Abstract
BACKGROUND
The most common CF-causing mutations interfere with CFTR trafficking from the endoplasmic reticulum (CFTR-F508del) or prematurely terminate transcription (CFTR-null). We suspected genotype-specific pattens of CFTR expression would have differential effects on smooth muscle cell calcium signaling and hence vascular tone. We hypothesized that compared to wild-type or CFTR-null aorta, aorta from CFTR-F508del (dF) piglets will have reduced endoplasmic reticulum calcium mobilization and decreased vasoconstriction.
METHODS
Aortic reactivity was assessed by myography, and ratiometric calcium imaging was performed in isolated vascular smooth muscle cells.
RESULTS
Aorta from dF piglets had reduced myogenic tone (P<0.001) and decreased constriction to KCl (P<0.05). Combined inhibition of ryanodine and IP3 receptors decreased wild-type and CFTR-null responses to levels seen in dF aorta. Compared to wild-type cells, dF-expressing smooth muscle cells had reduced calcium transients, while CFTR-null cells had decreased baseline intracellular calcium concentrations.
CONCLUSIONS
Expression of CFTR-F508del interferes with smooth muscle cell calcium handling and decreases aortic responsiveness.
Keywords: cystic fibrosis transmembrane conductance regulator, endoplasmic reticulum, inositol triphosphate receptor, vascular smooth muscle cell
INTRODUCTION
Cystic fibrosis (CF) is caused by more than one thousand different mutations of the cystic fibrosis transmembrane conductance regulator (CFTR) (1). The most common CF-causing mutation leads to deletion of phenylalanine at position 508 (CFTR-F508del), resulting in protein misfolding and ER-associated degradation (2, 3). The second most common CF-causing mutation (G542X) prematurely terminates CFTR transcription (1). In those homozygous for either mutation, the loss of epithelial cell CFTR leads to classic CF phenotypes, including pancreatic insufficiency and lung disease.
The direct cardiovascular effects of CFTR mutations are unknown. Clinically, CF-related hypotension and right ventricular dysfunction are well described, but these findings are typically attributed to the indirect effects of salt wasting and pulmonary disease (4, 5). However, recent studies have shown relatively healthy patients may have right ventricular dysfunction with little to no pulmonary hypertension (6), and heterozygote carriers of a CFTR-F508del mutation have lower blood pressures (7). Though a possible explanatory relationship between sweat chloride and blood pressure exists (7), there is no evidence of hemodynamically significant salt depletion or hypovolemia in CF patients (8, 9).
The vascular phenotype of patients with cystic fibrosis is likewise controversial. When arterial stiffness is indirectly assessed by pulse wave velocity analysis, CF patients have decreased aortic distensibility, but these measurements have been confounded by the presence of systemic inflammation and diabetes (10, 11). To more directly assess the vascular effects of CF mutations, McGrath and colleagues compared forearm blood flow following intra-arterial infusion of vasoactive agents and found CF patients tend to have diminished vasodilation to nitroprusside (P=0.06) but not acetylcholine, suggesting the presence of smooth muscle cell dysfunction (12). Further, human airway smooth muscle cells isolated from CF patients have decreased agonist-induced calcium transients, again suggesting a direct effect of the CFTR-F508del mutation on myocyte function (13). Given the importance of ER calcium mobilization in VSMC-mediated vasoconstriction and the differential ER expression of CFTR in WT, CFTR-null and CFTR-F508del aorta (3), we hypothesized that piglets with cystic fibrosis would have genotype-specific alterations in calcium handling and aortic tone.
METHODS
Animal model
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the University of Iowa Animal Care and Use Committee (Permit Numbers: 1108172 and 1002025). CFTR+/+ (WT), CFTR−/− (null), CFTR+/F508del (dF Het) and CFTRF508del/F508del (dF) piglets were obtained from Exemplar Genetics (Sioux Center, IA). Because cystic fibrosis piglets are born with meconium ileus and intestinal obstruction, they were euthanized with pentobarbital sodium-phenytoin sodium (Euthasol, Virbac, Fort Worth, TX) within 24 h after birth (14). The descending thoracic aorta was stored in chilled physiologic buffer solution until same-day assessment.
Aortic reactivity
The aorta was sectioned into 5mm segments and mounted in 18ml myograph chambers containing a physiologic saline solution (PSS) constantly aerated with a mixture of 95% O2 - 5% CO2. The composition of the PSS was (in mmol/L): 119 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO47 H2O, 25 NaHCO3, 2.5 CaCl22 H20, 5.5 dextrose and 0.03 CaNa2-EDTA. Initial studies assessed the relationship between resting tension and myogenic tone. Individual arterial segments were incubated in buffer alone or buffer containing ryanodine (1 μM; ryanodine receptor antagonist at this concentration) (15), 2-aminoethoxydiphenyl borate (2-APB; 50 μM; inositol triphosphate receptor (IP3R) antagonist), ryanodine plus 2-APB, or barium (100 μM, KIR antagonist) (16). The concentration of 2-APB just exceeds the IC50 value for 2-APB inhibition of IP3-induced calcium release (17). Additional segments were incubated in calcium-free buffer with EGTA (1 mM) to chelate any residual extracellular calcium. During the assessment of myogenic responses, the buffer was neither altered nor refreshed. After one hour of incubation, some of the arterial segments were constricted with either noradrenaline (10 μM) or KCl (5 to 60mM). All compounds were acquired from Sigma Chemical (St. Louis, MO) with the exception of ryanodine which was supplied by Tocris (Bristol, UK).
