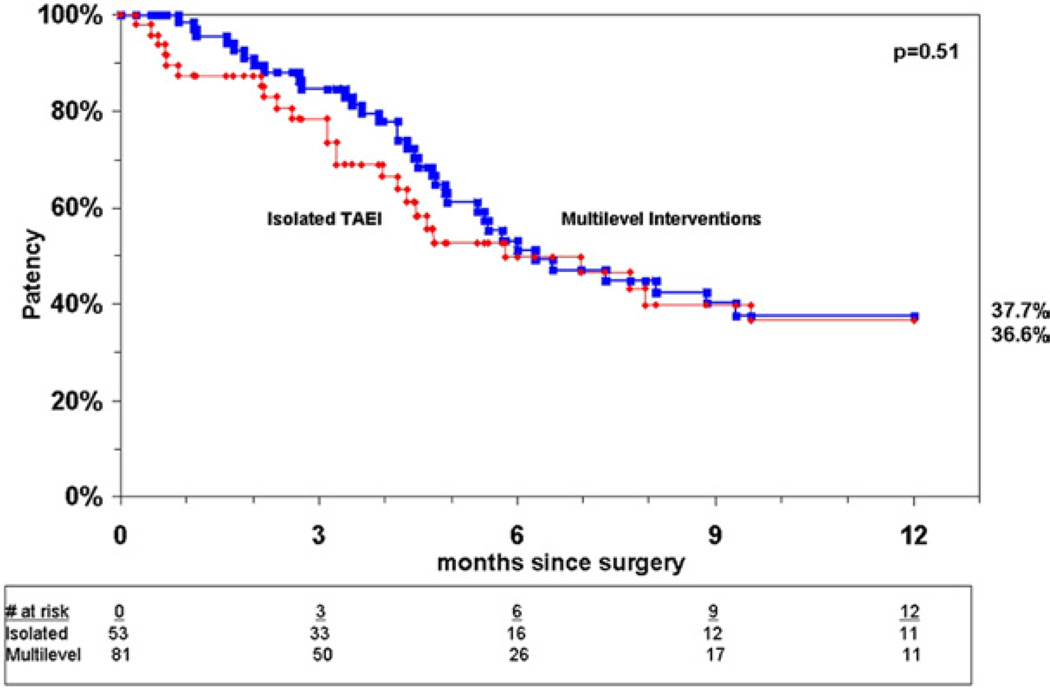
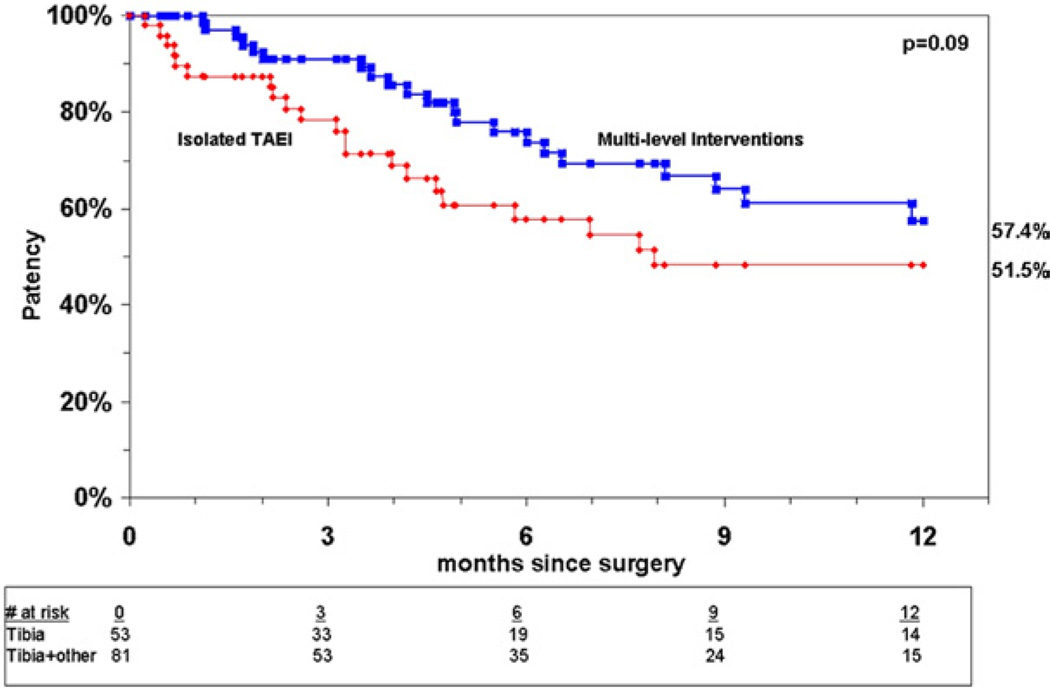
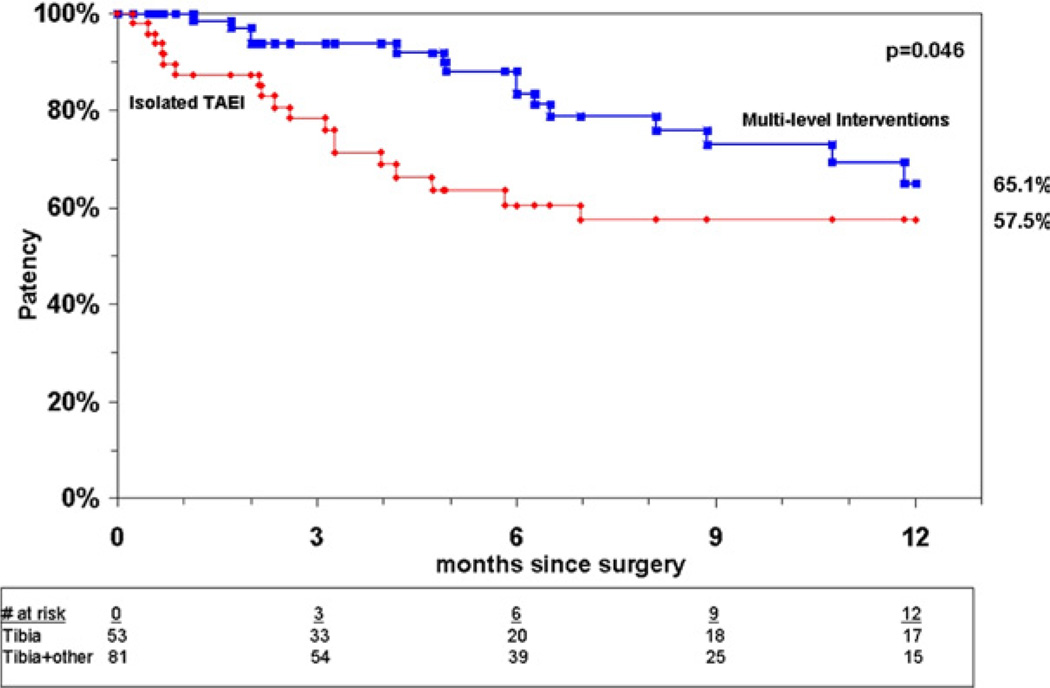
Analysis of patency for all limbs undergoing TAEI at 12 months showed a primary patency rate of 37%, a primary-assisted patency rate of 54%, and a secondary patency rate of 63% (Fig 2). Patency rates were comparable between both groups at 1 year by Kaplan-Meier analysis, with primary patency rates of 37% in group I vs 38% in group II (P = .42) (Fig 3). There was a trend toward improved primary-assisted patency for group II compared with isolated TAEI (58% vs 52%) (P = .09) (Fig 4), but the secondary patency rates were significantly better for limbs undergoing multilevel interventions, 65% vs 58% (P = .046) (Fig 5).
Fig 2.
Patency rates for all limbs undergoing tibial artery endovascular intervention.
Fig 3.
Cumulative primary patency for group I and group II.
Fig 4.
Cumulative primary-assisted patency for group I and group II.
Fig 5.
Cumulative secondary patency for group I and group II.
Reinterventions
In the course of follow-up, a total of 39 patients (10 in group I and 29 in group II) underwent a reintervention, with the most common indications, including failure of wound healing and duplex ultrasound evidence of recurrent severe stenosis in a treated segment (Table VIII). The characteristics and indications for repeat intervention are summarized in Table IX. Reinterventions were multilevel in both groups, as some patients in group I required femoral or popliteal interventions in addition to tibial reinterventions. Additionally, a total of eight patients went on to require surgical bypass (five in group I and three in group II) and their outcomes are summarized in Table X.
