Abstract
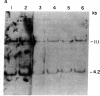
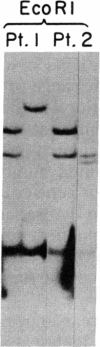
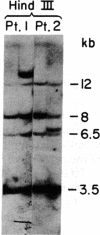
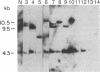
A cDNA clone representing the gene encoding the beta chain of the human T-cell antigen receptor has been isolated recently. By using fragments of this cDNA as hybridization probes in Southern blot analysis of restriction endonuclease-digested genomic DNA, we have now examined the structure of the gene in DNA from 26 patients with acute leukemia and from 23 normal individuals. We have found that the T-cell antigen receptor gene has undergone somatic rearrangement in 14 of 14 patients with the phenotypic diagnosis of T-cell acute lymphoblastic leukemia. In this group of patients, similar patterns of rearrangement appear to occur in different patients. This finding suggests that there is either a limited repertoire of possible rearrangements or an association between the development of leukemia and specific patterns of rearrangement. DNA from 6 patients with acute myeloblastic leukemia, 6 patients with non-B, non-T acute lymphoblastic leukemia, and 23 nonleukemic individuals showed no rearrangement or polymorphism. One case of T-cell acute lymphoblastic leukemia, however, showed rearrangement of both the T-cell receptor beta chain and the constant region of the immunoglobulin gene. Studies with mixtures of DNAs from leukemic bone marrow cells and cultured skin fibroblasts, as well as with remission and relapse marrow DNAs from the same patients, indicate that this technique can detect 1% leukemic cells in a mixed population. In addition, DNA from the marrow of a patient in relapse contains a similar rearrangement to that found in the marrow sample taken at the time of diagnosis, which suggests that the original clone of leukemic cells was responsible for relapse. Our results indicate that assessment of rearrangement of the T-cell antigen receptor gene will be valuable in the diagnosis and management of leukemia and can be used to evaluate clonality in T-cell neoplasia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold A., Cossman J., Bakhshi A., Jaffe E. S., Waldmann T. A., Korsmeyer S. J. Immunoglobulin-gene rearrangements as unique clonal markers in human lymphoid neoplasms. N Engl J Med. 1983 Dec 29;309(26):1593–1599. doi: 10.1056/NEJM198312293092601. [DOI] [PubMed] [Google Scholar]
- Clark S. P., Yoshikai Y., Taylor S., Siu G., Hood L., Mak T. W. Identification of a diversity segment of human T-cell receptor beta-chain, and comparison with the analogous murine element. 1984 Sep 27-Oct 3Nature. 311(5984):387–389. doi: 10.1038/311387a0. [DOI] [PubMed] [Google Scholar]
- Ford A. M., Molgaard H. V., Greaves M. F., Gould H. J. Immunoglobulin gene organisation and expression in haemopoietic stem cell leukaemia. EMBO J. 1983;2(6):997–1001. doi: 10.1002/j.1460-2075.1983.tb01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella J. F., Keys C., VarsanyiBreiner A., Kao F. T., Jones C., Puck T. T., Housman D. Isolation and localization of DNA segments from specific human chromosomes. Proc Natl Acad Sci U S A. 1980 May;77(5):2829–2833. doi: 10.1073/pnas.77.5.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha K., Minden M., Hozumi N., Gelfand E. W. Immunoglobulin mu-chain gene rearrangement in a patient with T cell acute lymphoblastic leukemia. J Clin Invest. 1984 Apr;73(4):1232–1236. doi: 10.1172/JCI111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J., Arnold A., Bakhshi A., Ravetch J. V., Siebenlist U., Hieter P. A., Sharrow S. O., LeBien T. W., Kersey J. H., Poplack D. G. Immunoglobulin gene rearrangement and cell surface antigen expression in acute lymphocytic leukemias of T cell and B cell precursor origins. J Clin Invest. 1983 Feb;71(2):301–313. doi: 10.1172/JCI110770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Ravetch J. V., Poplack D. G., Waldmann T. A., Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch E. A. Stem cells in normal and leukemic hemopoiesis (Henry Stratton Lecture, 1982). Blood. 1983 Jul;62(1):1–13. [PubMed] [Google Scholar]
- McGrath M. S., Weissman I. L. AKR leukemogenesis: identification and biological significance of thymic lymphoma receptors for AKR retroviruses. Cell. 1979 May;17(1):65–75. doi: 10.1016/0092-8674(79)90295-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Siu G., Clark S. P., Yoshikai Y., Malissen M., Yanagi Y., Strauss E., Mak T. W., Hood L. The human T cell antigen receptor is encoded by variable, diversity, and joining gene segments that rearrange to generate a complete V gene. Cell. 1984 Jun;37(2):393–401. doi: 10.1016/0092-8674(84)90369-6. [DOI] [PubMed] [Google Scholar]
- Smith L. J., Curtis J. E., Messner H. A., Senn J. S., Furthmayr H., McCulloch E. A. Lineage infidelity in acute leukemia. Blood. 1983 Jun;61(6):1138–1145. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Toyonaga B., Yanagi Y., Suciu-Foca N., Minden M., Mak T. W. Rearrangements of T-cell receptor gene YT35 in human DNA from thymic leukaemia T-cell lines and functional T-cell clones. 1984 Sep 27-Oct 3Nature. 311(5984):385–387. doi: 10.1038/311385a0. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Yanagi Y., Suciu-Foca N., Mak T. W. Presence of T-cell receptor mRNA in functionally distinct T cells and elevation during intrathymic differentiation. Nature. 1984 Aug 9;310(5977):506–508. doi: 10.1038/310506a0. [DOI] [PubMed] [Google Scholar]