Morphometrics
Additional aortic segments were incubated for 10 min in PSS containing 100 μM sodium nitroprusside to allow maximal vasodilatation. The rings were then fixed and paraffin embedded, microtomed in true cross section, and stained with hematoxylin and eosin. Sections were viewed under a brightfield microscope (Nikon Optiphot-2) and imaged using a digital camera and Spot software (Diagnostic Instruments, Sterling Heights, MI). Lumen diameter and mural thickness were averaged from three sections per vessel for the calculation of wall to lumen ratios, as previously described (18).
Smooth muscle cell culture
Aortic segments were cleansed of adherent connective tissue and placed in ice cold PSS with penicillin G (100 U/ml) and streptomycin sulfate (100 μg/ml). The artery was then subjected to enzymatic dispersion in minimal essential medium containing elastase (125 μg/ml), collagenase (1 mg/ml), bovine serum albumin (2 mg/ml), and soybean trypsin inhibitor (375 μg/ml). Cells were plated onto collagen coated dishes bathed in Dulbecco's modified Eagle's high-glucose medium containing 10% fetal bovine serum. The culture dishes were subsequently placed in a humidified 95% air-5% CO2 incubator set at 37°C. The incubation media was changed at frequent intervals until second passage cells reached 80% confluence (over 7–9 days). Cells were identified as myocytes given positive staining for alpha-smooth muscle actin, as previously described (19).
CFTR mRNA expression
VSMC RNA was purified with RNeasy kits (Qiagen, Valencia, CA), then quantified using a NanoDrop ND-1000 spectrophotometer (Labtech International, East Sussex, UK). Reverse-transcription reactions were performed on 0.5 μg total RNA. PCR was performed with the CFTR primer set: forward, 5'-CTGGAGCCTTCAGAGGGTAAAAT-3'; reverse, 5'-AGTTGGCACGCTTTGATGACACTCC-3'. To exclude contamination by genomic DNA, the reaction was run without reverse transcriptase and no band was seen.
Ratiometric calcium imaging
Cytosolic calcium concentrations ([Ca2+]i) were measured by fura-2 fluorescence using a videomicroscopy imaging system (IonOptix, Milton, MA). Calcium binding to fura-2 produces a shift in fluorescence intensity peak from 380 to 340 nm, and thus an increase in ([Ca2+]i) produces an increase in the 340/380 ratio. Individual VSMC were loaded with fura-2 (5 μM, Molecular Probes, Eugene, OR) for 30 min at 37°C. [Ca2+]i was computed from the 340 nm and 380 nm excitation images acquired prior to and following stimulation with angiotensin II (1 μM). As first described by Grynkiewicz (20), the system was calibrated with 11 pre-diluted buffers with calcium concentrations ranging from 0 to 39 μM with R2=0.99 (F-6774, Molecular Probes).
Data analysis
Data are presented as mean +/− SEM. The relationship between resting tension and myogenic tone was assessed by Pearson product moment correlation. All other results were compared using Student's t-test (with two-tailed significance at P < 0.05). To test our a priori hypothesis that CF-causing mutations differentially alter vascular function, separate t-tests were performed for null versus WT and dF versus WT responses. All analyses were performed using SigmaStat 3.0 (SPSS Inc., Chicago, IL).
RESULTS
Vascular reactivity
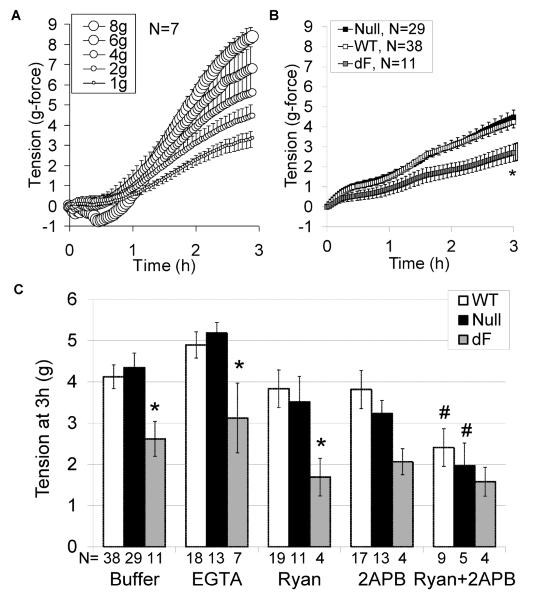
A direct relationship was seen between resting tension (passive stretch) and the myogenic force generated during 3h incubation of aortic segments in buffer alone (Figure 1A, R = 0.99, P<0.001). To approximate a physiologic neonatal piglet blood pressure of 52 mmHg (21), subsequent studies consistently utilized 2 g-force resting tension (2 g applied by 32 gauge wires over a surface area of 3.2 mm2 yields a pressure of 62.5 g-force/cm2 or 52 mmHg at 37°C). Compared to aorta from WT piglets, aorta from dF piglets had decreased myogenic tone after 3h incubation in PSS buffer alone (Figure 1B), PSS with ryanodine or calcium free buffer with EGTA (Figure 1C, all at 2g-force resting tension). Combined inhibition of ryanodine receptors and IP3 receptors with both ryanodine and 2-APB reduced the tone of WT and CFTR-null aorta, thereby approximating the tone generated by dF aorta (Figure 1C).
Figure 1.
