Abstract
Central neuropathic pain (CNP) is a debilitating consequence of central nervous system (CNS) damage for which current treatments are ineffective. To explore mechanisms underlying CNP, we developed a rat model involving T13/L1 dorsal root avulsion. The resultant dorsal horn damage creates bilateral below-level (L4-6) mechanical allodynia. This allodynia, termed spinal neuropathic avulsion pain (SNAP), occurs in the absence of confounding paralysis. To characterize this model, we undertook a series of studies aimed at defining whether SNAP could be reversed by any of 3 putative glial activation inhibitors, each with distinct mechanisms of action. Indeed, the phosphodiesterase inhibitor propentofylline, the macrophage migration inhibitory factor (MIF) inhibitor ibudilast, and the toll-like receptor 4 (TLR4) antagonist (+)-naltrexone each reversed below-level allodynia bilaterally. Strikingly, none of these impacted SNAP upon first administration but required 1–2 wk of daily administration before pain reversal was obtained. Given reversal of CNP by each of these glial modulatory agents, these results suggest that glia contribute to the maintenance of such pain and enduring release of MIF and endogenous agonists of TLR4 is important for sustaining CNP. The markedly delayed efficacy of all 3 glial modulatory drugs may prove instructive for interpretation of apparent drug failures after shorter dosing regimens.
Keywords: avulsion, TLR4, dextro-naltrexone, glia, pain
Introduction
Central neuropathic pain (CNP) is a common and debilitating consequence of a variety of different central nervous system (CNS) traumas including spinal cord injury (SCI), stroke, traumatic brain injury (TBI), and multiple sclerosis (MS). The percentage of patients that develop central pain following trauma range from 8% in stroke, 25% in MS, 50% in TBI, and up to 66% in SCI.9, 56, 68 Mechanisms underlying CNP are poorly understood, and current pharmacotherapies for treating this type of pain are not effective.
CNP is extremely difficult to treat, and current therapies have limited response rates, provide only minor pain relief, and often have intolerable side effects. Classes of drugs commonly used to treat CNP include tricyclic antidepressants (TCAs), calcium channel trafficking inhibitors, anticonvulsants, and opioids.24 Using SCI as an example, a clinical SCI study found that gabapentin, which inhibits calcium channel trafficking,35, 45 was no more effective than the active placebo and that the TCA amitriptyline was slightly more effective than active placebo but resulted in undesirable side effects (nausea, bladder problems, constipation).63 Another clinical study found that the anticonvulsant drug lamotrigine did not relieve central pain in patients with MS.10 Although opioids are one of the most widely used and effective central pain treatments,85 the side effects and addiction problems that can arise from chronic use cause many pain patients to discontinue treatment.6 In addition, recent literature has shown that morphine given shortly after SCI can have deleterious effects on recovery.36 One commonality between all of these drugs is that their mechanisms of action are thought to be largely neuronal, but it is well known that glial cells that become activated after a traumatic inflammatory event play a large role in the induction and maintenance of a variety of chronic pain states of peripheral origin.13, 27 Examining the potential role of glia in mechanisms underlying CNP may potentially lead to the development of more efficacious CNP treatments as well as improve our understanding of the mechanisms that underlie this type of pain.
Since the complexities of CNS traumas make them extremely difficult to treat, it is important to consider not only neuronal targeting compounds, but also those treatments that target other cell types such as glia and macrophages. The current series of studies examined the effects of administering three different putative glial activation inhibitors, propentofylline (PPF; a phosphodiesterase (PDE) inhibitor),55, 71 ibudilast (some PDE actions but considered predominantly as a MIF inhibitor),18 and (+)-naltrexone (a non-opioid TLR4 antagonist),39 in a novel model of unilateral T13/L1 dorsal root avulsion SCI. Dorsal root avulsions are common after automobile and motorcycle accidents7, 14 and can cause below-level pain.20, 23 Our avulsion model creates a discrete dorsal horn spinal cord lesion resulting in bilateral below-level neuropathic pain (termed spinal neuropathic avulsion pain [SNAP]), but does not cause paralysis, gross white matter damage, or urinary tract infections that are inherent, at least in part, in most models of stroke, TBI, MS, and contusion and hemisection SCI.84 We developed this avulsion model in order to study pain behavior in isolation from such complicating factors so that we may better understand the specific mechanisms underlying CNP.
Materials and Methods
Animals
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado at Boulder. The care and use of the animals also conformed to guidelines of the International Association for the Study of Pain. Pathogen-free male Sprague-Dawley rats (325–350g; Harlan Laboratories, Madison, WI, USA) were used for all experiments. Rats were pair-housed prior to surgery and then single-housed after surgery with standard rat chow and water available ad libitum. Housing was in a temperature-controlled room that was maintained at 23+/−2°C with a 12hr light/dark cycle (lights on at 0700 hr). The rats were allowed a minimum of 1 wk to habituate to the colony room before initiating the experiment. All procedures were done during the light cycle.
Drugs
Propentofylline (PPF; a gift from MediciNova, San Diego, CA, USA and Solace/Patheon UK Ltd, Wiltshire, England) was dissolved in sterile endotoxin-free isotonic saline (Abbott Laboratories, Abbott Park, IL, USA) and administered at dose of 10 mg/kg per intraperitoneal (i.p.) injection. Controls received i.p. equivolume (1 ml/kg) saline. Ibudilast (MN-166; a gift from MediciNova, San Diego, CA, USA) was dissolved in 100% corn oil (Mazola) and administered at 10 mg/kg per subcutaneous (s.c.) injection. Controls received s.c. equivolume (0.5 ml/kg) corn oil. (+)-Naltrexone (a gift from NIDA and NIAAA, Rockville, MD, USA) was dissolved in sterile endotoxin-free isotonic saline (Abbott Laboratories, Abbott Park, IL, USA) and administered at 6 mg/kg per s.c. injection. Controls received equivolume (1 ml/kg) saline. All drugs were given systemically as they are all known to cross the blood brain barrier.25, 39, 46
Spinal Neuropathic Avulsion Pain (SNAP) Surgery
Unilateral (left) T13/L1 dorsal root avulsion was performed under isoflurane anesthesia, as previously described in detail.83 Briefly, laminectomy was performed at the T12 vertebral level and the dura mater was incised over the dorsal root entry zone. The T13 and L1 dorsal rootlets were carefully isolated and then clamped at the dorsal root entry zone and briskly pulled out (avulsed). Sterile saline-moistened surgical sponge was placed over the exposed spinal cord to protect it, the muscle was sutured in layers with sterile 3-0 silk, and the skin was closed with stainless steel wound clips. Immediately following surgery, rats were single-housed in a cage with foam padding for a few hours to protect their spinal cord from further trauma due to the brief ataxic period that follows recovery from anesthesia. Sham operated rats were treated identically, except for avulsing of the rootlets. Combi-Pen-48 antibiotic (0.2 ml; Bimeda, Inc., Le Sueur, MN, USA) was administered at the time of surgery and daily for 4 days after surgery.
Low Threshold Mechanical Allodynia Testing
Prior to surgery, rats were habituated to the testing environment for 4 consecutive days prior to recording of behavioral responses. All von Frey assessments were performed blind with respect to drug and surgery assignments. Assessment of von Frey thresholds occurred before surgery (baseline) and across a timecourse beginning two weeks after surgery. The von Frey test was performed on the plantar surface of each hind paw as previously described in detail.53 A logarithmic series of 10 calibrated Semmes–Weinstein monofilaments (Stoelting) were sequentially applied to the left and right hind paws in random order, each for 8 s at constant pressure to determine the stimulus intensity threshold stiffness required to elicit a paw withdrawal response. Log stiffness of the hairs is determined by log10 (milligrams x10). The range of monofilaments used in these experiments (0.407–15.136 g) produces a logarithmically graded slope when interpolating a 50% response threshold of stimulus intensity (expressed as log10 (milligrams x10)).15 The stimulus intensity threshold to elicit a paw withdrawal response was used to calculate the 50% paw withdrawal threshold (absolute threshold) using the maximum-likelihood fit method to fit a Gaussian integral psychometric function.34 This method normalizes the withdrawal threshold for parametric analyses.34 The general von Frey testing rubric is as follows. Rats are baselined before surgery and are allowed a 14 day recovery period. Rats are tested on a weekly basis on days 14, 21, and 28 post-surgery. Drug administration begins after the day 28 behavioral test, with one exception ((+)-naltrexone) where drug administration began on day 32 post-surgery due to extenuating circumstances. Behavioral testing then occurs once per week until the conclusion of the study, except for when a more detailed timecourse was necessary to define when reversal of enhanced mechanical reactivity (allodynia) began to occur. In these studies, behavioral testing occurs every 2–3 days after drug administration.