Table VIII.
Indication for repeat endovascular interventions
| Indication | Group I (n = 10) |
Group II (n = 29) |
|---|---|---|
| Failure of wound healing | 60% (6) | 48% (14) |
| Duplex evidence of recurrence | 20% (2) | 31% (9) |
| Recurrent ulceration | NA | 7% (2) |
| Recurrent rest pain | 20% (2) | 14% (4) |
Table IX.
Summary of repeat interventions by level treated, type of intervention, and type of patency
| Primary-assisted patency |
Secondary patency |
|
|---|---|---|
| Level of intervention | ||
| Group I | (n = 7) | (n = 3) |
| Superficial femoral artery | NA | NA |
| Popliteal artery | 14% (1) | NA |
| Original tibial vessel | 100% (7) | 100% (3) |
| Alternate tibial vessel | 29% (2) | NA |
| Group II | (n = 18) | (n = 11) |
| Iliac | 6% (1) | NA |
| Superficial femoral artery | 56% (10) | 36% (4) |
| Popliteal artery | 44% (8) | 73% (8) |
| Original tibial vessel | 72% (13) | 91% (10) |
| Alternate tibial vessel | 17% (3) | 9% (1) |
| Type of intervention | ||
| Group I | ||
| Angioplasty | 100% (7) | 100% (3) |
| Laser atherectomy | 14% (1) | NA |
| Stenting | NA | NA |
| Group II | ||
| Angioplasty | 94% (17) | 100% (11) |
| Laser atherectomy | 11% (2) | 9% (1) |
| Silverhawk atherectomy | NA | 9% (1) |
| Stenting (fem pop) | 33% (6) | 55% (6) |
| Cryoplasty | 11% (2) | NA |
Table X.
Summary of patients requiring subsequent bypass
| Patient | Initial intervention | Bypass | Outcome |
|---|---|---|---|
| Group I | |||
| 1 | PTA PT | Pop-AT bypass | Healed TMA |
| 2 | PTA PT | Pop-pedal bypass | Healed toe amputation |
| 3 | PTA peroneal | Pop-pedal bypass | Healed toe gangrene |
| 4 | Laser atherectomy and PTA peroneal | Pop-peroneal | Open TMA (healing at time of death) |
| 5 | PTA AT | Pop-DP bypass | Healed toe ulcer |
| Group II | |||
| 1 | PTA Pop, TPT, and peroneal | Fem-peroneal bypass | Healed toe amputation |
| 2 | PTA/stent SFA, atherectomy and PTA TPT/peroneal | Fem-AT bypass | AKA |
| 3 | PTA SFA, TPT, and peroneal | Pop-pedal bypass | Bypass failed, ulcers stable at last follow-up |
AKA, Above-knee amputation; AT, anterior tibial artery; Pop, popliteal artery; PT, posterior tibial artery; PTA, percutaneous transluminal angioplasty; TMA, transmetatarsal amputation.