Genotype-specific myogenic tone. Aortic segments from 7 piglets (2 WT, 3 CFTR-null, 2 CFTR-F508del) were incubated at 1, 2, 4, 6 or 8g-force resting tension and myogenic tone was assessed (A, symbol size is proportionate to resting tension). Aortic segments from additional WT (open symbols, N=38), null (black symbols, N=29), and dF piglets (gray symbols, N=11) were incubated at 2g resting tension for 3 hours and the ensuing tension was recorded (B). Compared to aorta from WT piglets, aorta from dF piglets had deceased constriction during incubation in PSS buffer, calcium free buffer with EGTA or PSS with ryanodine (C). Combined inhibition of ryanodine and IP3 receptors with both ryanodine (1 μM) and 2-APB (50 μM) reduced the tone of aorta from WT or CFTR-null piglets to approximate the tone of dF aorta. *P<0.05 versus WT, #P<0.05 versus buffer alone.
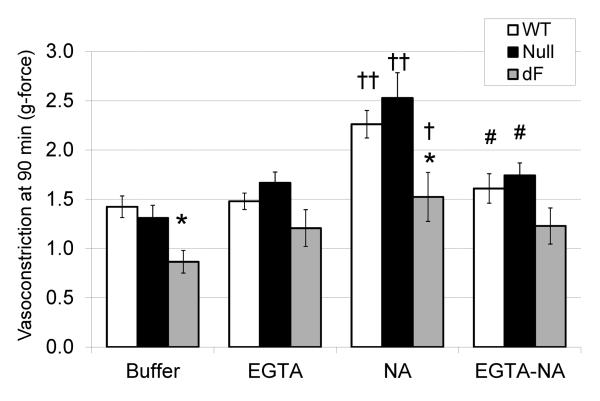
To assess for an additional effect of CFTR on receptor-mediated vasoconstriction, noradrenaline was added to the myograph chambers after one hour incubation in PSS or calcium free buffer with EGTA. During noradrenaline exposure, aorta from WT and CFTR-null aorta constricted more than aorta from dF piglets. Calcium-free buffer with EGTA attenuated this NA-augmented constriction, but did not eliminate the underlying myogenic tone (Figure 2). These results suggest NA-stimulated constriction is influenced by extracellular calcium content, and the dF mutation may suppress intracellular calcium release more than extracellular calcium entry. On further analysis of the subgroup of piglets that had aortic segments simultaneously incubated in the presence and absence of noradrenaline (N=12 null and 14 wild-type), CFTR-null aorta had increased relative NA contractility, assessed as a % of the response to buffer alone (null: 201 +/− 22%; WT 147 +/− 13%, P=0.04). Because extracellular calcium influx can occur through voltage dependent channels, we next assessed aortic constriction to increasing concentrations of KCl.
Figure 2.
Aortic response to adrenergic stimulation. Aortic constriction to noradrenaline (NA, 10 μM) was assessed for WT (open bars, N=17), CFTR-null (black bars, N=13), and dF piglets (gray bars, N=6) incubated in either PSS buffer alone or calcium-free buffer with EGTA. Aorta from dF piglets had decreased constriction during the 90 minute incubation, regardless of NA addition 30 minutes prior to study completion. Elimination of extracelluar calcium eliminated the heightened tone of WT and CFTR-null aorta. *P<0.05 versus WT, ‡P<0.01 or †P<0.05 for NA versus buffer, #P<0.05 for NA versus EGTA-NA.
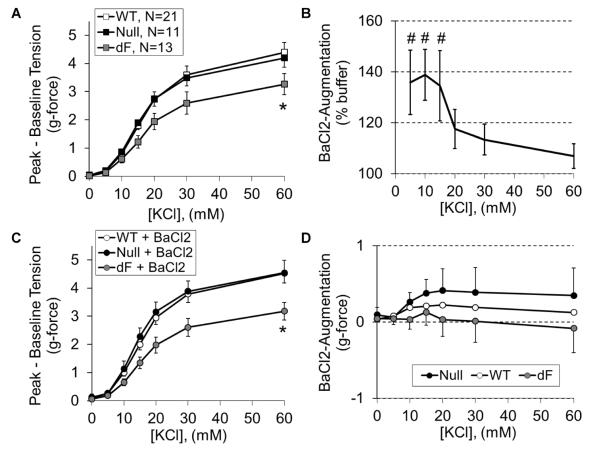
Given prior studies showing CFTR-mediated membrane hyperpolarization through an enhancement in KIR activity (22), we simultaneously assessed the response to KCl in the presence and absence of the KIR antagonist barium chloride. As seen with noradrenaline, aorta from dF piglets had impaired KCl-induced vasoconstriction (Figure 3A). While barium accentuated constriction to <20 mM KCl independent of CFTR genotype (Figure 3B), it did not correct the impaired constriction seen from CFTR-F508del aorta (Figure 3C) or elicit genotype-specific effects (Figure 3D). Notably, the KCl response failed to plateau, consistent with ongoing myogenic constriction throughout the 60 to 90 minutes that elapsed during completion of the KCl concentration-response protocol. Taken together, our myography data demonstrate CFTR-F508del dependent reductions in vasoconstriction to passive stretch, adrenergic receptor agonists and membrane depolarization, suggesting an intrinsic change in vessel morphology and/or calcium mobilization.
Figure 3.
Aortic response to membrane depolarization. Vasoconstriction to cumulative concentrations of KCl was assessed for WT (open symbols, N=21), CFTR-null (black symbols, N=11), and dF piglet aorta (gray symbols, N=13) following one hour incubation in PSS buffer alone (A) or PSS with BaCl2 (B–D). Independent of BaCl2, aorta from dF piglets had decreased KCl-induced constriction (A). Independent of genotype, KIR antagonism with BaCl2 increased aortic constriction to 5, 10 and 15 mM KCl (B), but this did not normalize the KCl responsiveness of dF aorta (C and D). *P<0.05 versus WT, #P<0.05 versus PSS without BaCl2.