Pharmacological Manipulations
Propentofylline (PPF) Timecourses
Daily PPF dosing began after development of SNAP was confirmed by von Frey testing 14, 21, and 28 days after surgery. PPF (10 mg/kg) or equivolume vehicle (saline) was administered once daily for either 14 days or 35 days. PPF was administered between 1600–1800 hr, and behavioral testing occurred approximately 15 hr later (0700–0900 hr), so to parallel previous publications.73 This delayed testing timepoint is standard when looking at glial effects of PPF in order to allow time for second messenger signaling cascades to exert their effects.31, 54, 60, 72, 74 Rats were behaviorally tested to define their mechanical response thresholds every 2–7 days until the final behavioral test 15 hr after the last dose of PPF or vehicle.
Ibudilast Timecourse Later in the Development of SNAP
Daily ibudilast dosing began after development of SNAP was confirmed by von Frey testing 14, 21, and 28 days after surgery. Ibudilast (10 mg/kg) or equivolume vehicle (corn oil) was administered once daily for 35 days. Ibudilast was administered between 0800–1000 hr each morning and testing occurred 1–2 hr later, so to parallel previous publications.47 Rats were behaviorally tested to define their mechanical response thresholds every 7 days until the final behavioral test 1–2 hr after the last dose of ibudilast or vehicle.
Ibudilast Timecourse Early in the Development of SNAP
Daily ibudilast dosing began after development of SNAP was confirmed by von Frey testing 14 days post-surgery. Ibudilast (10 mg/kg) or equivolume vehicle (corn oil) was administered once daily for 21 days. Ibudilast was administered between 0800–1000 hr each morning and testing occurred 1–2 hr later, as described above. Rats were behaviorally tested to define their mechanical response thresholds every 7 days until the final behavioral test after the last dose of ibudilast or vehicle.
(+)-Naltrexone Timecourse
Daily (+)-naltrexone dosing began after development of SNAP was confirmed by von Frey testing 14, 21, 28, and 32 days after surgery. Given its relatively short half-life compared to ibudilast and PPF, (+)-naltrexone (6 mg/kg) or equivolume vehicle (saline) was administered three times a day for 14 days. (+)-Naltrexone was administered at approximately 0900, 1200, and 1500 hr and testing occurred 1 hr after the second injection, based on our previous studies.39 Rats were behaviorally tested to define their mechanical response thresholds every 7 days until the final behavioral test after the last dose of (+)-naltrexone or vehicle.
(+)-Naltrexone Drug Cessation Timecourse
Daily (+)-naltrexone dosing began after development of SNAP was confirmed by von Frey testing 14, 21, and 28, days after surgery. Given its relatively short half-life compared to ibudilast and PPF, (+)-naltrexone (6 mg/kg) or equivolume vehicle (saline) was administered three times a day for 6 days. (+)-Naltrexone was administered at approximately 0900, 1200, and 1500 hr and testing occurred 1 hr after the second injection, based on our previous studies. Rats were behaviorally tested to define their mechanical response thresholds daily until the final behavioral test after the last dose of (+)-naltrexone or vehicle on day 33. Rats were then behaviorally tested every 2–6 days, at approximately 1300hr, until they returned back to pre-drug allodynia levels. Since we already observed that (+)-naltrexone had no effect on sham operated rats in the previous (+)-naltrexone study, and to conserve drug, sham groups were not included in this study.
Statistical Analysis
All data were expressed as mean ± standard error of the mean (SEM). Ipsilateral and contralateral behavioral data were analyzed individually. Behavioral measures were normalized as described above and group differences were analyzed by comparing area under the curve (AUC), as previously described by Jones and Sorkin.42 AUC values (GraphPad Prism 5.01; GraphPad software Inc., San Diego, CA) were calculated from absolute threshold values, from 3.56 (the lowest threshold response value in the data set) up to the threshold response of each rat across time. Decreased AUC reflects an increase in mechanical allodynia. For all studies, the AUC measures across time were collapsed into a single timepoint for each animal, thus there is not a repeated measurement. For baseline measurements, a two-way ANOVA at that single timepoint was the statistic used. For pre-drug statistics, a t-test was performed on the AUC values as there were only two groups, sham and SNAP. For statistics during the drug administration period, a two-way ANOVA was then performed on the AUC values, with the exception of the studies examining the effects of (+)-naltrexone on SNAP. For the (+)-naltrexone drug timecourse in Figure 5, a one-way ANOVA was used because it is not a 2x2 design. For the study looking at behavior after stopping (+)-naltrexone administration in Figure 6, a t-test was used because there are only two groups, SNAP+Vehicle and SNAP+(+)-Naltrexone. The appropriate AUC statistic was performed on days 35–63 for Figure 1, days 35–42 for Figure 2, days 35–63 for Figure 3, days 14–28 for Figure 4, days 35–46 for Figure 5, days 29–33 for Figure 6B, and days 35–42 for Figure 6C. A Bonferroni post hoc test for multiple comparisons was used where appropriate. For all tests, p<0.05 was considered statistically significant.
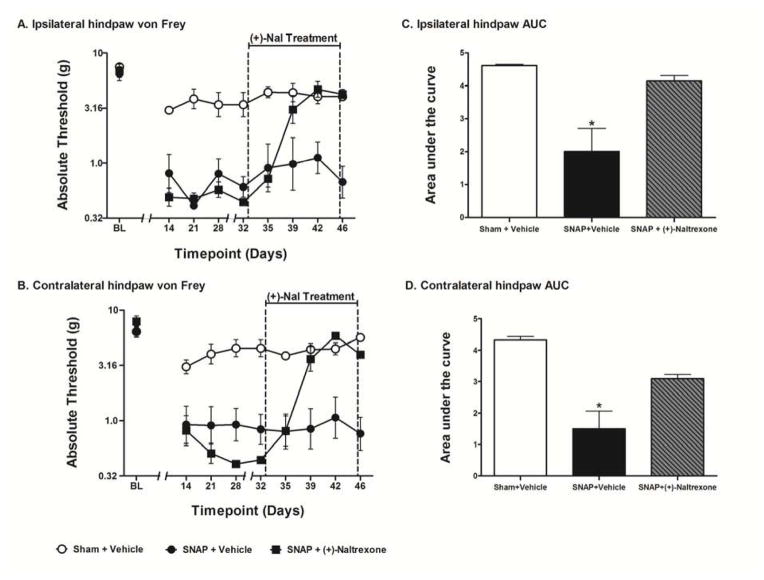
Figure 5.
Assessment of the effects of (+)-naltrexone on SNAP. Rats were tested for mechanical allodynia across a timecourse on both the ipsilateral (A) and contralateral (B) hindpaw. Rats that received (+)-naltrexone (6 mg/kg, s.c.) for 14 days beginning 32 days after surgery were significantly less allodynic than rats that received vehicle in both the ipsilateral (C) and contralateral (D) hindpaw. Data are presented as mean ± SEM and analyzed using a one-way ANOVA on the AUCs, n=6 per group. *p<0.05 compared to all other groups.
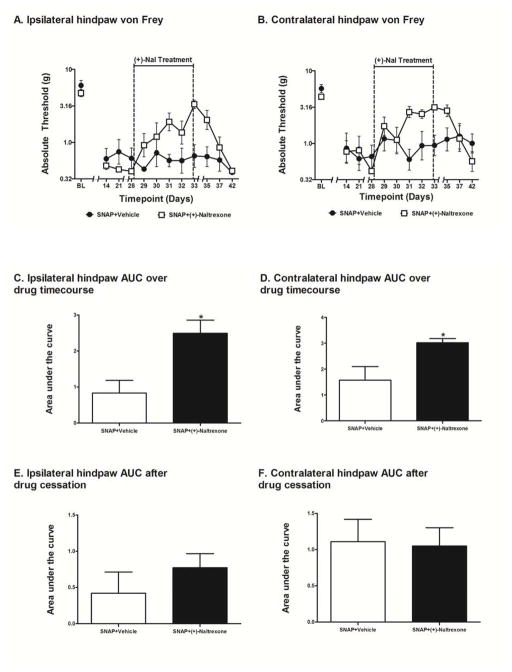
Figure 6.
Assessment of the effects of ceasing (+)-naltrexone on SNAP. Rats were tested for mechanical allodynia across a timecourse on both the ipsilateral (A) and contralateral (B) hindpaw. Rats that received (+)-naltrexone (6 mg/kg, s.c.) for 6 days beginning 28 days after surgery were significantly less allodynic than rats that received vehicle in both the ipsilateral (C) and contralateral (D) hindpaw. After stopping (+)-naltrexone administration on day 33, rats that were receiving (+)-naltrexone returned to pre-drug allodynia thresholds in both the ipsilateral (E) and contralateral (F) hindpaw. Data are presented as mean ± SEM and analyzed using a t-test on the AUCs, n=6 per group. *p<0.05 compared to all other groups.