DISCUSSION
Endovascular treatment of CLI has become the first-line approach in many centers.2,5,8 However, despite the fact that tibial interventions for CLI have been extensively described, results have been inconclusive with no clear definition of which patients represent the best anatomic and physiological candidates.1,9 A recent meta-analysis by Romiti et al of infrapopliteal angioplasty for the treatment of CLI showed an overall primary patency and secondary patency rates of 58% and 68%, respectively, at 1 year, with a limb salvage rate of 86% and patient survival of 98%.10 However, this study did not look at the wound healing as an endpoint of TAEI. In the current series, 80% of patients who were treated for tissue loss either healed or had improvement in their wounds. When comparing multilevel interventions with isolated tibial interventions, there was a strong trend toward complete or improved wound healing in group II (87%) vs 69% in group I (P = .055). Although there was no difference between the two treatment groups in the rates of complete wound healing, the time to wound healing was significantly longer (14.6 months vs 9.8 months) in the isolated tibial group compared with the multilevel intervention group (P = .02). A possible explanation to such findings include the fact that patients who undergo an isolated tibial intervention may have a greater local disease burden, which would increase the chance of recurrence and limit the effectiveness of single-level intervention. Alternatively, isolated tibial disease may represent a different, more aggressive disease process with more frequent small vessel disease and poorer outflow, although we have noted a similar proportion of diabetic patients in both treatment groups. Wound healing was not included in a recent report by Sadek et al, but they also reported trends toward improved limb salvage and primary patency as well as significantly improved secondary patency when multilevel interventions involving the tibial vessels were compared with isolated tibial artery interventions.5
Similar to our previously reported outcomes with TAEI for CLI,11 all of the limbs in this series were treated for rest pain or tissue loss. In the current study, an isolated tibial intervention and multiple synchronous tibial interventions were predictive of limb loss, and a multilevel intervention was associated with improved limb salvage at 1 year. These findings can also be explained by a more aggressive local disease burden in patients with isolated tibial disease. In addition, the treatment of TASC C and D tibial lesions was associated with a lower rate of limb loss. This finding is likely related to the small numbers of TASC A and B lesions treated, limiting the power of a comparative analysis. We were not able to find a significant difference in limb salvage based on wound location, heel vs forefoot, or the type of wound treated, ulcer vs gangrene. In addition, although we believe that wound size on presentation is potentially an important predictor of limb salvage, we were not able to retrospectively obtain enough data on wound size to perform any meaningful analysis of the effect of the amount of tissue loss on the effectiveness of TAEI for wound healing.
In the report by Sadek et al, a limb salvage rate of 81% at 12 months was reported, which is similar to our study. Additionally, they did have a trend toward improved limb salvage with multilevel interventions but felt that their study was underpowered to detect a true difference.5 Our results are also comparable to those reported by Giles et al for CLI. They noted a limb salvage rate of 84% at 12 months,12 which was similar to limb salvage rates with bypass reported in the Prevent III trial (88% at 1 year).13 There were no instances of limb loss as a consequence of an endovascular intervention or periprocedural complication in this series. Although all attempts at limb salvage were made, some patients did require a major amputation for tissue loss after they were offered a last effort endovascular intervention. Such patients typically have challenging advanced cardiac comorbidities and/or inadequate pedal target vessel or saphenous or arm vein conduit for bypass.
In addition to improved limb salvage and reduced time to healing with multilevel interventions, our current series identified improved patency in the multilevel cohort when compared with isolated tibial interventions. Although the primary patency rates were similar, we did see a trend toward improved primary-assisted patency and did find a significantly improved secondary patency with multilevel interventions. This is similar to findings described by Sadek et al5 However, they did report improved primary patency with multilevel vs isolated tibial disease at 1 year. One possible explanation for this discrepancy is that while all patients in our series were Rutherford category 4 to 6, 25% of the limbs treated in the report by Sadek et al were < Rutherford 4.
The primary limitation of this report is inherent to its retrospective nature. This included incomplete data available for wound-healing analysis. Additionally, the approach to treatment of CLI was left to the discretion of the operating surgeon. As such, there was no standard approach to the types of revascularization modality performed. Although most patients were treated as part of an “endo first” approach, some were referred for an endovascular revascularization because of the lack of a bypass target, lack of adequate vein conduit, or severe comorbidities precluding surgery. As such, this has resulted in a heterogenous patient population with different comorbidities and disease distribution.
Finally, longer follow-up is essential and ongoing to determine the durability of TAEI. This is particularly important in patients with tissue loss treated with TAEI since longer follow-up is often needed to achieve complete wound healing. As such, favorable wound outcomes may not be reflected in patients with short, yet ongoing follow-up.14 A prospective cohort is therefore needed to compare the effectiveness of the different available endovascular techniques and their impact on wound healing and limb salvage. Nevertheless, based on the current data, patients with CLI, even when presenting with tissue loss, should not be denied endovascular revascularization in the setting of diffuse multilevel disease distribution. In fact, such patients seem to achieve better limb salvage and wound healing than patients with isolated tibial disease.
CONCLUSIONS
Tibial artery endovascular intervention results in acceptable rates of limb salvage and wound healing with appropriate wound care in patients undergoing multilevel interventions for CLI, despite low patency rates at 1 year. Patients with isolated tibial disease appear to have a higher incidence of limb loss secondary to poor initial pedal runoff and more extensive local disease. Other therapeutic strategies should be considered in these patients, including pedal bypass if fit enough for bypass.