Morphology
To assess aortic morphology, segments were fixed during maximal dilation. While the aorta from CFTR-null piglets tended to have increased lumen diameters and decreased wall to lumen ratios, these did not reach statistical significance (Figure 4, P>0.20 versus WT). Aorta from dF piglets had intermediate values between those of CFTR-null and WT aorta.
Figure 4.
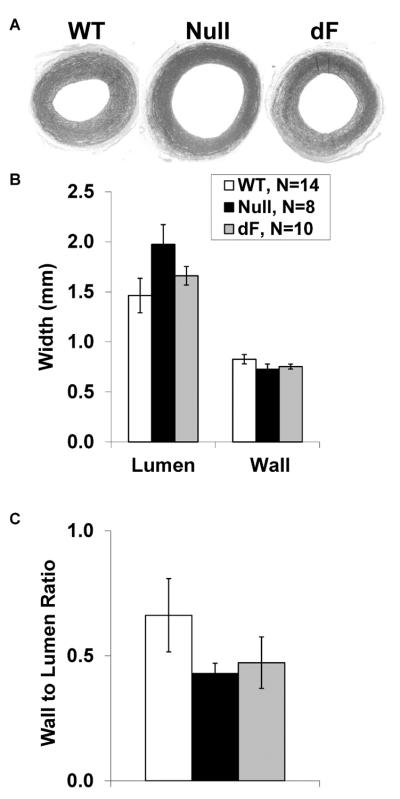
Genotype-specific aortic morphology. Following dilation with sodium nitroprusside, aortic segments from WT (left, N=14), Null (middle, N=8) and dF piglets (right, N=10) were fixed and stained for morphometric analysis (A). While the aorta from CFTR-null piglets tended to have increased lumen diameters (B) and decreased wall to lumen ratios (C), these did not approach statistical significance.
Calcium imaging
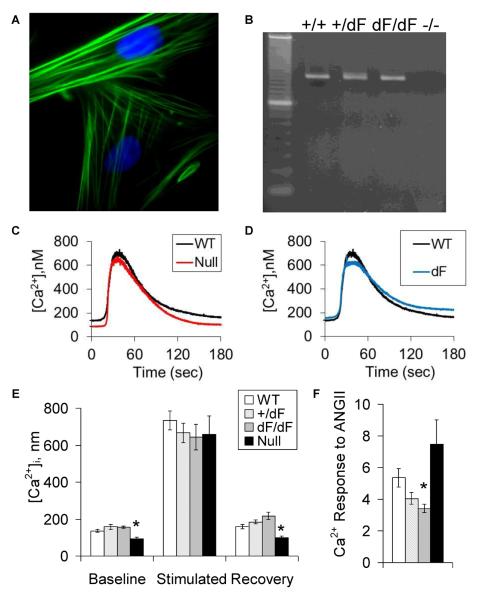
Cultured cells were uniformly identified as VSMC based on their positive immunoreactivity for alpha-smooth muscle actin (Figure 5A). CFTR mRNA was identified in VSMC expressing wild-type and/or CFTR-F508del, but not VSMC isolated from CFTR-null piglets (Figure 5B). By fura-2 imaging, VSMC from null piglets had decreased baseline [Ca2+]i (Figures 5C and 5E), while VSMC from CFTR-F508del piglets had blunted increases in cytosolic calcium in response to angiotensin II (P=0.01 for dF versus wild-type and P=0.07 for dF Het versus wild-type. Figure 5D and 5F).
Figure 5.
Vascular smooth muscle cell CFTR expression and its effect on calcium transients. VSMC were isolated from neonatal piglet aorta and identified by positive immunofluorescence for alpha-smooth muscle actin (A, with DAPI nuclear counterstain). CFTR mRNA was identified in aortic VSMC from WT, dF Het and dF/dF, but not CFTR-null piglets (B, dominant markers in left lane: 200 and 100 bp, expected size: 155 bp). Mean angiotensin II-stimulated calcium transients are provided for WT versus null (C) and WT versus dF genotypes (D). Compared to WT (white bars), VSMC from null piglets (black bars) had decreased baseline [Ca2+]i (E), while VSMC from dF piglets (gray bars) had decreased calcium responses to angiotensin II (F). N= 8 to 17, *P<0.05 versus wild-type.
DISCUSSION
While classically considered an epithelial cell-specific chloride channel, recent investigations have shown functional CFTR expression in non-epithelial cells, including smooth muscle cells, Schwann cells and skeletal muscle cells (13), (23). In rodents, wild-type CFTR activation antagonizes IP3-mediated smooth muscle cell constriction and promotes vasodilation (24)–(25). In contrast, mouse CFTR-null aorta have enhanced calcium release and vasoconstriction (26). Our studies on isolated arterial segments and VSMC explored the physiologic relevance of CFTR expression in newborn piglet aorta.
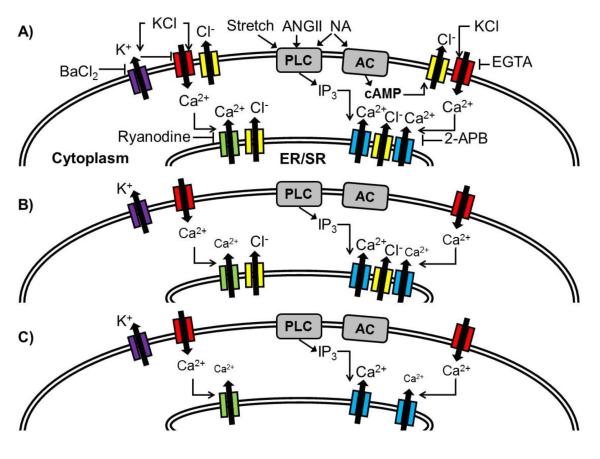
Intriguingly, CFTR null aortic responses did not differ from those of wild-type aorta, suggesting CFTR expression may have a limited role in the regulation of wild-type aortic tone. Alternatively, the dual loss of cell surface and intracellular CFTR may elicit counterbalancing physiologic effects. In stark contrast, CFTR-F508del aortas had impaired calcium mobilization that correlated with a significant and highly consistent decrease in vasoconstrictor response to stretch, adrenergic receptor stimulation or membrane depolarization. Taken together, these studies suggest a reduction in VSMC intracellular calcium signaling related to gain of CFTR function in CFTR-F508del piglets. A model of the proposed genotype-specific effects of CFTR on vascular function is provided in Figure 7.