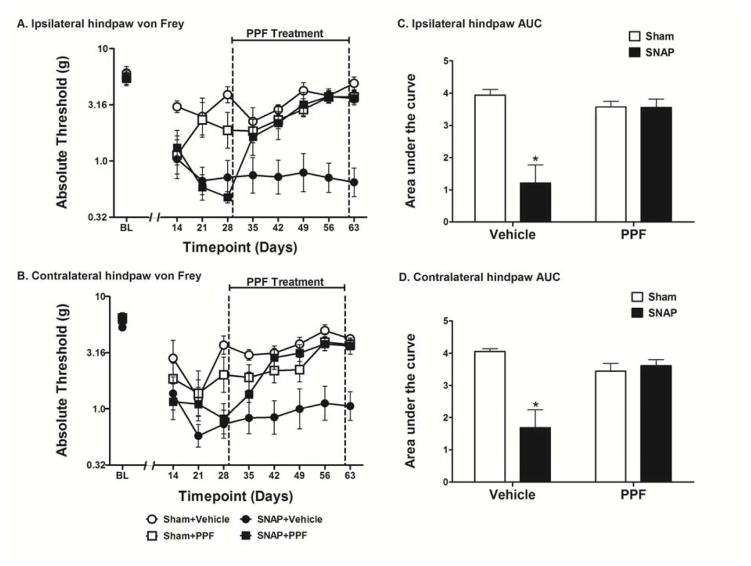
Figure 1.
Assessment of the effects of propentofylline (PPF) on SNAP. Rats were tested for mechanical allodynia across a timecourse on both the ipsilateral (A) and contralateral (B) hindpaw. Rats that received PPF (10 mg/kg, i.p.) for 35 days beginning 28 days after surgery were significantly less allodynic than rats that received vehicle in both the ipsilateral (C) and contralateral (D) hindpaw. Data are presented as mean ± SEM and analyzed using a two-way ANOVA on the AUCs, n=6–8/group. *p<0.05 compared to all other groups.
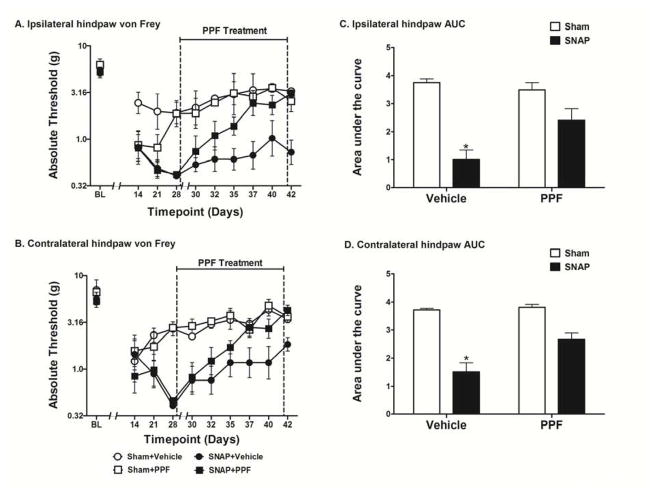
Figure 2.
Detailed timecourse of the effects of PPF on SNAP. Rats were tested for mechanical allodynia across a timecourse on both the ipsilateral (A) and contralateral (B) hindpaw. Rats that received PPF (10 mg/kg, i.p.) for 14 days beginning 28 days after surgery were significantly less allodynic than rats that received vehicle in both the ipsilateral (C) and contralateral (D) hindpaw. Data are presented as mean ± SEM and analyzed using a two-way ANOVA on the AUCs, n=6 per group. *p<0.05 compared to all other groups
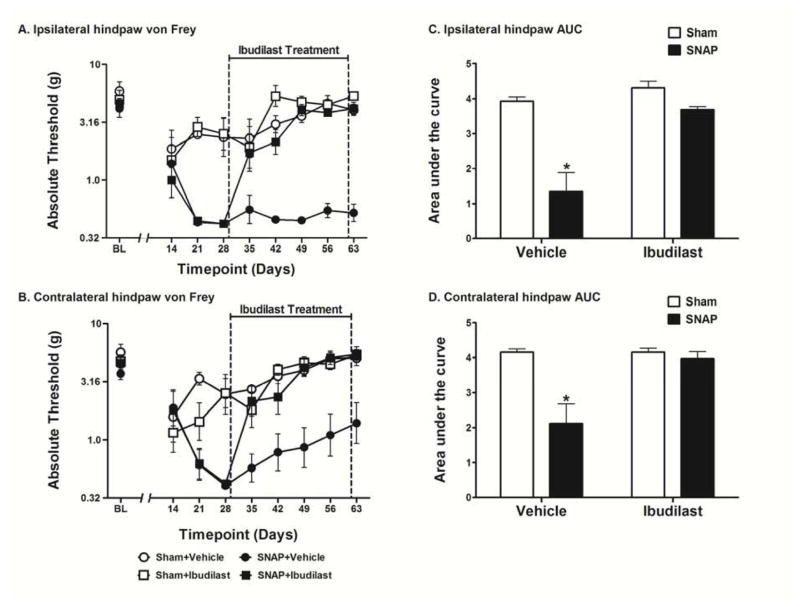
Figure 3.
Assessment of the effects of ibudilast administered late in the development of SNAP. Rats were tested for mechanical allodynia across a timecourse on both the ipsilateral (A) and contralateral (B) hindpaw. Rats that received ibudilast (10 mg/kg, s.c.) for 35 days beginning 28 days after surgery were significantly less allodynic than rats that received vehicle in both the ipsilateral (C) and contralateral (D) hindpaw. Data are presented as mean ± SEM and analyzed using a two-way ANOVA on the AUCs, n=6–8 per group. *p<0.05 compared to all other groups.
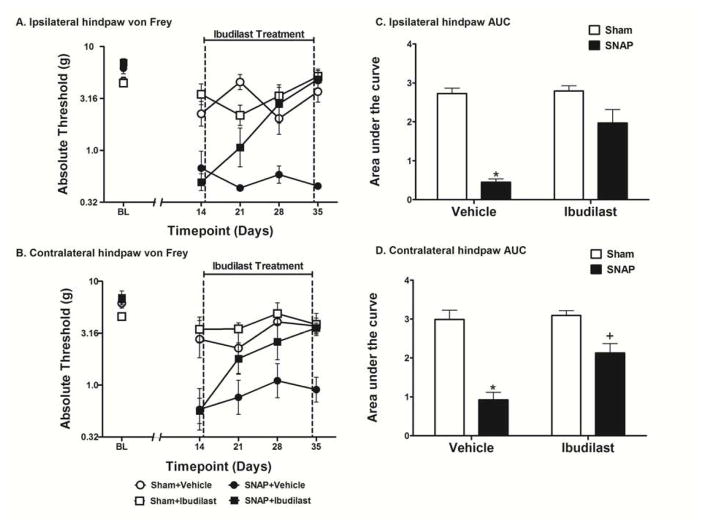
Figure 4.
Assessment of the effects of ibudilast administered early in the development of SNAP. Rats were tested for mechanical allodynia across a timecourse on both the ipsilateral (A) and contralateral (B) hindpaw. Rats that received ibudilast (10 mg/kg, s.c.) for 21 days beginning 14 days after surgery were significantly less allodynic than rats that received vehicle in both the ipsilateral (C) and contralateral (D) hindpaw. Data are presented as mean ± SEM and analyzed using a two-way ANOVA on the AUCs, n=5–6 per group. *p<0.05 compared to all other groups, +p<0.05 compared to Sham+Vehicle and Sham+Ibudilast.
Results
Effect of Administering Propentofylline on SNAP
In this first study, once daily PPF was administered i.p. at 10 mg/kg for 35 days beginning 28 days post-surgery. No differences were observed between groups in the response thresholds recorded for the hindpaw ipsilateral (Fig 1A) or contralateral (Fig 1B) to the avulsion injury pre-surgery [baseline (BL)]. No differences were observed between the SNAP groups on either the ipsilateral or contralateral hindpaw pre-drug, recorded 14, 21, and 28 days after surgery; that is, prior to initiation of PPF treatment. PPF had no effect on the response thresholds of sham operated rats, which showed mild and transient allodynia compared to avulsion. The SNAP group was significantly more allodynic than the sham group pre-drug (days 14–28) on both the ipsilateral (t30 = 4.396; p<0.001) and contralateral (t29 = 2.9; p<0.01) hindpaw. The two-way ANOVA comparing the AUC of Sham+Vehicle, Sham+PPF, SNAP+Vehicle, and SNAP+PPF over the drug treatment timecourse (days 35–63) showed a significant interaction in both the ipsilateral (F1,32 = 16.29; p<0.001; Fig 1C) and contralateral (F1,32 = 15.78; p<0.001; Fig 1D) hindpaw. There was also a significant main effect of surgery in both the ipsilateral (F1,32 = 16.57; p<0.001) and contralateral (F1,32 = 11.94; p<0.01) hindpaw as well as a significant main effect of drug treatment in both the ipsilateral (F1,32 = 8.603; p<0.01) and contralateral (F1,32 = 4.232; p<0.05) hindpaw. Bonferroni post hoc analysis of the AUCs revealed that the SNAP+Vehicle group was significantly more allodynic than all other groups (p<0.05) in both the ipsilateral and contralateral hindpaw. Furthermore, there were no significant differences between the other three groups in both the ipsilateral and contralateral hindpaw (p>0.05).