Acknowledgments
The authors would like to thank Dr Faith Selzer, PhD, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh Medical Center, for her assistance with statistical analysis.
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design: RC
Analysis and interpretation: NF, RM, LM, RR, SL, MM, RC
Data collection: NF, RM, RC
Writing the article: NF, RM, LM, RR, SL, MM, RC
Critical revision of the article: NF, LM, RR, SL, MM, RC
Final approval of the article: RC
Statistical analysis: Not applicable
Obtained funding: Not applicable
Overall responsibility: NF, RC
Competition of interest: none.
Presented at the Twenty-third Annual Meeting of the Eastern Vascular Society, Philadelphia, Pa, September 24–26, 2009.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a competition of interest.
REFERENCES
- 1.Kudo T, Chandra FA, Ahn SS. The effectiveness of percutaneous transluminal angioplasty for the treatment of critical limb ischemia: a 10 year experience. J Vasc Surg. 2005;41:423–435. doi: 10.1016/j.jvs.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 2.DeRubertis BG, Faries PL, McKinsey JF, Chaer RA, Pierce M, Karwowski J, et al. Shifting paradigms in the treatment of lower extremity vascular disease-a report of 1000 percutaneous interventions. Ann Surg. 2007;246:415–424. doi: 10.1097/SLA.0b013e31814699a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudo T, Chandra FA, Kwun WH, Haas BT, Ahn SS. Changing pattern of surgical revascularization for critical limb ischemia over 12 years: endovascular versus open bypass surgery. J Vasc Surg. 2006;44:304–313. doi: 10.1016/j.jvs.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 4.Norgren L, Hiatt WR, Dormandy JA, et al. TASC II Working Group. Inter-Society Consensus for the Management of peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Sadek M, Ellozy SH, Turnbull IC, Lookstein RA, Marin ML, Faries PL. Improved outcomes are associated with multilevel endovascular interventions involving the tibial vessels compared with isolated tibial intervention. J Vasc Surg. 2009;49:638–644. doi: 10.1016/j.jvs.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Baril DT, Rhee RY, Kim J, Makaroun MS, Chaer RA, Marone LK. Duplex criteria for determination of in-stent stenosis after angioplasty and stenting of the superficial femoral artery. J Vasc Surg. 2009;49:133–139. doi: 10.1016/j.jvs.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 7.Dormandy JA, Rutherford RB TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). Management of peripheral arterial disease (PAD) J Vasc Surg. 2000;31:S1–S296. [PubMed] [Google Scholar]
- 8.Black JH, 3rd, LaMuraglia GM, Kwolek CJ, Brewster DC, Watkins MT, Cambria RP. Contemporary results of angioplasty-based infrainguinal percutaneous interventions. J Vasc Surg. 2005;42:932–939. doi: 10.1016/j.jvs.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Parsons RE, Suggs WD, Lee JJ, Sanchez LA, Lyon RT, Veith FJ. Percutaneous transluminal angioplasty for the treatment of limb threatening ischemia: do the results justify an attempt before bypass grafting. J Vasc Surg. 1998;28:1066–1071. doi: 10.1016/s0741-5214(98)70033-3. [DOI] [PubMed] [Google Scholar]
- 10.Romiti M, Albers M, Brochado-Neto FC, Durazzo AE, Pereira CA, De Luccia N. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008;47:975–981. doi: 10.1016/j.jvs.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez N, McEnaney R, Marone LK, Rhee RY, Leers S, Makaroun M, et al. RR17. Predictors of failure and success of tibial interventions for critical limb ischemia. J Vasc Surg. 2009;49:S51. doi: 10.1016/j.jvs.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giles KA, Pomposelli FB, Spence TL, Hamdan AD, Blattman SB, Panossian H, et al. Infrapopliteal angioplasty for critical limb ischemia: relation of TransAtlantic Intersociety Consensus class to outcome in 176 limbs. J Vasc Surg. 2008;48:128–136. doi: 10.1016/j.jvs.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 13.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT; III A multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–751. doi: 10.1016/j.jvs.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez N, McEnaney R, Marone LK, Rhee RY, Leers S, Makaroun M, et al. Predictors of failure and success of tibial interventions for critical limb ischemia. J Vasc Surg. 2010;52:834–842. doi: 10.1016/j.jvs.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]