The generation of unrelenting myogenic tone was an unexpected attribute of the newborn piglet aorta. We have demonstrated myogenic responses in a majority of our myography studies, but these responses typically fade over time. That was not the case with newborn piglet aorta. In fact, these strong myogenic responses provided a short window early in the protocol when secondary responses to noradrenaline or KCl could be elicited, and the strong myogenic tone that soon developed precluded assessment of vasodilatory responsiveness. We defined the pathways contributing to the vigorous myogenic tone by preincubating aortic segments in a variety of pharmacologic inhibitors. The use of a calcium-free buffer with EGTA excluded a role of extracellular calcium uptake in either the inception or propagation of the myogenic response. Instead, myogenic tone was attenuated only after combined inhibition of ryanodine and IP3 receptors. This is consistent with the prevailing view that myogenic responses can be mediated by endoplasmic reticulum IP3 receptor activation (29). Notably, the evolutionarily-related ER/SR calcium channels (IP3 receptors and ryanodine receptors) share a feed-forward mechanism whereby increased cytosolic calcium concentration induces further calcium release (30), possibly explaining the need to inhibit both receptor types to fully recapitulate the detrimental effects of the dF mutation.
The attenuated myogenic tone generated by dF aorta was reminiscent of the impaired response seen when wild-type aorta was incubated with either a 50% reduction in passive tension or inhibitors of both ryanodine and IP3 receptors. Unlike the results reported for piglet trachea (31), light microscopy did not reveal any ultrastructural changes in the aorta from dF piglets. Because IP3 receptor activation is a final common pathway for a number of vasoactive agents, we further interrogated aortic responsiveness to receptor and voltage-mediated calcium channel activation.
While aortic segments incubated in buffer alone again developed myogenic tone, the addition of noradrenaline significantly augmented arterial tone of segments obtained from both wild-type and CF piglets, with the greatest effect seen in CFTR-null aorta. This noradrenaline-augmented tone, but not the underlying myogenic tone, was significantly attenuated by the removal of extracellular calcium, suggesting an important role for calcium influx in this process. In this regard, the lack of CFTR-F508del-specific noradrenaline responsiveness strengthens the hypothesis that the effect of CFTR-F508del is related more to endoplasmic reticulum expression/degradation than the lack of plasma membrane expression.
Arterial constriction to KCl was of greater magnitude then that seen with either myogenic stretch alone or noradrenaline, possibly due to augmentation of extracellular calcium influx by calcium-induced intracellular calcium release. Interestingly, the CFTR-F508del induced decrease in KCl elicited vasoconstriction was not evident until the concentration of KCl exceeded 10mM. Because KIR may elicit membrane hyperpolarization, barium chloride was added to select myograph chambers to potentially unmask an independent effect of CFTR on KCl sensitivity. Even in the presence of barium, dF aorta had decreased vasoconstriction. Because barium blocks the extracellular pore of potassium channels, including KIR, and an effect of CFTR on intracellular membrane potential has been reported, future studies could investigate the potential of CFTR-F508del to inhibit intracellular calcium release through endoplasmic reticulum hyperpolarization. Relevant to this hypothesis, recent investigations have shown a lack of CFTR effect on the rate of late endosome acidification as a consequence of high intrinsic membrane permeability (32). This contrasts with investigations showing an effect of CFTR-F508del on epithelial cell ER/SR calcium release that is dependent on chloride channel activity, with the differential results potentially related to subcellular compartmentalization (27).
Investigation of aortic morphology and aortic smooth muscle cell calcium transients complemented the myography results. Independent of genotype, aorta from piglets with cystic fibrosis tended to have decreased wall to lumen thickness, but this did not approach statistical significance. While CF-related alterations in aortic morphology have not been reported, reductions in birth weight and tracheal dimensions have been reported in both CF humans and pigs (31). The reduction in IGF-1 seen in humans and piglets with CF is intriguing in this regard (33), as IGF-1 levels correlate with aortic intima-media thickness (34).
Given investigations showing CFTR-mediated smooth muscle cell dysfunction, we hypothesized VSMC alterations were responsible for the aortic phenotypes. Indeed, the CFTR-F508del mutation decreased calcium transients with a possible gene-dosage effect seen in heterozygous aorta. The presence of an intermediate phenotype in dF Het aorta would argue in favor of a non-gating effect of the mutation on VSMC function as 50% WT expression should be adequate to provide the chloride current needed for charge neutralization. While experimental data show intracellular potassium or chloride channels may contribute to the charge neutralization required during rapid and sustained ER calcium release (35) (36), additional studies utilizing impermeant ions and mathematical modeling suggest a majority of the charge neutralization may be mediated by the ER calcium channels themselves (37). Although Hybiske and colleagues did not identify statistically significant differences in calcium handling when CFTR-F508del homozygous epithelial cells were transfected with lacZ versus wild-type CFTR, dF versus WT comparisons were not performed (38). Support for an effect of ER-specific CFTR expression on calcium handling is found in recent investigations by Balghi and colleagues showing ER retention of CFTR increases store-operated calcium entry through an Orai1-dependent mechanism (28). In those investigations, the inhibition of cell surface WT CFTR did not reproduce the results seen in dF cells, and further studies are necessary to determine if the presence of CFTR-F508del within the ER or the absence of plasma membrane CFTR expression mediate the effects of the chloride channel on not only on calcium entry, but also intracellular calcium release.