Detailed Timecourse of Administering Propentofylline on SNAP
Given that PPF was able to reverse SNAP, the next step was to determine how quickly PPF reversed the pain. To answer this question and to provide a replication of the full reversal of allodynia reported above, PPF was administered for 14 days and tested every 2–4 days over the drug timecourse. No differences were observed between groups in the response thresholds recorded for the hindpaw ipsilateral (Fig 2A) or contralateral (Fig 2B) to the avulsion injury pre-surgery [baseline (BL)]. No differences were observed between the SNAP groups on either the ipsilateral or contralateral hindpaw pre-drug, recorded 14, 21, and 28 days after surgery; that is, prior to initiation of PPF treatment. PPF had no effect on the response thresholds of sham operated rats, which showed mild and transient allodynia compared to SNAP. The SNAP group was significantly more allodynic than the sham group pre-drug (days 14–28) on both the ipsilateral (t22 = 4.155; p<0.001) and contralateral (t22 = 4.154; p<0.001) hindpaw. The two-way ANOVA comparing the AUC of Sham+Vehicle, Sham+PPF, SNAP+Vehicle, and SNAP+PPF over the drug treatment timecourse (days 35–42) showed a significant interaction in both the ipsilateral (F1,20 = 7.854; p<0.05; Fig 2C) and contralateral (F1,20 = 6.996; p<0.05; Fig 2D) hindpaw. There was also a significant main effect of surgery in both the ipsilateral (F1,20 = 40.65; p<0.0001) and contralateral (F1,20 = 67.61; p<0.0001) hindpaw as well as a significant main effect of drug treatment in both the ipsilateral (F1,20 = 7.442; p<0.05) and contralateral (F1,20 = 9.668; p<0.01) hindpaw. Bonferroni post hoc analysis of the AUCs revealed that SNAP+Vehicle group was significantly more allodynic than all other groups (p<0.05) in both the ipsilateral and contralateral hindpaw. Furthermore, there were no significant differences between the other three groups in both the ipsilateral and contralateral hindpaw (p>0.05). Replicating the effects reported above, PPF again completely reversed allodynia, such that response thresholds for the SNAP+PPF group were comparable to those of sham controls.
Effect of Administering Ibudilast Late in the Development of SNAP
To define whether similar results could be achieved using a different putative glial activation inhibitor with a distinct mechanism of action, ibudilast was chosen for test. Ibudilast was studied here as it is known to have MIF inhibitor and toll like receptor 4 (TLR4) inhibitor mechanisms of action for neuropathic pain reversal64 beyond its action as a PDE inhibitor.47 Ibudilast was administered s.c. once daily at 10 mg/kg for 35 days beginning 28 days post-surgery. No differences were observed between groups in the response thresholds recorded for the hindpaw ipsilateral (Fig 3A) or contralateral (Fig 3B) to the avulsion injury pre-surgery [baseline (BL)]. No differences were observed between the SNAP groups on either the ipsilateral or contralateral hindpaw pre-drug, recorded 14, 21, and 28 days after surgery, prior to initiation of ibudilast dosing. Ibudilast had no effect on the response thresholds of sham operated rats, which showed mild and transient allodynia compared to SNAP. The SNAP group was significantly more allodynic than the sham group pre-drug (days 14–28) on both the ipsilateral (t26 = 6.366; p<0.0001) and contralateral (t27 = 3.767; p<0.001) hindpaw. The two-way ANOVA comparing the AUC of Sham+Vehicle, Sham+Ibudilast, SNAP+Vehicle, and SNAP+Ibudilast over the drug treatment timecourse (days 35–63) showed a significant interaction in both the ipsilateral (F1,28 = 11.01; p<0.01; Fig 3C) and contralateral (F1,28 = 9.033; p<0.01; Fig 3D) hindpaw. There was also a significant main effect of surgery in both the ipsilateral (F1,28 = 28.92; p<0.0001) and contralateral (F1,28 = 13.03; p<0.01) hindpaw as well as a significant main effect of drug treatment in both the ipsilateral (F1,28 = 20.97; p<0.0001) and contralateral (F1,28 = 8.975; p<0.01) hindpaw. Bonferroni post hoc analysis of the AUCs revealed that SNAP+Vehicle group was significantly more allodynic than all other groups (p<0.05) in both the ipsilateral and contralateral hindpaw. Furthermore, there were no significant differences between the other three groups in both the ipsilateral and contralateral hindpaw (p>0.05).
Effect of Administering Ibudilast Early in the Development of SNAP
Since two different putative glial activation inhibitors could reverse established chronic SNAP, here it was tested whether ibudilast would prove effective when administration began at an earlier timepoint after surgery. In order to examine this issue, ibudilast was administered once daily s.c. at 10 mg/kg for 21 days beginning 14 days post-surgery. No differences were observed between groups in the response thresholds recorded for the hindpaw ipsilateral (Fig 4A) or contralateral (Fig 4B) to the avulsion injury pre-surgery [baseline (BL)]. Ibudilast had no effect on the response thresholds of sham operated rats, which showed mild and transient allodynia compared to SNAP. The two-way ANOVA comparing the AUC of Sham+Vehicle, Sham+Ibudilast, SNAP+Vehicle, and SNAP+Ibudilast over the drug treatment timecourse (days 14–35) showed a significant interaction in both the ipsilateral (F1,20 = 12.79; p<0.01; Fig 4C) and contralateral (F1,20 = 7.268; p<0.05; Fig 4D) hindpaw. There was also a significant main effect of surgery in both the ipsilateral (F1,20 = 58.24; p<0.0001) and contralateral (F1,20 = 55.06; p<0.001) hindpaw as well as a significant main effect of drug treatment in both the ipsilateral (F1,20 = 15.18; p<0.001) and contralateral (F1,20 = 10.20; p<0.01) hindpaw. Bonferroni post hoc analysis of the AUCs revealed that SNAP+Vehicle group was significantly more allodynic than all other groups (p<0.05) in both the ipsilateral and contralateral hindpaw. Furthermore, there were no significant differences between the other three groups in the ipsilateral hindpaw (p>0.05). While the ipsilateral and contralateral hindpaws of the SNAP+Ibudilast group did not statistically differ, the contralateral hindpaw of the SNAP+Ibudilast group, given its tighter SEMs, was found to be statistically different from both the Sham+Vehicle group and the Sham+Ibudilast group (p<0.05), supportive of a modestly reliable but incomplete reversal of allodynia contralaterally.
Effect of Administering (+)-Naltrexone on SNAP
The success of ibudilast and PPF, above, raises the question of whether a glial modulator with no known PDE activity can also resolve SNAP. As one of the mechanisms of action of ibudilast is as a TLR4 inhibitor,64 this study sought to define whether TLR4 inhibition would be sufficient to resolve SNAP. Given that TLR4 is activated by endogenous substances released by cellular stress, damage and death,11, 38 inhibition of TLR4 was chosen from the known ibudilast mechanisms of action for test here given the neuropathology associated with SNAP. (+)-Naltrexone was chosen for test as it is a non-opioid, blood-brain barrier permeable, highly selective TLR4 antagonist75 that has been shown to reverse peripheral neuropathic pain.39 Here we administered 3x daily (+)-naltrexone s.c. at 6 mg/kg for 14 days beginning 32 days post-surgery. To extend the detailed timecourse reported for PPF above, behavior was again recorded every 3–4 days of (+)-naltrexone dosing so to define the rapidity with which allodynia reversal would occur in the absence of PDE inhibition. No differences were observed between groups in the response thresholds recorded for the hindpaw ipsilateral (Fig 5A) or contralateral (Fig 5B) to the avulsion injury pre-surgery [baseline (BL)]. No differences were observed between the SNAP groups on either the ipsilateral or contralateral hindpaw pre-drug recorded 14, 21, 28, and 32 days after surgery; that is prior to initiation of (+)-naltrexone dosing. (+)-Naltrexone had no effect on response thresholds of sham operated rats, which showed mild and transient allodynia compared to SNAP. The SNAP group was significantly more allodynic than the sham group pre-drug (days 14–32) on both the ipsilateral (t16 = 6.928; p<0.0001) and contralateral (t16 = 7.444; p<0.0001) hindpaw. The one-way ANOVA comparing the AUC of Sham+Vehicle, SNAP+Vehicle, and SNAP+(+)-Naltrexone over the drug treatment timecourse (days 35–46) was significant in both the ipsilateral (F2,15 = 11.76; p<0.001; Fig 5C) and contralateral (F2,15 = 16.82; p<0.001; Fig 5D) hindpaw. Bonferroni p post hoc analysis of the AUCs revealed that SNAP+Vehicle group was significantly more allodynic than all other groups (p<0.05) in both the ipsilateral and contralateral hindpaw. Furthermore, there were no significant differences between the other two groups in both the ipsilateral and contralateral hindpaw (p>0.05).
Effect of Ceasing (+)-Naltrexone Administration on SNAP
In order to determine whether the reversal of allodynia observed with such glial modulators may be sustained after elimination of the drug, a final study was undertaken using (+)-naltrexone as a test compound for this purpose. Here we administered 3x daily (+)-naltrexone s.c. at 6 mg/kg for 6 days beginning 28 days post-surgery, then stopped administering the drug after day 33 and continued to record behavior until the rats were back at pre-drug allodynia pain thresholds. No differences were observed between groups in the response thresholds recorded for the hindpaw ipsilateral (Fig 6A) or contralateral (Fig 6B) to the avulsion injury pre-surgery [baseline (BL)]. No differences were observed between the SNAP groups on either the ipsilateral or contralateral hindpaw pre-drug recorded 14, 21, and 28 days after surgery; that is prior to initiation of (+)-naltrexone dosing. The t-test comparing the AUC of SNAP+Vehicle and SNAP+(+)-Naltrexone over the drug treatment timecourse (days 29–33) was significant in both the ipsilateral (t9 = 3.267; p<0.01; Fig 6C) and contralateral (t9 = 2.833; p<0.05; Fig 6D) hindpaw. The t-test comparing the AUC of SNAP+Vehicle and SNAP+(+)-Naltrexone over the drug cessation period (days 35–42) was not significant in either the ipsilateral (p>0.05; Fig 6E) or contralateral (p>0.05; Fig 6F) hindpaw.
Discussion
Here we show that three different putative glial inhibitors with distinct mechanisms of action, PPF, ibudilast, and (+)-naltrexone, are all able to reverse SNAP. Thus, converging lines of evidence from testing multiple inhibitors commonly assumed to have some glial mechanisms of action suggest a role for glia in this phenomenon. Importantly, none of these inhibitors reversed allodynia upon first administration, but required multiple days of treatment to achieve full reversal. Taken together, these data suggest that treating CNP with inhibitors that have some action on glia may prove to be a fruitful strategy for improving clinical pain control in such cases.
One strategy for controlling CNP is increasing cyclic adenosine monophosphate (cAMP) by inhibiting phosphodiesterases (PDEs) which hydrolyze cAMP and/or cyclic guanosine monophosphate (cGMP).8 PDE inhibitors reverse CCI-induced allodynia and hyperalgesia,79 and rolipram has been shown to decrease proinflammatory cytokine levels in TBI3 and SCI.59 Further, selective PDE4 inhibitors decrease hindpaw allodynia from compression SCI by reducing immune cell infiltration/activation and free radical formation.5 PPF is a methyl-xanthine selective PDE4 inhibitor,72 an isoform expressed in both microglia and astrocytes.51 Although neurons express PDE4, PDE4 is the major isoform expressed in immune and inflammatory cells.41 Further, microglial activation is regulated by PDE466 and PPF decreases release of proinflammatory cytokines in microglia.65 In addition, systemic PPF decreases both microglia and astrocyte activation in a rat peripheral neuropathy model.72 The present study is the first to show that systemic PPF is effective in reversing allodynia in a CNP model. The only other neuropathic pain studies using PPF administered it intrathecally in a rat model of hemisection SCI, which also reversed mechanical allodynia.28 Notably, the half life of PPF and its active metabolite is very short, ~1 hr,72 therefore the anti-allodynic effects seen in the current studies at ~15 hr post administration suggest that persistent intracellular alterations have occurred, in keeping with the conclusions of Sweitzer et al.72 Studies have shown that increases in cAMP results in increases of the anti-inflammatory cytokine IL-10,2, 21, 69 including from microglia.86 Notably, IL-10 has been recognized for its therapeutic potential in SCI.76, 89 The resulting ratio of decreased proinflammatory signaling to increased anti-inflammatory signaling could explain the sustained pain reversal seen even when propentofylline is not present at the time of testing.
Although ibudilast, like PPF, is a PDE inhibitor (inhibits PDE 1, 2, 3, and 4 in rat),46 PDE inhibition alone cannot account for the anti-inflammatory effects of ibudilast.18 Therefore, there must be an alternative mechanism.64 Ibudilast is now known to also inhibit macrophage migration inhibitory factor (MIF)64 and is effective in treating SNL,47 SCI (present data),33 and CCI pain.33 MIF stimulates the release of IL-1 and TNF and is required for IL-1 and TNF-induced MAPK activation.77 MIF itself is now recognized as a pro-inflammatory cytokine1 and has been implicated in multiple central neuropathies including stroke,80 MS,32 TBI,78 and SCI.57 In studies examining the role of MIF in SCI, MIF knock-out mice with compression SCI recovered motor function faster than wild-type controls,57 and a clinical pilot study found that SCI patients with chronic pain had higher levels of serum MIF compared to both non-injured controls as well as SCI patients who did not have a history of chronic pain.70 It is important to note that these increased circulating levels of MIF were seen long after the initial injury, providing evidence that there is enduring release of MIF that could then be sustaining the CNP state we observe in SNAP which is reversed by ibudilast administration. Taken together, these studies suggest that since MIF is up-regulated in multiple CNP models, MIF inhibitors are potential candidates for successful CNP therapeutics.
Another potential CNP treatment that has not been as well studied is (+)-naltrexone, a selective TLR4 antagonist, as evidenced by in vivo and in vitro studies as well as in silico modeling.37, 39, 40, 75 In contrast to PPF and ibudilast, (+)-naltrexone has no known PDE or MIF inhibitory actions. Although we have shown that (+)-naltrexone reverses peripheral CCI pain,39 no one has shown reversal of CNP using (+)-naltrexone until now. (+)-Naltrexone is unique compared to the (−)-naltrexone isomer in that it does not bind classical μ-opioid receptors.75 As a result, (+)-naltrexone does not cause many of the undesirable side effects seen with many other opiate-based analgesics,39, 75 making it a more desirable treatment option. Since TLR4 in the CNS is found predominantly on glial cells37 and blocking TLR4 reverses allodynia, it is very likely that the pain reversal seen here after (+)-naltrexone administration is glially mediated. Importantly, as observed with PPF and ibudilast, it took multiple days of (+)-naltrexone administration to see full reversal, indicating that acute dosing with glial modulators may not be ideal for treating established CNP. In addition, the anti-allodynic effects of (+)-naltrexone gradually dissipated once the drug was no longer being administered, which has also been observed previously for PPF29 and ibudilast.47 This suggests that although these drugs are perhaps able to attenuate glial activation during administration, they are not inducing permanent changes that can overcome the proinflammatory environment and persistent glial activation caused by the original injury and subsequent central sensitization.
One reason that many drugs fail clinically could be that they are not given for a sufficiently long duration, and there are examples in the literature of this with all three of the drugs tested here. Reversal of spinal nerve ligation (SNL)-induced allodynia failed to occur when PPF was administered for only 7 days,58 whereas it took 10 days of daily PPF administration to both prevent and reverse SNL-induced allodynia.72 In the CCI and spinal nerve ligation models, only transient pain reversal occurred with one day of systemic ibudilast, and ~5 days of dosing was required for full reversal of CCI allodynia.47 Similarly, only transient reversal (~1 hr) of allodynia occurs with a single intrathecal injection of (+)-naltrexone, whereas chronic intrathecal infusion of (+)-naltrexone completely reversed allodynia only when administered for 4 days.39 In the current studies it took multiple days of systemic administration to see significant allodynic reversal with all 3 of the drugs tested, which suggests that had these drugs been administered as a single injection or for only a couple of days they would have failed.81
Furthermore, treatments are often more effective if they are administered early on in pain development. For instance, disease modifying anti-rheumatic drugs for rheumatoid arthritis are only effective for treating pain and other symptoms if administered within the first three months symptoms appear.50 Another example of successful early pharmacological intervention is in migraine patients, where administering triptans within the first 20–120 minutes after symptom onset can actually prevent allodynia from developing.12 Unfortunately, there are many cases where pain patients do not or are not able to seek treatment until they have been in chronic pain for months. Thus, it is important to find therapeutics that are effective not only when administered upon first pain development but also after the symptoms have become chronic. Here we gave ibudilast in the early stages of pain development as well as in a later stage of chronic pain development, and saw complete reversal of allodynia in both cases. These data suggest that glia are likely involved both in the early stages of neuropathic pain development as well as the long-term maintenance of chronic neuropathic pain, and that at least ibudilast has the potential to treat patients at various stages of their pain progression. Ibudilast has recently been identified as a toll-like receptor 4 (TLR4) antagonist40 (Johnson, unpub. obs., 2012), and ibudilast as well as PPF and (+)-naltrexone were able to treat below-level contralateral pain as well, increasing their attractiveness as therapeutics.