Compared to dF-expressing cells, CFTR-null cells had diametrically opposed results, with a trend towards increased calcium transients that was strongly influenced by a significant decrease in baseline calcium levels. The suppression in baseline [Ca2+]i seen in only CFTR-null aorta implies a role for either absent ER expression or a compensatory cellular response to the mutation, further studies are needed to define the origin and physiologic implications of this alteration with a loss of charge neutralization across the ER membrane a possibility worth considering (27). It is possible the reduction in baseline [Ca2+]i sufficiently reduced basal actinmyosin interaction such that subsequent agonist-induced calcium responses elicit a disproportionately greater increase in force generation, and this is supported by both the relative increase in baseline CFTR-null aortic diameters and the relative increase in noradrenaline-induced vasoconstriction. Although the baseline calcium concentrations we measured from wild-type myocytes are consistent with the literature (39), absolute precision could be improved if future studies utilize a system calibrated with permeabilized cells.
The findings in VSMC do not rule-out an additional role of other vascular cell types, including CFTR-expressing endothelial cells (40), in the observed aortic phenotypes, and they do not identify the intracellular or extracellular source of the altered calcium transients. Indeed, skeletal muscle cells from CFTR-null mice have ryanodine and IP3 receptor-dependent increases in the intracellular calcium response to KCl (41), and CFTR inhibition acutely increases the extracellular calcium uptake of spontaneously beating ventricular myocytes (42). Further studies are needed to determine whether these alterations in calcium handling are related to changes in plasma membrane electrochemical gradients and/or altered endoplasmic reticulum calcium channel distribution, potentially as a consequence of a CFTR-F508del induced ER stress response that could attenuate IP3 receptor function through a combination of spacial distortion of the ER/SR membrane and/or accelerated IP3R degradation in activated proteasome complexes (Figure 6). However, despite the degradation of CFTR-F508del by an unfolded protein response (43), investigations by Ribeiro and Boucher suggest, at least in epithelial cells, there is a relatively low level of ER stress in the absence of inflammatory stimuli (44).
Figure 6.
Simplified model of the potential impact of CFTR mutations on VSMC function. Wild-type CFTR (yellow) localizes within the plasma and ER/SR membranes (A). Increasing extracellular [KCl] leads to membrane depolarization and activation of voltage-dependent calcium channels (red), a process that is attenuated by the calcium chelator EGTA and inward rectifying potassium channels (purple, blocked by barium). An increase in [Ca2+]i induces further calcium release through ryanodine receptors (green, blocked by ryanodine) and IP3 receptors (blue, blocked by 2-APB). Countercurrent chloride efflux from ER/SR CFTR channels may provide charge neutralization (27). Membrane stretch, angiotensin II (AngII) and noradrenaline (NA) mediate vasoconstriction in part by activation of phospholipase C (PLC), leading to the release of IP3 and subsequent IP3 receptor activation. In addition to PLC activation, NA activates adenylate cyclase (AC) leading to the production of cAMP, with subsequent CFTR activation potentially leading to plasma membrane depolarization and enhanced calcium entry. VSMC expressing CFTR-F508del lack plasma membrane CFTR expression (B), potentially contributing to membrane hyperpolarization (28) and a reduction in calcium-induced calcium release. CFTR-null cells also lack ER/SR CFTR expression, and this may contribute to the observed decrease in baseline calcium concentration (C).
In conclusion, while wild-type CFTR expression appears to have a negligible effect on aortic reactivity, the CFTR-F508del mutation is associated with decreased aortic tone and aortic VSMC calcium release. If widespread in conduit and resistance arteries, this reduction in myocyte reactivity may contribute to the cardiovascular phenotypes increasingly reported in CF patients. Further investigations centered on VSMC-specific CFTR-F508 expression are needed to clarify the causal relationship between CFTR mutations and the reported reductions in arterial pressure.
As therapeutic interventions increasingly focus on systemic delivery of CFTR-corrector therapy, there is increased emphasis on improved understanding of the extra-pulmonary effects of CFTR expression. If our findings in piglet aorta translate to human arterioles, the reduction in vascular tone might contribute to the lower blood pressure seen in those with heterozygous or homozygous CFTR-F508del expression. A restoration of the CFTR trafficking in patients with an F508del mutation might thereby influence blood pressure and more importantly, systemic and pulmonary blood flow. Assessment of indirect and direct measures of tissue perfusion should be considered in future therapeutic trials of small molecule CFTR correctors.
Acknowledgements
Dr. Michael Welsh provided invaluable guidance and assisted with manuscript preparation.
Role of the funding source
These investigations were supported by the Howard Hughes Medical Institute which had no involvement in the study design, data analysis, manuscript preparation or the decision to submit the manuscript for publication.
Abbreviations
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- dF
CFTR-F508del
- ER
endoplasmic reticulum
- IP3
inositol triphosphate
- PSS
physiologic saline solution
- VSMC
vascular smooth muscle cell
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data were presented in part at the North American Cystic Fibrosis Conference, October 11, 2012, Orlando, FL (45)
Conflict of interest statement
The authors have no personal or financial conflicts of interest.
REFERENCES
- 1.Bobadilla JL, Macek M, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations—correlation with incidence data and application to screening. Hum Mutat. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- 2.Cheng SH, Gregory RJ, Marshall J, et al. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 3.Kopito RR. Biosynthesis and degradation of CFTR. Physiol Rev. 1999;79:S167–73. doi: 10.1152/physrev.1999.79.1.S167. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman J, Rodbard S. Low blood pressure in young adults with cystic fibrosis: an effect of chronic salt loss in sweat? Ann Intern Med. 1975;82:806–808. doi: 10.7326/0003-4819-82-6-806. [DOI] [PubMed] [Google Scholar]
- 5.Fraser KL, Tullis DE, Sasson Z, Hyland RH, Thornley KS, Hanly PJ. Pulmonary hypertension and cardiac function in adult cystic fibrosis: role of hypoxemia. Chest. 1999;115:1321–1328. doi: 10.1378/chest.115.5.1321. [DOI] [PubMed] [Google Scholar]
- 6.Baño-Rodrigo A, Salcedo-Posadas A, Villa-Asensi JR, Tamariz-Martel A, Lopez-Neyra A, Blanco-Iglesias E. Right ventricular dysfunction in adolescents with mild cystic fibrosis. J Cyst Fibros. 2012;11:274–280. doi: 10.1016/j.jcf.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Super M, Irtiza-Ali A, Roberts SA, et al. Blood pressure and the cystic fibrosis gene: evidence for lower pressure rises with age in female carriers. Hypertension. 2004;44:878–883. doi: 10.1161/01.HYP.0000145901.81989.46. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal A, Button LN, Khaw KT. Blood volume changes in patients with cystic fibrosis. Pediatrics. 1977;59:588–594. [PubMed] [Google Scholar]
- 9.Brown MB, McCarty NA, Millard-Stafford M. High-sweat Na+ in cystic fibrosis and healthy individuals does not diminish thirst during exercise in the heat. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1177–R1185. doi: 10.1152/ajpregu.00551.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hull JH, Ansley L, Bolton CE, et al. The effect of exercise on large artery haemodynamics in cystic fibrosis. J Cyst Fibros. 2011;10:121–127. doi: 10.1016/j.jcf.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Buehler T, Steinmann M, Singer F, et al. Increased arterial stiffness in children with cystic fibrosis. Eur Respir J. 2012;39:1536–1537. doi: 10.1183/09031936.00212511. [DOI] [PubMed] [Google Scholar]
- 12.McGrath LT, McCall D, Hanratty CG, et al. Individuals with cystic fibrosis do not display impaired endothelial function or evidence of oxidative damage in endothelial cells exposed to serum. Clin Sci (Lond) 2001;101:507–513. [PubMed] [Google Scholar]
- 13.Michoud MC, Robert R, Hassan M, et al. Role of the cystic fibrosis transmembrane conductance channel in human airway smooth muscle. Am J Respir Cell Mol Biol. 2009;40:217–222. doi: 10.1165/rcmb.2006-0444OC. [DOI] [PubMed] [Google Scholar]
- 14.Stoltz DA, Rokhlina T, Ernst SE, et al. Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J Clin Invest. 2013;123:2685–2693. doi: 10.1172/JCI68867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutko JL, Airey JA, Welch W, Ruest L. The pharmacology of ryanodine and related compounds. Pharmacol Rev. 1997;49:53–98. [PubMed] [Google Scholar]
- 16.Orie NN, Fry CH, Clapp LH. Evidence that inward rectifier K+ channels mediate relaxation by the PGI2 receptor agonist cicaprost via a cyclic AMP-independent mechanism. Cardiovasc Res. 2006;69:107–115. doi: 10.1016/j.cardiores.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 18.Roghair RD, Segar JL, Sharma RV, Zimmerman MC, Jagadeesha DK, Segar EM, Scholz TD, Lamb FS. Newborn lamb coronary artery reactivity is programmed by early gestation dexamethasone before the onset of systemic hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1169–76. doi: 10.1152/ajpregu.00369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roghair RD, Volk KA, Lamb FS, Segar JL. Impact of maternal dexamethasone on coronary PGE(2) production and prostaglandin-dependent coronary reactivity. Am J Physiol Regul Integr Comp Physiol. 2012;303:R513–R519. doi: 10.1152/ajpregu.00658.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50. [PubMed] [Google Scholar]
- 21.Rootwelt T, Løberg EM, Moen A, Oyasaeter S, Saugstad OD. Hypoxemia and reoxygenation with 21% or 100% oxygen in newborn pigs: changes in blood pressure, base deficit, and hypoxanthine and brain morphology. Pediatr Res. 1992;32:107–113. doi: 10.1203/00006450-199207000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Lu M, Leng Q, Egan ME, et al. CFTR is required for PKA-regulated ATP sensitivity of Kir1.1 potassium channels in mouse kidney. J Clin Invest. 2006;116:797–807. doi: 10.1172/JCI26961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamhonwah AM, Bear CE, Huan LJ, Kim Chiaw P, Ackerley CA, Tein I. Cystic fibrosis transmembrane conductance regulator in human muscle: Dysfunction causes abnormal metabolic recovery in exercise. Ann Neurol. 2010;67:802–808. doi: 10.1002/ana.21982. [DOI] [PubMed] [Google Scholar]
- 24.Robert R, Thoreau V, Norez C, et al. Regulation of the cystic fibrosis transmembrane conductance regulator channel by beta-adrenergic agonists and vasoactive intestinal peptide in rat smooth muscle cells and its role in vasorelaxation. J Biol Chem. 2004;279:21160–21168. doi: 10.1074/jbc.M312199200. [DOI] [PubMed] [Google Scholar]