Although the studies here focus on the glial mechanisms underlying CNP, we cannot rule out the fact that neurons and other cell types are also likely involved. Chew et al. report increased ipsilateral bilateral glial activation in the dorsal horn following L3–L6 dorsal root avulsion16 and increased ipsilateral mechanical hypersensitivity following L5 dorsal and ventral root avulsion,17 which lends support to what we see in the current studies. However, they also see significant increases in bilateral infiltrating macrophages in the dorsal horn, which could also be helping to maintain the neuropathic pain.16, 17 Inhibiting AMPA receptors attenuates mechanical allodynia and neuronal hyperexciteability following SCI.30 In addition, injecting NMDA near a SCI contusion injury site delays functional recovery22 and administering NMDA antagonists shortly after SCI can improve functional recovery and increase pain thresholds.44 A recent study found that co-administering PPF and a neuronal NMDA receptor inhibitor had additive effects on attenuating peripherally induced-chronic pain.54 It is thought that MIF triggers ERK/NMDA dependent plasticity in sensory neurons1 and it has been shown that ibudilast suppresses apoptosis in nerve cells during ischemic brain injury.48 Lastly, there are studies that report that TLR4 is expressed in primary sensory neurons and can contribute to neuropathic pain.19
Since the drugs used here were administered systemically and are putative glial inhibitors, we recognize that their actions may not be exclusively on glia and could be interacting with other cell types, including resident peripheral immune cells or cells recruited to the injured spinal tissue. PPF suppresses the production of TNF-α43 and reactive oxygen species in macrophages,4, and can also counteract neutrophil activation by blocking the removal of adenosine.88 Both PPF and ibudilast can prevent kainite-induced cell death in oligodendroglia,87 and ibudilast can also inhibit platelet aggregation in the presence of endothelial cells.62 Furthermore, ibudilast reduces inflammatory cell infiltration into the dorsal spinal cord in EAE,26 which could also be happening in SNAP, along with attenuating resident microglial activation. Since TLR4 can also be expressed on neurons19 and peripheral49, 52 or recruited immune cells,67 (+)-naltrexone could be exerting some effects on these cells as well. Since we use a pain model of central origin and multiple pain studies using these compounds suggest the anti-allodynic effects are glially mediated,33, 39, 47, 61, 72, 73, 82 it is likely that these drugs exert at least some of their anti-allodynic effects by inhibiting glial activation. However, these observations highlight the importance of considering the contributions of neurons, peripheral/infiltrating immune cells, and glia to CNP.
In conclusion, it is clear that glia play an important role in the pathogenesis of CNP. We were able to show, using our dorsal root avulsion model of SCI (SNAP), that administering putative glial inhibitors reverses SCI-induced allodynia both at the onset of pain as well as after chronic neuropathic pain had developed, although it takes at least a week of daily dosing to achieve full reversal. Treating CNP with inhibitors that have some mechanistic action on glial cells has the potential to optimize treatment and dramatically increase the quality of life for thousands of chronic pain patients.
Perspective.
CNP that develops after trauma is often described by patients as severe and intolerable. Unfortunately, current treatments are not effective. This work suggests that using pharmacological treatments that target glial cells could be an effective clinical treatment for CNP.
Acknowledgments
The authors would like to thank Dr. Kenner Rice for providing the (+)-naltrexone, Dr. Kirk Johnson for providing the ibudilast, and MediciNova and Solace/Patheon for providing the propentofylline.
Footnotes
All animal work was done on the University of Colorado Boulder campus
Disclosures
Financial and material support was given by Craig Hospital and DA024044. A portion of this work was supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism. The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexander JK, Cox GM, Tian JB, Zha AM, Wei P, Kigerl KA, Reddy MK, Dagia NM, Sielecki T, Zhu MX, Satoskar AR, McTigue DM, Whitacre CC, Popovich PG. Macrophage migration inhibitory factor (MIF) is essential for inflammatory and neuropathic pain and enhances pain in response to stress. Exp Neurol. 2012;236:351–362. doi: 10.1016/j.expneurol.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez Y, Municio C, Alonso S, Sanchez Crespo M, Fernandez N. The induction of IL-10 by zymosan in dendritic cells depends on CREB activation by the coactivators CREB-binding protein and TORC2 and autocrine PGE2. J Immunol. 2009;183:1471–1479. doi: 10.4049/jimmunol.0900312. [DOI] [PubMed] [Google Scholar]
- 3.Atkins CM, Oliva AA, Jr, Alonso OF, Pearse DD, Bramlett HM, Dietrich WD. Modulation of the cAMP signaling pathway after traumatic brain injury. Exp Neurol. 2007;208:145–158. doi: 10.1016/j.expneurol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banati RB, Schubert P, Rothe G, Gehrmann J, Rudolphi K, Valet G, Kreutzberg GW. Modulation of intracellular formation of reactive oxygen intermediates in peritoneal macrophages and microglia/brain macrophages by propentofylline. J Cereb Blood Flow Metab. 1994;14:145–149. doi: 10.1038/jcbfm.1994.19. [DOI] [PubMed] [Google Scholar]
- 5.Bao F, Fleming JC, Golshani R, Pearse DD, Kasabov L, Brown A, Weaver LC. A selective phosphodiesterase-4 inhibitor reduces leukocyte infiltration, oxidative processes, and tissue damage after spinal cord injury. J Neurotrauma. 2011;28:1035–1049. doi: 10.1089/neu.2010.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berland D, Rodgers P. Rational use of opioids for management of chronic nonterminal pain. Am Fam Physician. 2012;86:252–258. [PubMed] [Google Scholar]
- 7.Berman JS, Birch R, Anand P. Pain following human brachial plexus injury with spinal cord root avulsion and the effect of surgery. Pain. 1998;75:199–207. doi: 10.1016/s0304-3959(97)00220-0. [DOI] [PubMed] [Google Scholar]
- 8.Bland ST, Hutchinson MR, Maier SF, Watkins LR, Johnson KW. The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release. Brain Behav Immun. 2009;23:492–497. doi: 10.1016/j.bbi.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boivie J. Chapter 48 Central post-stroke pain. Handb Clin Neurol. 2006;81:715–730. doi: 10.1016/S0072-9752(06)80052-7. [DOI] [PubMed] [Google Scholar]
- 10.Breuer B, Pappagallo M, Knotkova H, Guleyupoglu N, Wallenstein S, Portenoy RK. A randomized, double-blind, placebo-controlled, two-period, crossover, pilot trial of lamotrigine in patients with central pain due to multiple sclerosis. Clin Ther. 2007;29:2022–2030. doi: 10.1016/j.clinthera.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan MM, Hutchinson M, Watkins LR, Yin H. Toll-like receptor 4 in CNS pathologies. J Neurochem. 2010;114:13–27. doi: 10.1111/j.1471-4159.2010.06736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol. 2004;55:19–26. doi: 10.1002/ana.10786. [DOI] [PubMed] [Google Scholar]
- 13.Calvo M, Bennett DL. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp Neurol. 2012;234:271–282. doi: 10.1016/j.expneurol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Carlstedt T. Root repair review: basic science background and clinical outcome. Restor Neurol Neurosci. 2008;26:225–241. [PubMed] [Google Scholar]
- 15.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 16.Chew DJ, Carlstedt T, Shortland PJ. A comparative histological analysis of two models of nerve root avulsion injury in the adult rat. Neuropathol Appl Neurobiol. 2011;37:613–632. doi: 10.1111/j.1365-2990.2011.01176.x. [DOI] [PubMed] [Google Scholar]
- 17.Chew DJ, Murrell K, Carlstedt T, Shortland PJ. Segmental spinal root avulsion in the adult rat: a model to study avulsion injury pain. J Neurotrauma. 2013;30:160–172. doi: 10.1089/neu.2012.2481. [DOI] [PubMed] [Google Scholar]