- 25.Robert R, Savineau JP, Norez C, Becq F, Guibert C. Expression and function of cystic fibrosis transmembrane conductance regulator in rat intrapulmonary arteries. Eur Respir J. 2007;30:857–864. doi: 10.1183/09031936.00060007. [DOI] [PubMed] [Google Scholar]
- 26.Robert R, Norez C, Becq F. Disruption of CFTR chloride channel alters mechanical properties and cAMP-dependent Cl- transport of mouse aortic smooth muscle cells. J Physiol. 2005;568:483–495. doi: 10.1113/jphysiol.2005.085019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins JR, Kongsuphol P, Sammels E, et al. F508del-CFTR increases intracellular Ca(2+) signaling that causes enhanced calcium-dependent Cl(−) conductance in cystic fibrosis. Biochim Biophys Acta. 2011;1812:1385–1392. doi: 10.1016/j.bbadis.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Balghi H, Robert R, Rappaz B, Zhang X, Wohlhuter-Haddad A, Evagelidis A, Luo Y, Goepp J, Ferraro P, Roméo P, Trebak M, Wiseman PW, Thomas DY, Hanrahan JW. Enhanced Ca2+ entry due to Orai1 plasma membrane insertion increases IL-8 secretion by cystic fibrosis airways. FASEB J. 2011;25:4274–91. doi: 10.1096/fj.11-187682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayanan J, Imig M, Roman RJ, Harder DR. Pressurization of isolated renal arteries increases inositol trisphosphate and diacylglycerol. Am J Physiol. 1994;266:H1840–H1845. doi: 10.1152/ajpheart.1994.266.5.H1840. [DOI] [PubMed] [Google Scholar]
- 30.Stathopulos PB, Seo MD, Enomoto M, Amador FJ, Ishiyama N, Ikura M. Themes and variations in ER/SR calcium release channels: structure and function. Physiology. 2012;27:331–342. doi: 10.1152/physiol.00013.2012. [DOI] [PubMed] [Google Scholar]
- 31.Meyerholz DK, Stoltz DA, Namati E, et al. Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children. Am J Respir Crit Care Med. 2010;182:1251–1261. doi: 10.1164/rccm.201004-0643OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barriere H, Bagdany M, Bossard F, et al. Revisiting the role of cystic fibrosis transmembrane conductance regulator and counterion permeability in the pH regulation of endocytic organelles. Mol Biol Cell. 2009;20:3125–3141. doi: 10.1091/mbc.E09-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogan MP, Reznikov LR, Pezzulo AA, et al. Pigs and humans with cystic fibrosis have reduced insulin-like growth factor 1 (IGF1) levels at birth. Proc Natl Acad Sci U S A. 2010;107:20571–20575. doi: 10.1073/pnas.1015281107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koklu E, Ozturk MA, Kurtoglu S, Akcakus M, Yikilmaz A, Gunes T. Aortic intima-media thickness, serum IGF-I, IGFBP-3, and leptin levels in intrauterine growth-restricted newborns of healthy mothers. Pediatr Res. 2007;62:704–709. doi: 10.1203/PDR.0b013e318157caaa. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Best PM. Characterization of the potassium channel from frog skeletal muscle sarcoplasmic reticulum membrane. J Physiol. 1994;477:279–90. doi: 10.1113/jphysiol.1994.sp020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rousseau E, Roberson M, Meissner G. Properties of single chloride selective channel from sarcoplasmic reticulum. Eur Biophys J. 1988;16:143–51. doi: 10.1007/BF00261900. [DOI] [PubMed] [Google Scholar]
- 37.Gillespie D, Fill M. Intracellular calcium release channels mediate their own countercurrent: the ryanodine receptor case study. Biophys J. 2008;95:3706–14. doi: 10.1529/biophysj.108.131987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hybiske K, Fu Z, Schwarzer C, Tseng J, Do J, Huang N, Machen TE. Effects of cystic fibrosis transmembrane conductance regulator and DeltaF508CFTR on inflammatory response, ER stress, and Ca2+ of airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1250–60. doi: 10.1152/ajplung.00231.2007. [DOI] [PubMed] [Google Scholar]
- 39.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508:199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tousson A, Van Tine BA, Naren AP, Shaw GM, Schwiebert LM. Characterization of CFTR expression and chloride channel activity in human endothelia. Am J Physiol. 1998;275:C1555–C1564. doi: 10.1152/ajpcell.1998.275.6.C1555. [DOI] [PubMed] [Google Scholar]
- 41.Divangahi M, Balghi H, Danialou G, et al. Lack of CFTR in skeletal muscle predisposes to muscle wasting and diaphragm muscle pump failure in cystic fibrosis mice. PLoS Genet. 2009;5:e1000586. doi: 10.1371/journal.pgen.1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sellers ZM, De Arcangelis V, Xiang Y, Best PM. Cardiomyocytes with disrupted CFTR function require CaMKII and Ca(2+)-activated Cl(−) channel activity to maintain contraction rate. J Physiol. 2010;588:2417–2429. doi: 10.1113/jphysiol.2010.188334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartoszewski R, Rab A, Jurkuvenaite A, et al. Activation of the unfolded protein response by deltaF508 CFTR. Am J Respir Cell Mol Biol. 2008;39:448–457. doi: 10.1165/rcmb.2008-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ribeiro CM, Boucher RC. Role of endoplasmic reticulum stress in cystic fibrosis-related airway inflammatory responses. Proc Am Thorac Soc. 2010;7:387–94. doi: 10.1513/pats.201001-017AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roghair R, McCray P, Welsh M, Santillan D, Santillan M, Stoltz DA. Direct cardiovascular effects of the CFTR △F508 mutation. Pulmonology. 2012;47:288. [Google Scholar]