- 18.Cho Y, Crichlow GV, Vermeire JJ, Leng L, Du X, Hodsdon ME, Bucala R, Cappello M, Gross M, Gaeta F, Johnson K, Lolis EJ. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc Natl Acad Sci U S A. 2010;107:11313–11318. doi: 10.1073/pnas.1002716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res. 2011;90:759–764. doi: 10.1177/0022034511400225. [DOI] [PubMed] [Google Scholar]
- 20.Edgar RE, Best LG, Quail PA, Obert AD. Computer-assisted DREZ microcoagulation: posttraumatic spinal deafferentation pain. J Spinal Disord. 1993;6:48–56. [PubMed] [Google Scholar]
- 21.Eigler A, Siegmund B, Emmerich U, Baumann KH, Hartmann G, Endres S. Anti-inflammatory activities of cAMP-elevating agents: enhancement of IL-10 synthesis and concurrent suppression of TNF production. J Leukoc Biol. 1998;63:101–107. doi: 10.1002/jlb.63.1.101. [DOI] [PubMed] [Google Scholar]
- 22.Faden AI, Simon RP. A potential role for excitotoxins in the pathophysiology of spinal cord injury. Ann Neurol. 1988;23:623–626. doi: 10.1002/ana.410230618. [DOI] [PubMed] [Google Scholar]
- 23.Falci S, Best L, Bayles R, Lammertse D, Starnes C. Dorsal root entry zone microcoagulation for spinal cord injury-related central pain: operative intramedullary electrophysiological guidance and clinical outcome. J Neurosurg. 2002;97:193–200. doi: 10.3171/spi.2002.97.2.0193. [DOI] [PubMed] [Google Scholar]
- 24.Finnerup NB. A review of central neuropathic pain states. Curr Opin Anaesthesiol. 2008;21:586–589. doi: 10.1097/ACO.0b013e32830a4c11. [DOI] [PubMed] [Google Scholar]
- 25.Frampton M, Harvey RJ, Kirchner V. Propentofylline for dementia. Cochrane Database Syst Rev. 2003:CD002853. doi: 10.1002/14651858.CD002853. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto T, Sakoda S, Fujimura H, Yanagihara T. Ibudilast, a phosphodiesterase inhibitor, ameliorates experimental autoimmune encephalomyelitis in Dark August rats. J Neuroimmunol. 1999;95:35–42. doi: 10.1016/s0165-5728(98)00251-3. [DOI] [PubMed] [Google Scholar]
- 27.Graeber MB, Christie MJ. Multiple mechanisms of microglia: a gatekeeper’s contribution to pain states. Exp Neurol. 2012;234:255–261. doi: 10.1016/j.expneurol.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Gwak YS, Crown ED, Unabia GC, Hulsebosch CE. Propentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. Pain. 2008;138:410–422. doi: 10.1016/j.pain.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gwak YS, Hulsebosch CE. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience. 2009;161:895–903. doi: 10.1016/j.neuroscience.2009.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gwak YS, Kang J, Leem JW, Hulsebosch CE. Spinal AMPA receptor inhibition attenuates mechanical allodynia and neuronal hyperexcitability following spinal cord injury in rats. J Neurosci Res. 2007;85:2352–2359. doi: 10.1002/jnr.21379. [DOI] [PubMed] [Google Scholar]
- 31.Gwak YS, Unabia GC, Hulsebosch CE. Activation of p-38alpha MAPK contributes to neuronal hyperexcitability in caudal regions remote from spinal cord injury. Exp Neurol. 2009;220:154–161. doi: 10.1016/j.expneurol.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagman S, Raunio M, Rossi M, Dastidar P, Elovaara I. Disease-associated inflammatory biomarker profiles in blood in different subtypes of multiple sclerosis: prospective clinical and MRI follow-up study. J Neuroimmunol. 2011;234:141–147. doi: 10.1016/j.jneuroim.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Hama AT, Broadhead A, Lorrain DS, Sagen J. The antinociceptive effect of the asthma drug ibudilast in rat models of peripheral and central neuropathic pain. J Neurotrauma. 2012;29:600–610. doi: 10.1089/neu.2011.1863. [DOI] [PubMed] [Google Scholar]
- 34.Harvey LO. Efficient estimation of sensory thresholds. Behav Res Methods Inst Comp. 1986;18:623–632. [Google Scholar]
- 35.Heblich F, Tran Van Minh A, Hendrich J, Watschinger K, Dolphin AC. Time course and specificity of the pharmacological disruption of the trafficking of voltage-gated calcium channels by gabapentin. Channels (Austin) 2008;2:4–9. doi: 10.4161/chan.2.1.6045. [DOI] [PubMed] [Google Scholar]
- 36.Hook MA, Liu GT, Washburn SN, Ferguson AR, Bopp AC, Huie JR, Grau JW. The impact of morphine after a spinal cord injury. Behav Brain Res. 2007;179:281–293. doi: 10.1016/j.bbr.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci. 2012;32:11187–11200. doi: 10.1523/JNEUROSCI.0684-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63:772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin SL, Ding SL, Lin SC. Phosphodiesterase 4 and its inhibitors in inflammatory diseases. Chang Gung Med J. 2012;35:197–210. doi: 10.4103/2319-4170.106152. [DOI] [PubMed] [Google Scholar]
- 42.Jones TL, Sorkin LS. Calcium-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptors mediate development, but not maintenance, of secondary allodynia evoked by first-degree burn in the rat. J Pharmacol Exp Ther. 2004;310:223–229. doi: 10.1124/jpet.103.064741. [DOI] [PubMed] [Google Scholar]
- 43.Jung S, Donhauser T, Toyka KV, Hartung HP. Propentofylline and iloprost suppress the production of TNF-alpha by macrophages but fail to ameliorate experimental autoimmune encephalomyelitis in Lewis rats. J Autoimmun. 1997;10:519–529. doi: 10.1006/jaut.1997.0159. [DOI] [PubMed] [Google Scholar]
- 44.Kim Y, Cho HY, Ahn YJ, Kim J, Yoon YW. Effect of NMDA NR2B antagonist on neuropathic pain in two spinal cord injury models. Pain. 2012;153:1022–1029. doi: 10.1016/j.pain.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Kukkar A, Bali A, Singh N, Jaggi AS. Implications and mechanism of action of gabapentin in neuropathic pain. Arch Pharm Res. 2013;36:237–251. doi: 10.1007/s12272-013-0057-y. [DOI] [PubMed] [Google Scholar]
- 46.Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW. Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opin Investig Drugs. 2007;16:935–950. doi: 10.1517/13543784.16.7.935. [DOI] [PubMed] [Google Scholar]
- 47.Ledeboer A, Liu T, Shumilla JA, Mahoney JH, Vijay S, Gross MI, Vargas JA, Sultzbaugh L, Claypool MD, Sanftner LM, Watkins LR, Johnson KW. The glial modulatory drug AV411 attenuates mechanical allodynia in rat models of neuropathic pain. Neuron Glia Biol. 2006;2:279–291. doi: 10.1017/S1740925X0700035X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JY, Cho E, Ko YE, Kim I, Lee KJ, Kwon SU, Kang DW, Kim JS. Ibudilast, a phosphodiesterase inhibitor with anti-inflammatory activity, protects against ischemic brain injury in rats. Brain Res. 2012;1431:97–106. doi: 10.1016/j.brainres.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Li HG, Zhou ZG, Li Y, Zheng XL, Lei S, Zhu L, Wang Y. Alterations of Toll-like receptor 4 expression on peripheral blood monocytes during the early stage of human acute pancreatitis. Dig Dis Sci. 2007;52:1973–1978. doi: 10.1007/s10620-006-9211-4. [DOI] [PubMed] [Google Scholar]
- 50.Li LC, Badley EM, MacKay C, Mosher D, Jamal SW, Jones A, Bombardier C. An evidence-informed, integrated framework for rheumatoid arthritis care. Arthritis Rheum. 2008;59:1171–1183. doi: 10.1002/art.23931. [DOI] [PubMed] [Google Scholar]
- 51.Madelian V, La Vigne E. Rapid regulation of a cyclic AMP-specific phosphodiesterase (PDE IV) by forskolin and isoproterenol in LRM55 astroglial cells. Biochem Pharmacol. 1996;51:1739–1747. doi: 10.1016/0006-2952(96)00167-0. [DOI] [PubMed] [Google Scholar]
- 52.Methe H, Kim JO, Kofler S, Weis M, Nabauer M, Koglin J. Expansion of circulating Toll-like receptor 4-positive monocytes in patients with acute coronary syndrome. Circulation. 2005;111:2654–2661. doi: 10.1161/CIRCULATIONAHA.104.498865. [DOI] [PubMed] [Google Scholar]
- 53.Milligan ED, O’Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morales F, Constandil L, Pelissier T, Hernandez A, Laurido C. Antinociceptive interaction of (+/−)-CPP and propentofylline in monoarthritic rats. Arthritis Res Ther. 2012;14:R196. doi: 10.1186/ar4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagata K, Ogawa T, Omosu M, Fujimoto K, Hayashi S. In vitro and in vivo inhibitory effects of propentofylline on cyclic AMP phosphodiesterase activity. Arzneimittelforschung. 1985;35:1034–1036. [PubMed] [Google Scholar]
- 56.Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA. 2008;300:711–719. doi: 10.1001/jama.300.6.711. [DOI] [PubMed] [Google Scholar]
- 57.Nishio Y, Koda M, Hashimoto M, Kamada T, Koshizuka S, Yoshinaga K, Onodera S, Nishihira J, Okawa A, Yamazaki M. Deletion of macrophage migration inhibitory factor attenuates neuronal death and promotes functional recovery after compression-induced spinal cord injury in mice. Acta Neuropathol. 2009;117:321–328. doi: 10.1007/s00401-008-0476-x. [DOI] [PubMed] [Google Scholar]
- 58.Obata H, Sakurazawa S, Kimura M, Saito S. Activation of astrocytes in the spinal cord contributes to the development of bilateral allodynia after peripheral nerve injury in rats. Brain Res. 2010;1363:72–80. doi: 10.1016/j.brainres.2010.09.105. [DOI] [PubMed] [Google Scholar]
- 59.Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 60.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 61.Rahn KA, McLaughlin PJ, Zagon IS. Prevention and diminished expression of experimental autoimmune encephalomyelitis by low dose naltrexone (LDN) or opioid growth factor (OGF) for an extended period: Therapeutic implications for multiple sclerosis. Brain Res. 2011;1381:243–253. doi: 10.1016/j.brainres.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 62.Rile G, Yatomi Y, Qi R, Satoh K, Ozaki Y. Potentiation of ibudilast inhibition of platelet aggregation in the presence of endothelial cells. Thromb Res. 2001;102:239–246. doi: 10.1016/s0049-3848(01)00258-4. [DOI] [PubMed] [Google Scholar]
- 63.Rintala DH, Holmes SA, Courtade D, Fiess RN, Tastard LV, Loubser PG. Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Arch Phys Med Rehabil. 2007;88:1547–1560. doi: 10.1016/j.apmr.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 64.Rolan P, Hutchinson M, Johnson K. Ibudilast: a review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expert Opin Pharmacother. 2009;10:2897–2904. doi: 10.1517/14656560903426189. [DOI] [PubMed] [Google Scholar]
- 65.Schubert P, Morino T, Miyazaki H, Ogata T, Nakamura Y, Marchini C, Ferroni S. Cascading glia reactions: a common pathomechanism and its differentiated control by cyclic nucleotide signaling. Ann N Y Acad Sci. 2000;903:24–33. doi: 10.1111/j.1749-6632.2000.tb06346.x. [DOI] [PubMed] [Google Scholar]
- 66.Sebastiani G, Morissette C, Lagace C, Boule M, Ouellette MJ, McLaughlin RW, Lacombe D, Gervais F, Tremblay P. The cAMP-specific phosphodiesterase 4B mediates Abeta-induced microglial activation. Neurobiol Aging. 2006;27:691–701. doi: 10.1016/j.neurobiolaging.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 67.Shichita T, Ago T, Kamouchi M, Kitazono T, Yoshimura A, Ooboshi H. Novel therapeutic strategies targeting innate immune responses and early inflammation after stroke. J Neurochem. 2012;123 (Suppl 2):29–38. doi: 10.1111/j.1471-4159.2012.07941.x. [DOI] [PubMed] [Google Scholar]
- 68.Siddall PJ, Taylor DA, McClelland JM, Rutkowski SB, Cousins MJ. Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain. 1999;81:187–197. doi: 10.1016/s0304-3959(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 69.Siegmund B, Eigler A, Moeller J, Greten TF, Hartmann G, Endres S. Suppression of tumor necrosis factor-alpha production by interleukin-10 is enhanced by cAMP-elevating agents. Eur J Pharmacol. 1997;321:231–239. doi: 10.1016/s0014-2999(96)00947-8. [DOI] [PubMed] [Google Scholar]
- 70.Stein A, Panjwani A, Sison C, Rosen L, Chugh R, Metz C, Bank M, Bloom O. Pilot Study: Elevated circulating levels of the pro-inflammatory cytokine macrophage migration inhibitory factor in chronic spinal cord injury patients. Arch Phys Med Rehabil. 2013 doi: 10.1016/j.apmr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 71.Sweitzer S, De Leo J. Propentofylline: glial modulation, neuroprotection, and alleviation of chronic pain. Handb Exp Pharmacol. 2011:235–250. doi: 10.1007/978-3-642-13443-2_8. [DOI] [PubMed] [Google Scholar]
- 72.Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J Pharmacol Exp Ther. 2001;297:1210–1217. [PubMed] [Google Scholar]
- 73.Tawfik VL, Nutile-McMenemy N, Lacroix-Fralish ML, Deleo JA. Efficacy of propentofylline, a glial modulating agent, on existing mechanical allodynia following peripheral nerve injury. Brain Behav Immun. 2007;21:238–246. doi: 10.1016/j.bbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 74.Tawfik VL, Regan MR, Haenggeli C, Lacroix-Fralish ML, Nutile-McMenemy N, Perez N, Rothstein JD, DeLeo JA. Propentofylline-induced astrocyte modulation leads to alterations in glial glutamate promoter activation following spinal nerve transection. Neuroscience. 2008;152:1086–1092. doi: 10.1016/j.neuroscience.2008.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM, Baumann MH, Hutchinson MR, Rice KC, Watkins LR, Shaham Y. Effect of Chronic Delivery of the Toll-like Receptor 4 Antagonist (+)-Naltrexone on Incubation of Heroin Craving. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thompson CD, Zurko JC, Hellenbrand DJ, Hanna B, Hanna A. The Therapeutic Role of Interleukin-10 after Spinal Cord Injury. J Neurotrauma. 2013 doi: 10.1089/neu.2012.2651. [DOI] [PubMed] [Google Scholar]
- 77.Toh ML, Aeberli D, Lacey D, Yang Y, Santos LL, Clarkson M, Sharma L, Clyne C, Morand EF. Regulation of IL-1 and TNF receptor expression and function by endogenous macrophage migration inhibitory factor. J Immunol. 2006;177:4818–4825. doi: 10.4049/jimmunol.177.7.4818. [DOI] [PubMed] [Google Scholar]
- 78.Truettner JS, Suzuki T, Dietrich WD. The effect of therapeutic hypothermia on the expression of inflammatory response genes following moderate traumatic brain injury in the rat. Brain Res Mol Brain Res. 2005;138:124–134. doi: 10.1016/j.molbrainres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 79.Vakili A, Shirvanian M, Safakhah H, Rashidy-Pour A. Pentoxifylline decreases allodynia and hyperalgesia in a rat model of neuropathic pain. Daru. 2011;19:306–311. [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L, Zis O, Ma G, Shan Z, Zhang X, Wang S, Dai C, Zhao J, Lin Q, Lin S, Song W. Upregulation of macrophage migration inhibitory factor gene expression in stroke. Stroke. 2009;40:973–976. doi: 10.1161/STROKEAHA.108.530535. [DOI] [PubMed] [Google Scholar]
- 81.Watkins LR, Hutchinson MR, Johnson KW. Commentary on Landry et al.: “Propentofylline, a CNS glial modulator, does not decrease pain in post-herpetic neuralgia patients: in vitro evidence for differential responses in human and rodent microglia and macrophages”. Exp Neurol. 2012;234:351–353. doi: 10.1016/j.expneurol.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 82.Whitehead KJ, Smith CG, Delaney SA, Curnow SJ, Salmon M, Hughes JP, Chessell IP. Dynamic regulation of spinal pro-inflammatory cytokine release in the rat in vivo following peripheral nerve injury. Brain Behav Immun. 2010;24:569–576. doi: 10.1016/j.bbi.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 83.Wieseler J, Ellis A, Maier SF, Watkins LR, Falci S. Unilateral T13 and L1 dorsal root avulsion: methods for a novel model of central neuropathic pain. Methods Mol Biol. 2012;851:171–183. doi: 10.1007/978-1-61779-561-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wieseler J, Ellis AL, McFadden A, Brown K, Starnes C, Maier SF, Watkins LR, Falci S. Below level central pain induced by discrete dorsal spinal cord injury. J Neurotrauma. 2010;27:1697–1707. doi: 10.1089/neu.2010.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Woller SA, Moreno GL, Hart N, Wellman PJ, Grau JW, Hook MA. Analgesia or addiction?: implications for morphine use after spinal cord injury. J Neurotrauma. 2012;29:1650–1662. doi: 10.1089/neu.2011.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoshikawa M, Suzumura A, Tamaru T, Takayanagi T, Sawada M. Effects of phosphodiesterase inhibitors on cytokine production by microglia. Mult Scler. 1999;5:126–133. doi: 10.1177/135245859900500210. [DOI] [PubMed] [Google Scholar]
- 87.Yoshioka A, Shimizu Y, Hirose G, Kitasato H, Pleasure D. Cyclic AMP-elevating agents prevent oligodendroglial excitotoxicity. J Neurochem. 1998;70:2416–2423. doi: 10.1046/j.1471-4159.1998.70062416.x. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y, Fredholm BB. Propentofylline enhancement of the actions of adenosine on neutrophil leukocytes. Biochem Pharmacol. 1994;48:2025–2032. doi: 10.1016/0006-2952(94)90501-0. [DOI] [PubMed] [Google Scholar]
- 89.Zhou Z, Peng X, Insolera R, Fink DJ, Mata M. IL-10 promotes neuronal survival following spinal cord injury. Exp Neurol. 2009;220:183–190. doi: 10.1016/j.expneurol.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]